Abstract
The extracellular matrix protein Laminin B1 (LamB1) regulates tumor cell migration and invasion. Carcinoma cells acquire invasive properties by epithelial to mesenchymal transition (EMT), which is a fundamental step in dissemination of metastatic cells from the primary tumor. Recently, we showed that enhanced translation of LamB1 upon EMT of malignant hepatocytes is mediated by an internal ribosome entry site (IRES). We demonstrated that the IRES transacting factor La binds the minimal IRES motif and positively modulates IRES activity of LamB1. Here, we show that platelet-derived growth factor (PDGF) enhances IRES activity of LamB1 by the increasing cytoplasmic localization of La during EMT. Accordingly, cells expressing dominant negative PDGF receptor display reduced cytoplasmic accumulation of La and show no elevation of IRES activity or endogenous LamB1 levels after stimulation with PDGF. Furthermore, La-mediated regulation of LamB1 IRES activity predominantly depends on MAPK/ERK signaling downstream of PDGF. Notably, LamB1 expression is not significantly downregulated by the impairment of the translation initiation factor eIF4E. In vivo, knockdown of La associated with decreased LamB1 expression and reduced tumor growth. Together, these data suggest that PDGF is required for the cytoplasmic accumulation of La that triggers IRES-dependent translation of LamB1 during EMT.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide with increasing incidence over recent years (1,2). The majority of HCC develops as a consequence of chronic liver inflammation and is preceded by liver fibrosis and cirrhosis (3). Main reasons for the high mortality rate of patients with HCC are the late diagnosis at an advanced stage of disease and intrahepatic metastasis (4). HCC derives from the neoplasia of hepatocytes that are highly differentiated and metabolically specialized epithelial cells (5). During HCC progression, trans-differentiation of hepatocytes frequently occurs through epithelial to mesenchymal transition (EMT), which is characterized by abrogation of cell–cell contacts and acquisition of a fibroblastoid, highly motile phenotype (6). EMT-transformed malignant hepatocytes show metastatic abilities by disseminating from the primary tumor and invading the surrounding tissue (7). Dissociation of adherence junctions is accompanied by loss of cell polarity and cytoskeletal rearrangements (8). It involves changes in integrin expression and distribution (9). Integrins are a family of heterodimeric transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton of the cell. In particular, integrin β1 complexes are upregulated in Ras-transformed cells that have undergone EMT in response to transforming growth factor (TGF)-β (9–11). They potentiate growth factor receptor-driven MAPK/ERK and PI3K/AKT signaling and activate latent TGF-β (12,13). Moreover, β1 integrin-mediated MAPK/p38 activation was found to be essential for EMT (14). The extracellular interaction partners of integrins are laminins, which are the main non-collagenous component of the ECM (15). Laminins are heterotrimeric glycoproteins composed of several different α-, β- and γ–subunits, which assemble in 15 laminin isoforms that are expressed in a tissue-specific manner (16). Laminin B1 (LamB1) is the β1-subunit of six laminin isoforms including laminin 1 that is expressed in hepatocytes (16,17). LamB1 mediates β1 integrin signaling and activates a specific p67kDa laminin receptor (LamR) that regulates cell migration, proliferation and survival (18,19). Proteome profiling of HCC patients showed elevated LamB1 levels in cirrhotic liver tissues and a further increased expression in HCC cells (20). Concomitantly, β1 integrin receptors and LamR were found to be upregulated in HCC correlating with increased tumor aggressiveness as well as poor patient survival (21,22). EMT-transformed cells rearrange their extracellular surrounding by enhanced secretion of ECM components as well as by cleavage through matrix metalloproteinases (23,24). This ECM remodeling facilitates tumor cell migration and invasion, thus essentially contributing to metastasis (24).
To study EMT of HCC cells, we previously established p19ARF-deficient immortalized hepatocytes that become tumorigenic upon expression of oncogenic H-Ras and further undergo EMT in response to TGF-β treatment (25). EMT-transformed hepatocytes establish an autocrine TGF-β signaling loop that upregulates platelet-derived growth factor (PDGF) signaling components including PDGF receptor α (PDGF-Rα) (26). LamB1 is translationally upregulated in hepatocytes that have undergone EMT (27) through an internal ribosome entry site (IRES) located in the 5′-untranslated region (UTR) of LamB1 mRNA. IRES-mediated translation of LamB1 is enhanced upon EMT by the cytoplasmic accumulation of the IRES transacting factor (ITAF) La that binds the minimal IRES motif (28). In this study, we show that the cytoplasmic accumulation of La is regulated by PDGF and downstream MAPK/ERK signaling in EMT-transformed cells. Cytoplasmic levels of La are essentially required for the enhanced IRES translation of LamB1 in mesenchymal hepatocytes. In vivo, stable knockdown of La results in reduced LamB1 expression and impaired tumor formation.
MATERIALS AND METHODS
Construction of plasmids
The bicistronic plasmid containing the 5′-UTR of LamB1 between β-galactosidase (β-gal) and chloramphenicol acetyltransferase (29) reporters was constructed as described recently (28). Primers were designed according to GenBank sequence NM_002291. The monocistronic vector contains the LamB1 5′-UTR upstream of a Firefly luciferase reporter as described recently (27). Vectors expressing 2A protease of rhinovirus serotype 2 (2Awt) or the inactive mutant C106A (2Amut) were generated as outlined (27).
Cell culture
Immortalized p19ARF-deficient MIM-1-4 hepatocytes were grown on collagen-coated culture dishes in RPMI 1640 plus 10% fetal calf serum, 40 ng/ml human TGF-α (Sigma, St Louis, USA), 30 ng/ml human insulin-like growth factor II (IGF-II, Sigma, St Louis, USA), 1.4 nM insulin (Sigma, St Louis, USA) and antibiotics, as described previously (30). Epithelial MIM-R, MIM-S35 or MIM-C40 hepatocytes were derived from MIM-1-4 cells by stable retroviral transmission with a bicistronic construct expressing either oncogenic v-Ha-Ras and green fluorescent protein (GFP) or the Ras mutants S35-V12-Ras or C40-V12-Ras, respectively (25). For interference with PDGF signaling, MIM-R cells were retrovirally transmitted with a bicistronic vector expressing the dominant negative (dn)PDGF receptor α and red fluorescent protein, resulting in MIM-RdnP (26,31). Treatment of the epithelial cell lines MIM-R, MIM-S35, MIM-C40 and MIM-RdnP with TGF-β1 (R&D Systems, MN, USA) for >2 weeks resulted in cells with a stable mesenchymal phenotype, namely MIM-RT, MIM-ST, MIM-CT and MIM-RTdnP, respectively. TGF-β1 was used at a concentration of 2.5 ng/ml for the first 72 h of EMT induction. For long-term treatment of epithelial hepatocytes, 1 ng/ml TGF-β1 was supplemented to the medium (25). Recombinant human PDGF-A/B (PeproTech, Rocky Hill, NJ, USA) was used at a concentration of 10 ng/ml. The pharmacological inhibitors LY294002 (Cell Signaling, MA, USA) against PI3K and UO126 (Cell Signaling) against MEK1/2 were used at a concentration of 10 µM. The inhibitor LY02109761 against TGF-β receptor I/II kinase (Lilly, IN, USA) was used at a concentration of 1 µM (32). All cells were kept at 37°C and 5% CO2 and routinely screened for the absence of mycoplasma.
RNA interference
MISSION® shRNA lentiviral transduction particles (Sigma, St Louis, USA) were used to stably knockdown La (NM_009278) in MIM-R and MIM-RT cells as outlined recently (28).
Western blot analysis
The preparation of cellular extracts, separation of proteins by SDS–PAGE and immunoblotting were performed as described (28). Cytoplasmic and nuclear cell fractions were generated using a Proteo-JET® kit (Fermentas, St Leon-Rot, Germany) according to the manufacturer’s description. Thirty micrograms protein extract was loaded onto gels and immunological detection of proteins was performed with the Super Signal detection system (Pierce Chemical Company, Rockford, IL, USA). The following primary antibodies were used: anti-LamB1 (Neo Markers, Fremont, CA, USA), 1:1000; anti-actin (Sigma, St Louis, USA), 1:2000; anti-La (Cell Signaling), 1:1000; anti-tubulin (Sigma), 1:1000; anti-nucleoporin (BD Biosciences, NJ, USA), 1:1000; anti-phospho-ERK (Cell Signaling), 1:1000; anti-ERK (Cell Signaling), 1:1000; anti-phospho-AKT (Cell Signaling), 1:1000; anti-AKT (BD Biosciences, NJ, USA), 1:1000; anti-phospho-eIF2 (Cell Signaling), 1:1000; anti-eIF2 (Cell Signaling), 1:1000; anti-phospho-p38 (Cell Signaling), 1:1000; anti-p38 (Santa Cruz Biotechnology, CA, USA), 1:1000; anti-PDGF-receptor α (Cell Signaling), 1:1000; anti-GFP, 1:1000 (Santa Cruz Biotechnology); anti-eIF4G, 1:2500 (33). Secondary antibodies (Calbiochem, LaJolla, CA, USA) were used at dilutions of 1:10 000. Signals were scanned and quantified with ImageQuant 5.0 (Amersham Biosciences, Little Chalfont, UK).
Quantitative polymerase chain reaction
RNA was extracted, DNaseI treated and reverse transcribed using a RNA isolation and cDNA synthesis kit (Quiagen, Hilden, Germany) as recommended by the manufacturer. Aliquots of cDNA were employed for Fast SYBR green quantitative polymerase chain reaction (qPCR) (Applied Biosystems, CA, USA) and quantified with the 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA). Primer sequences are shown in Supplementary Table S1.
Transient transfection and reporter assays
Cells were seeded on six-well plates and transfected after 24 h with Lipofectamine Plus as recommended by the manufacturer (Invitrogen, Carlsbad, CA, USA). Cells were lysed 48 h post-transfection and reporter activities were determined. Luciferase activity was measured with a Luminoskan microplate reader (Labsystems, Farnborough, UK) as described recently (27). β-Gal activity was photometrically determined using o-nitrophenyl-β-d-galactopyranoside and CAT activity was measured by ELISA (Roche, Mannheim, Germany) as recommended by the manufacturer. In assays analyzing the interference of cap-dependent translation, reporter activities were normalized to mRNA levels as quantified by qPCR. Otherwise, relative IRES activity of the bicistronic assay was calculated as the ratio of CAT/β-gal. Assays were performed in triplicate and results represent the average of three independent experiments.
Subcutaneous tumor formation
Cells were detached from tissue culture plates and washed with PBS. 1 × 106 cells in 100 µl Ringer solution were each subcutaneously injected into immunodeficient C.B-17 SCID mice. Each cell line was injected four times. Experiments were performed according to the Austrian guidelines for animal care and protection.
Immunohistochemistry
Mice were sacrificed and tumors were fixed in paraformaldehyde as previously described (31). Paraffin-embedded tumor sections of 4 µm were stained with hematoxylin and eosin (H&E). Primary antibodies against LamB1 (Neo Markers) and La (Cell Signaling) were used at a dilution of 1:100. Biotinylated secondary antibodies were diluted 1:200. The immunoperoxidase procedure was performed using a Vectastain Elite ABC kit (Vector Laboratories Inc., CA, USA) as described by the manufacturer. DAB-Ni substrate (Vector Laboratories Inc.) was employed for color development. Sections were counterstained with hematoxylin and mounted in Entellan®.
Immunocytochemistry
Cells were grown on collagen coated Superfrost® plus glass slides (Thermo Scientific, MA, USA) and fixed with paraformaldehyde. Primary antibodies against LamB1 (Neo Markers) and La (Cell Signaling) were used at a dilution of 1:100. Immunological stainings were performed as described for immunohistochemistry.
Statistical analysis
Data were expressed as means ± standard deviation. The statistical significance of differences was evaluated using a paired, non-parametric Student’s t-test. Significant differences between experimental groups were: *P < 0.05, **P < 0.01 or ***P < 0.005.
RESULTS
IRES-mediated translation of LamB1 during EMT is enhanced by cytoplasmic La
To study hepatocellular tumorigenesis, we employed a cellular HCC model based on p19ARF−/− hepatocytes transformed with oncogenic H-Ras (MIM-R), which synchronously underwent EMT in response to TGF-β treatment [MIM-RT; (25)]. Upon EMT, we found LamB1 to be translationally upregulated by an IRES element in the LamB1 5′-UTR (27,28). We identified La as an ITAF that interacts with the LamB1 IRES (28). Increased levels of La were bound to the minimal IRES motif in EMT-transformed cells suggesting a role of La in the regulation of LamB1 IRES translation during EMT. In this study, we verified the increase of LamB1 expression in mesenchymal cells by western blot analysis. Quantification of LamB1 protein levels revealed a 2.5-fold upregulation during EMT (Figure 1A). Accordingly, de novo protein synthesis of LamB1 is increased upon EMT (Supplementary Figure S1). In contrast, qPCR displayed similar LamB1 mRNA levels in epithelial MIM-R and mesenchymal MIM-RT cells (Figure 1B). Quantification of western blots further showed that shRNA-mediated La knockdown significantly reduced LamB1 expression in mesenchymal MIM-RT but not in epithelial MIM-R cells. These data indicate that La enhances LamB1 IRES activity after EMT [Figure 1A and Supplementary Figure S2 (28)].
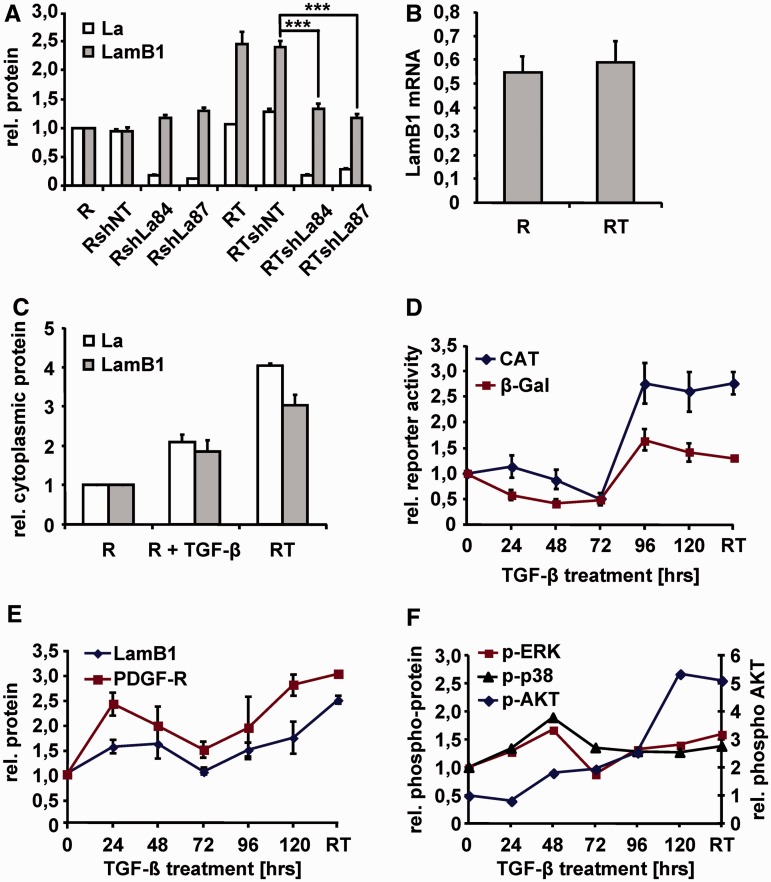
Figure 1.
LamB1 IRES translation is enhanced by La during EMT. (A) Expression of shRNA against La (sh84, sh87) resulted in a stable knockdown of La in epithelial MIM-R (R) and fibroblastoid MIM-RT (RT) cells. Non-target shRNA (shNT) was used as control. Protein levels of La and LamB1 were detected by western blotting and were quantified. The protein levels of MIM-R cells were set to a value of 1 for normalization. (B) qPCR of LamB1 mRNA levels in MIM-R and MIM-RT cells. (C) Cytoplasmic fractions of MIM-R and MIM-RT cells as well as MIM-R cells treated for 24 h with 2.5 ng/ml TGF-β were analyzed by western blotting and protein levels were quantified. The protein levels of MIM-R cells were set to a value of 1 for normalization (D) EMT of epithelial MIM-R cells was induced by administration of 2.5 ng/ml TGF-β in intervals of 24 h. Cells were transfected with the bicistronic vector containing the LamB1 5′-UTR between the β-gal and CAT reporter. β-Gal and CAT activities were detected 48 h after transfection and normalized to mRNA levels as quantified by qPCR. Relative reporter activities of MIM-R cells were set to a value of 1 for normalization. (E) Total protein or (F) phospho-protein levels were detected by western blotting and were quantified. The protein levels of MIM-R cells were set to a value of 1 for normalization. ***P < 0.005.
La is a nuclear protein, which has been described to shuttle to the cytoplasm during apoptosis or cellular stress, where it activates IRES translation of target mRNAs (34–38). We investigated the cytoplasmic localization of La during EMT by western blot analysis of subcellular fractions (Figure 1C and Supplementary Figure S3). Cytoplasmic fractions showed accumulation of La in EMT-transformed cells, whereas total La levels were not changed (Figure 1A and C). Interestingly, we found increased cytoplasmic La not only in completely EMT-transformed cells but also in epithelial cells after TGF-β treatment for 24 h (Figure 1C), suggesting LamB1 IRES activity after induction of EMT. To analyze IRES-mediated LamB1 translation during kinetics of EMT, we stimulated epithelial MIM-R cells with TGF-β and examined the expression of a bicistronic β-gal/CAT reporter (Figure 1D). β-Gal activity of the first reporter shows cap-dependent translation, whereas CAT activity indicates the translation of the second reporter that is regulated by the LamB1 IRES. Reporter activities were normalized to mRNA levels of the bicistronic transcript allowing an independent monitoring of cap- and IRES-dependent translation during EMT. Interestingly, β-gal levels decreased during the first 48 h of TGF-β treatment, indicating downregulation of cap-dependent translation. At later time points of TGF-β treatment as well as in EMT-transformed cells (RT), cap-dependent β-gal expression was even slightly but not significantly elevated (Figure 1D). These data indicate that epithelial cells respond to TGF-β treatment with a transient downregulation of cap-dependent translation that does not persist after EMT transformation. IRES-dependent LamB1 translation responded to TGF-β with a transient downregulation, however, increased at later time points of TGF-β treatment and showed a persistent upregulation in EMT-transformed cells. Taken together, we found no significant modulation of cap-dependent translation during EMT that would suggest predominant IRES-mediated translation.
PDGF was reported to modulate phosphorylation and subcellular localization of La in glioma (39). Recently, we described the upregulation of PDGF signaling components including PDGF-Rα in EMT-transformed hepatocytes [Supplementary Figure S4A and S4C; (26)]. Kinetics of TGF-β-induced EMT showed activation of PDGF-Rα expression at an early phase as well as in EMT-transformed cells (Figure 1E). Expression of PDGF-Rα correlated with LamB1 protein levels (Figure 1E) and IRES activity (Figure 1D), pointing to an involvement of PDGF signaling in the regulation of LamB1 IRES translation. Investigation of pathways downstream of PDGF that are activated during EMT revealed that MAPK/ERK rather than PI3K/AKT signaling (Figure 1F) correlated with the regulation of LamB1 expression (Figure 1E). In summary, these data show that LamB1 IRES translation is enhanced by La that accumulates in the cytoplasm of EMT-transformed cells. Stimulation of epithelial cells with TGF-β leads to a transient downregulation of cap-dependent translation and results in cytoplasmic localization of La. PDGF-Rα expression is induced by TGF-β during EMT and correlates with LamB1 expression and IRES activity, suggesting a role in the regulation of LamB1 translation.
The cytoplasmic accumulation of La in EMT-transformed cells is regulated by PDGF
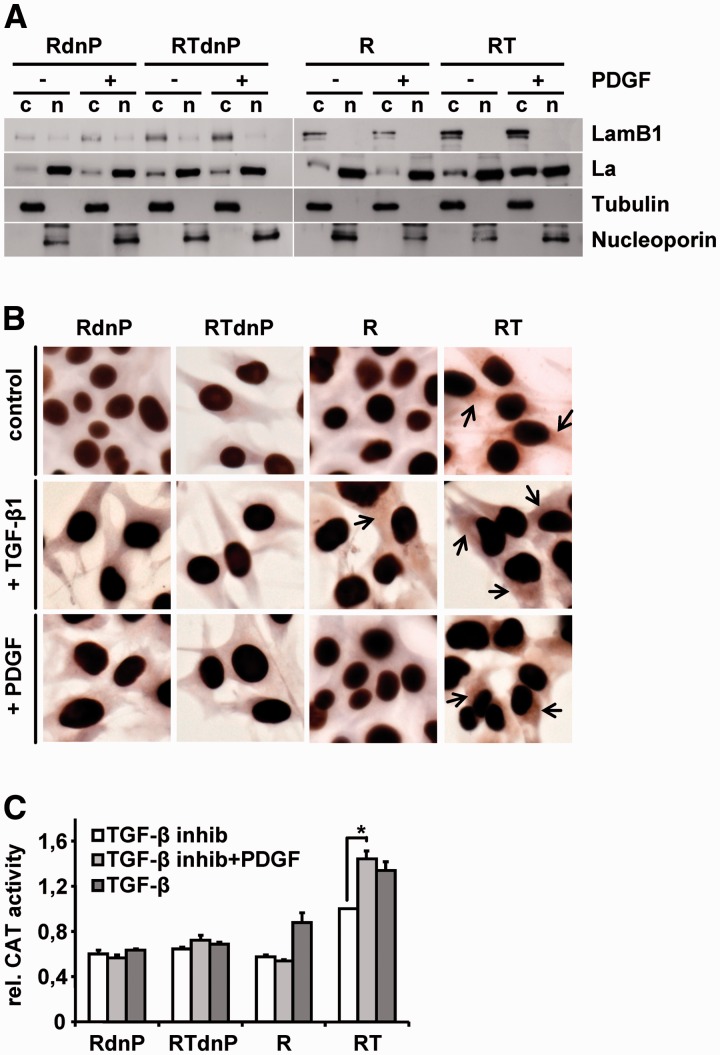
La was reported to translocate from the nucleus to the cytoplasm where it functions as an ITAF by enhancing IRES translation of target mRNAs (35,40,41). Multiple phosphorylation sites or cleavage of the C-terminal nuclear localization signal have been described to regulate the subcellular localization of La (38,42,43). Yet, less is known about the regulation of La distribution. To examine whether PDGF signaling is responsible for cytoplasmic accumulation of La and enhancement of LamB1 IRES translation, we used hepatocytes expressing a dn version of PDGF-Rα (dnP) (31). EMT-transformed cells show an autocrine TGF-β regulation and activate downstream PDGF signaling (26). During PDGF stimulation, cells were therefore simultaneously treated with the TGF-β receptor I/II kinase inhibitor LY02109761 to interfere with TGF-β-driven endogenous PDGF signaling and to exclude the involvement of other TGF-β-activated pathways. Western blot analysis of subcellular fractions revealed that cytoplasmic accumulation of La and enhanced LamB1 expression is induced by PDGF in mesenchymal MIM-RT but not in epithelial MIM-R cells (Figure 2A). Expression of dnP impaired the upregulation of cytoplasmic La and LamB1. Relative protein levels were quantified and normalized to tubulin resulting in a 3.2-fold higher level of cytoplasmic La and a 1.8-fold stronger expression of LamB1 in PDGF-stimulated RT cells as compared to RTdnP cells treated with PDGF. These data suggest a link between PDGF-Rα expression, cytoplasmic La and LamB1 levels. Immunocytochemical stainings confirmed the impact of PDGF signaling on the cytoplasmic localization of La (Figure 2B).
Figure 2.
The EMT-induced cytoplasmic accumulation of La is regulated by PDGF. Epithelial MIM-R and mesenchymal MIM-RT hepatocytes and those expressing dominant negative PDGF-Rα (dnP) were employed to study the subcellular localization of La. All cells were treated with 1 µM TGF-β receptor I/II kinase inhibitor LY02109761 or stimulated with 10 ng/ml PDGF for 32 h in the presence of 1 µM TGF-β inhibitor. (A) Cytoplasmic (c) and nuclear fractions (n) were analyzed by western blotting. The purity of subcellular fractions was assessed by detection of tubulin (c) and nucleoporin (n). (B) Immunocytochemical staining of La. Arrows indicate cytoplasmic localization of La. (C) The effect of impaired PDGF signaling on LamB1 IRES activity was analyzed with a bicistronic reporter assay. Cells were transfected with a bicistronic vector containing the LamB1 5′-UTR. β-Gal and CAT activities were detected 48 h after transfection. Relative reporter activities of MIM-RT cells treated with TGF-β inhibitor were set to a value of 1 for normalization. *P < 0.05.
To investigate whether the cytoplasmic localization of La affects IRES-mediated translation of LamB1, we assessed IRES activity with the bicistronic reporter assay. Importantly, cap-dependent translation did not change significantly as shown in Supplementary Figure S5. MIM-RTdnP (TGF-β inhibitor + PDGF) showed decreased LamB1 IRES activity when compared to MIM-RT (TGF-β inhibitor + PDGF), indicating a role of PDGF in LamB1 IRES regulation (Figure 2C). Impairment of TGF-β and downstream PDGF signaling by treatment with the TGF-β receptor I/II kinase inhibitor LY02109761 resulted in decreased LamB1 IRES activity in mesenchymal MIM-RT cells (RT: white bars) but not after dnP expression (RTdnP: white bars; Figure 2C). In contrast, LamB1 IRES activity was not reduced in MIM-RT cells (RT: light gray bars) upon the simultaneous treatment with TGF-β inhibitor and PDGF stimulation. These data indicate that PDGF is downstream of TGF-β signaling and that LamB1 IRES translation in EMT-transformed cells depends on PDGF signaling. Noteworthy, epithelial MIM-R cells express only low levels of PDGF-Rα. Thus, treatment with PDGF had no effect on LamB1 IRES translation (R, RdnP; Figure 2C), whereas stimulation with TGF-β induced PDGF-Rα expression and LamB1 IRES activity in MIM-R cells. As expected, expression of dnP impairs the TGF-β-mediated induction of LamB1 IRES translation (RdnP). Together, these data indicate that induction of EMT by TGF-β activates PDGF signaling, which triggers cytoplasmic accumulation of La to enhance LamB1 IRES translation.
PDGF signals via PI3K and MAPK pathways
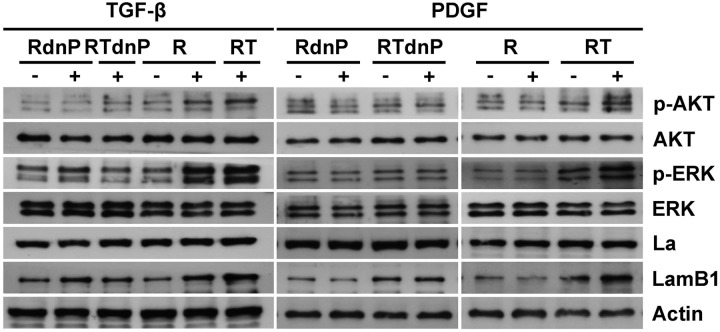
To characterize the pathways that are involved in the PDGF-mediated regulation of La, western blot analysis of epithelial MIM-R, mesenchymal MIM-RT cells and those expressing dnP (RdnP, RTdnP) was performed (Figure 3). Treatment with TGF-β resulted in the activation of PI3K/AKT as well as MAPK/ERK signaling and LamB1 expression in epithelial and EMT-transformed cells, the latter exhibiting autocrine TGF-β production (6). Expression of dnP significantly reduced activation of these pathways and impaired the enhancement of LamB1 expression, suggesting that PDGF contributes to TGF-β-induced PI3K and MAPK signaling and regulates LamB1 expression. In order to estimate the effect of PDGF stimulation, the TGF-β triggered endogenous PDGF expression was pharmacologically inhibited by intervention with upstream TGF-β signaling using the TGF-β receptor I/II kinase inhibitor LY02109761. In contrast to TGF-β, PDGF was exclusively able to induce AKT and ERK signaling in mesenchymal MIM-RT but not in epithelial MIM-R hepatocytes that express low levels of PDGF-Rα. Interference with PDGF signaling by dnP expression impaired PDGF-induced AKT as well as MAPK signaling and enhancement of LamB1 expression. In contrast, the expression of La was not changed (Figure 3). Interestingly, expression of dnP strongly impaired AKT and ERK signaling in response to PDGF. Thus, the correlation of both ERK and AKT phosphorylation with LamB1 expression point to their involvement in PDGF-mediated regulation of LamB1 IRES translation during EMT.
Figure 3.
PDGF activates PI3K and MAPK signaling in EMT-transformed cells. MIM-R and MIM-RT cells or those expressing dnP were employed to analyze PDGF-induced signaling by western blotting. MIM-R and MIM-RdnP cells were treated with 2.5 ng/ml TGF-β for 24 h (RdnP, R) or persistently cultured with 1 ng/ml TGF-β (RTdnP, RT) to analyze TGF-β signaling. Cells were cultured with 1 µM TGF-β receptor I/II kinase inhibitor LY02109761 and simultaneously treated with 10 ng/ml PDGF for 32 h to study PDGF signaling.
LamB1 IRES translation is predominantly regulated by MAPK signaling
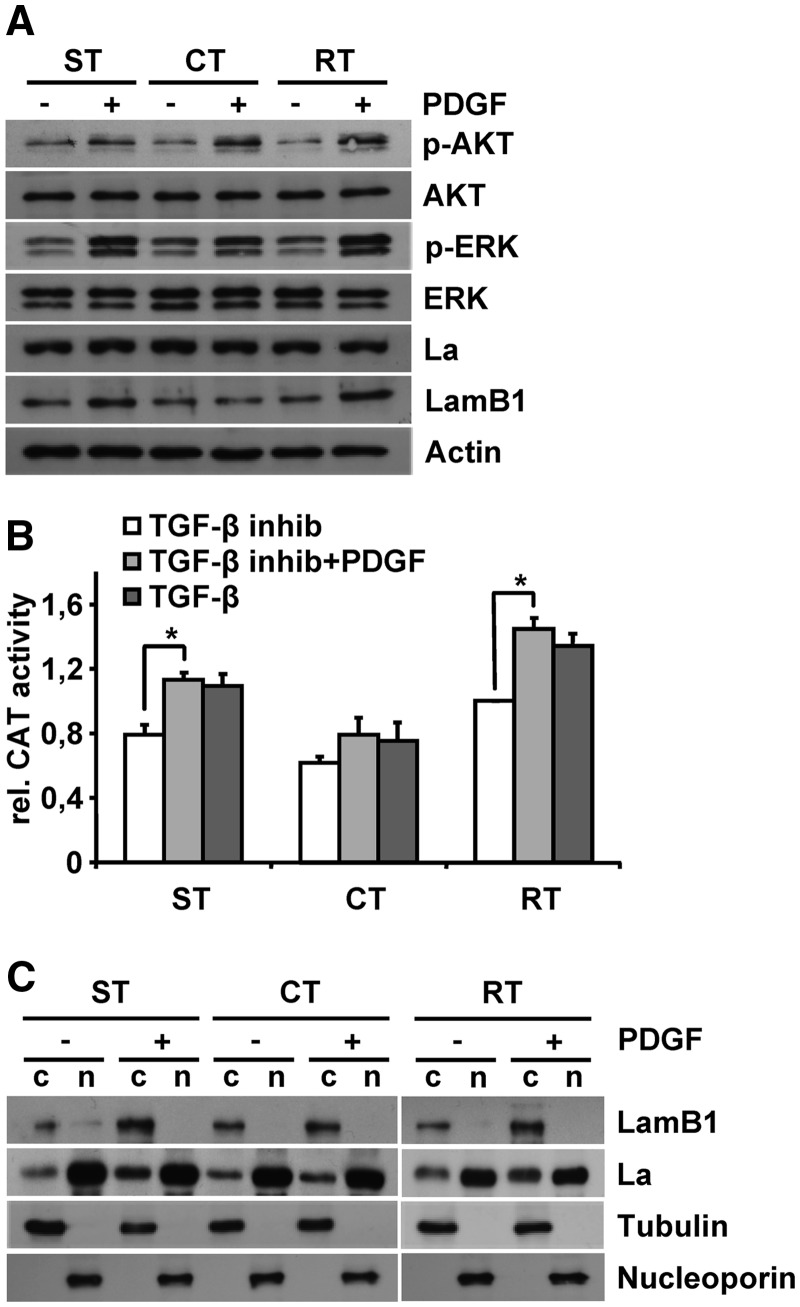
To analyze PDGF downstream signaling for the regulation of LamB1 IRES translation, we used cell lines expressing mutated Ras versions. C40-V12-Ras predominantly activates PI3K/AKT, whereas S35-V12-Ras induces the MAPK/ERK signaling (25). Cells that activate the MAPK/ERK pathway undergo TGF-β-induced EMT resulting in MIM-ST cells, whereas those activating PI3K/AKT signaling acquire a scattering phenotype in response to TGF-β and fail to undergo complete EMT [MIM-CT; (25)]. Cells were treated with TGF-β receptor I/II kinase inhibitor LY02109761 and PDGF to trigger PDGF but not TGF-β-induced signaling. As expected, PDGF treatment stimulated PI3K/AKT and MAPK/ERK signaling (Figure 4A). Activation of AKT was stronger in MIM-CT and MIM-RT (2.5- and 3.2-fold respectively, as compared to 1.5-fold in MIM-ST), whereas activation of ERK was higher in MIM-ST and MIM-RT cells (3.1- and 2.8-fold, respectively, as compared to 1.7-fold in MIM-CT). All cell lines expressed comparable levels of total La, thus being not regulated in response to PDGF treatment. LamB1 expression was increased in PDGF-stimulated MIM-ST but not in MIM-CT cells, suggesting the involvement of MAPK/ERK signaling in the regulation of LamB1 expression. Accordingly, PDGF treatment significantly elevated LamB1 IRES translation in MIM-ST and MIM-RT cell lines but showed less effect in MIM-CT cells (Figure 4B and Supplementary Figure S5). In agreement with these data, cytoplasmic localization of La was induced by PDGF treatment in MIM-ST and MIM-RT but not in MIM-CT cells (Figure 4C and Supplementary Figure S6). These data suggest that the PDGF-induced IRES-mediated translation of LamB1 is predominantly regulated by MAPK/ERK signaling, which triggers the cytoplasmic accumulation of La.
Figure 4.
LamB1 IRES translation is enhanced by PDGF-driven MAPK signaling. Hepatocytes expressing S35-V12-Ras undergo EMT in response to TGF-β (MIM-ST), whereas cells expressing C40-V12-Ras acquire a scattering phenotype (MIM-CT). All cells were treated with 1 µM TGF-β receptor I/II kinase inhibitor LY02109761 or stimulated with 10 ng/ml PDGF for 32 h in the presence of 1 µM TGF-β inhibitor. (A) Western blot analysis of cells in response to PDGF. (B) Cells were transfected with a bicistronic vector containing the LamB1 5′-UTR to analyze LamB1 IRES activity. β-Gal and CAT reporter activities were measured 48 h post-transfection. Relative reporter activities of MIM-RT cells treated with TGF-β inhibitor were set to a value of 1 for normalization. (C) The subcellular localization of La was analyzed by western blotting of cytoplasmic (c) and nuclear (n) fractions. Tubulin and nucleoporin served as cytoplasmic or nuclear marker, respectively. *P < 0.05.
PI3K affects MAPK-dependent regulation of cytoplasmic La localization
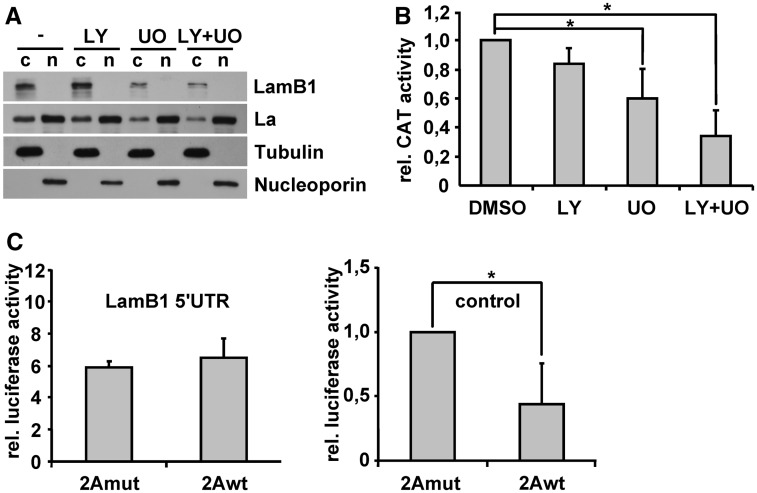
To further examine the PDGF signaling on LamB1 IRES translation, we selectively inhibited PI3K/AKT or MAPK/ERK signaling in EMT-transformed MIM-RT cells using small molecule inhibitors targeting PI3K or MEK1/2. Inhibition of the PI3K/AKT signaling did neither affect LamB1 expression nor cytoplasmic levels of La (Figure 5A). In contrast, MEK1/2 inhibition resulted in a significant reduction of LamB1 and cytoplasmic La levels, which was even more lowered by combined inhibitor treatment. In accordance, inhibition of MEK1/2 reduced LamB1 IRES activity that was even more inhibited through the combined treatment, whereas interference with PI3K/AKT alone had no significant effect (Figure 5B and Supplementary Figure S5). These results suggest that cytoplasmic accumulation of La and the enhanced IRES-driven expression of LamB1 is mainly regulated by MAPK/ERK signaling with an additional impact of the PI3K/AKT pathway.
Figure 5.
Cytoplasmic accumulation of La depends on the cooperation of MAPK and PI3K signaling. MIM-RT cells were treated with 10 µM of PI3K (LY) and/or MAPK (UO) inhibitor. DMSO was used as control. (A) Western blotting of cytoplasmic (c) and nuclear (n) fractions to analyze subcellular localization of La. (B) Cells were transfected with the bicistronic vector containing the LamB1 5′-UTR to study LamB1 IRES activity. CAT reporter activities were measured 48 h after transfection and normalized to mRNA levels as quantified by qPCR. Relative reporter activities of MIM-RT cells treated with DMSO were set to a value of 1 for normalization. (C) MIM-RT cells were co-transfected with a vector expressing 2A protease (2Awt) or an inactive mutant (2Amut) together with either a plasmid containing the LamB1 5′-UTR in front of a Firefly luciferase reporter (left panel) or the empty Firefly luciferase reporter without 5′-UTR as control (right panel). Relative reporter activities of MIM-RT cells that were co-transfected with the 2Amut and Firefly luciferase control vector were set to a value of 1 for normalization. *P < 0.05.
Inhibition of the MAPK/ERK pathway leads to reduced eIF4E activity (44) that could be involved in the downregulation of cap-dependent LamB1 expression. To estimate whether decreased eIF4E availability could influence the expression of LamB1, a reporter assay was performed in the presence of 2A protease, which cleaves eIF4G (Supplementary Figure S7). This enzyme cleaves the eIF4E binding site on eIF4G and therefore disrupts the association of eIF4E with the translation preinitiation complex (45). We expressed 2A protease (2Awt) or the inactive mutant (2Amut) together with a reporter plasmid containing the LamB1 5′-UTR in front of a Firefly luciferase reporter (Figure 5C, left panel). Firefly luciferase activity therefore reflects both the cap-dependent and IRES-mediated translation of LamB1. To detect the impairment of cap-dependent translation by 2A protease, 2Awt or 2Amut was co-transfected with the empty reporter vector expressing Firefly luciferase (Figure 5C, right panel). Interestingly, cap-dependent translation was significantly reduced by 2A protease activity, whereas translation of the LamB1 5′-UTR containing reporter was not impaired (Figure 5C). The translation of LamB1, consisting in this experimental setting of both cap-dependent and IRES-mediated translation, therefore remains not affected by reduced eIF4E availability. Recently, we performed a comparable experiment with a bicistronic vector showing that 2A protease activity leads to increased IRES translation of LamB1 (27). Together, these results suggest that reduced eIF4E availability leads to enhanced IRES-mediated translation of LamB1, which compensates a possible reduction of cap-dependent translation and stabilizes protein expression of LamB1. Notably, the decrease of eIF4E activity has been reported to reduce cap-dependent and to induce IRES-mediated translation (46). Diminished LamB1 expression upon inhibition of MAPK/ERK signaling can therefore not be explained by the reduction of cap-dependent translation. Thus, inhibition of MAPK/ERK does not induce a possible switch to IRES translation of LamB1. Rather, MAPK/ERK pathway regulates IRES-mediated translation of LamB1 by the modulation of La translocation.
PDGF-regulated subcellular localization of La correlates with LamB1 levels in vivo
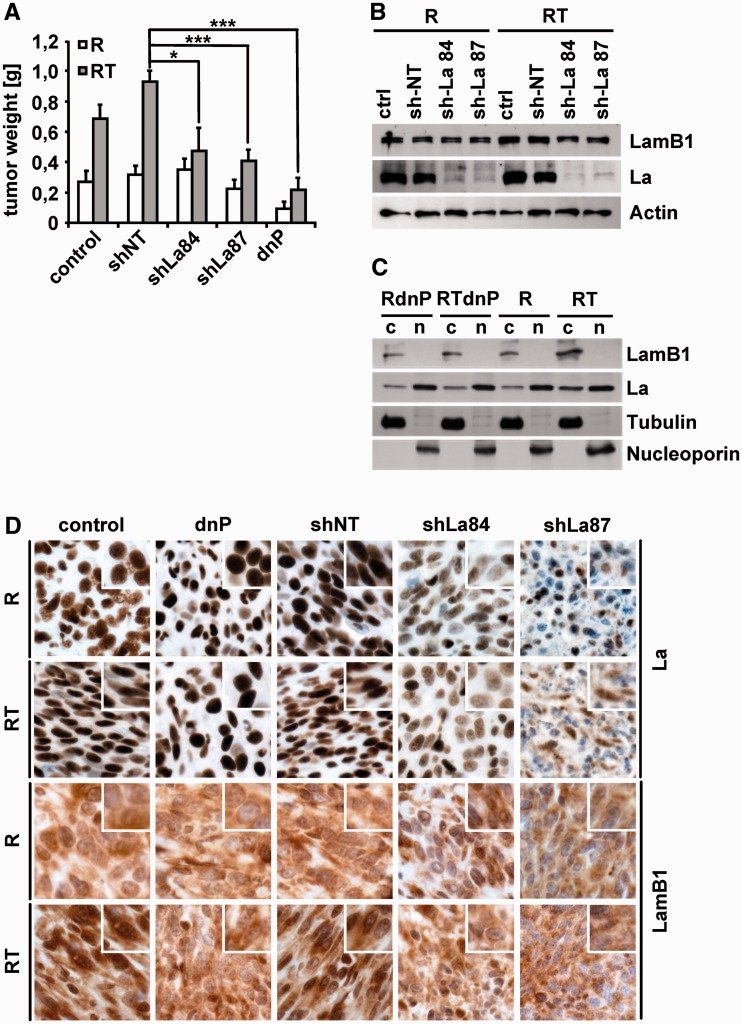
We next addressed the question whether the localization of La correlates with LamB1 expression and the tumor formation of EMT-transformed cells in vivo. Therefore, epithelial MIM-R or mesenchymal MIM-RT cells as well as those expressing dnP or a stable knockdown of La (shLa84, shLa87) or the non-target control (shNT) were xenografted for subcutaneous tumor formation. Interestingly, the knockdown of La as well as the intervention with PDGF signaling significantly reduced subcutaneous tumor growth of EMT-transformed cells (Figure 6A and Supplementary Figure S8). In contrast, epithelial MIM-R-derived tumors remained unaffected by the knockdown of La. Although expression of dnP had no effect on in vitro properties of epithelial MIM-R cells (R) such as proliferation and migration (31), a reduced tumor growth was observed, which might be explained by the complex tumor stroma crosstalk in vivo. Expression of LamB1 was analyzed in cell lysates or subcellular fractions obtained from tumor tissues. The knockdown of La in EMT-transformed cells decreased LamB1 expression, showing that enhanced LamB1 expression depends on La even under in vivo conditions (Figure 6B). Analysis of subcellular fractions from mesenchymal MIM-RT-derived tumors revealed a cytoplasmic accumulation of La and increased LamB1 expression in vivo (Figure 6C). Furthermore, intervention with PDGF signaling decreased the cytoplasmic accumulation of La as well as LamB1 expression. No changes in La localization could be observed in cell lysates from tumors generated by epithelial cells (data not shown). Immunohistochemical analysis further confirmed the link between cytoplasmic La and increased LamB1 levels in tumors derived from EMT-transformed cells. Tumors generated from MIM-RT cells expressing dnP showed a lack of cytoplasmic La that correlated with lowered LamB1 expression upon EMT as compared to control or shNT (Figure 6D). Similarly, reduced levels of LamB1 were observed in tumors with a knockdown of La. In summary, these data provide strong evidence that endogenous, tumor-derived TGF-β levels are sufficient to induce PDGF-mediated accumulation of La and to enhance LamB1 expression upon hepatocellular EMT.
Figure 6.
Stable knockdown of La reduces LamB1 expression and impairs tumor formation of EMT-transformed hepatocytes. Epithelial (MIM-R, R) or mesenchymal (MIM-RT, RT) hepatocytes expressing dnP or a stable knockdown of La (sh84, sh87) or the non-target control (shNT) were subcutaneously injected into SCID mice. (A) Mean weight of subcutaneous tumors after 16 days of growth in SCID mice. (B) Western blot analysis of tumor tissue. (C) Western blotting of cytoplasmic (c) and nuclear (n) fractions isolated from tumor tissue. (D) Histological staining of subcutaneous tumors. Insets show a higher magnification. *P < 0.05 or ***P < 0.005.
DISCUSSION
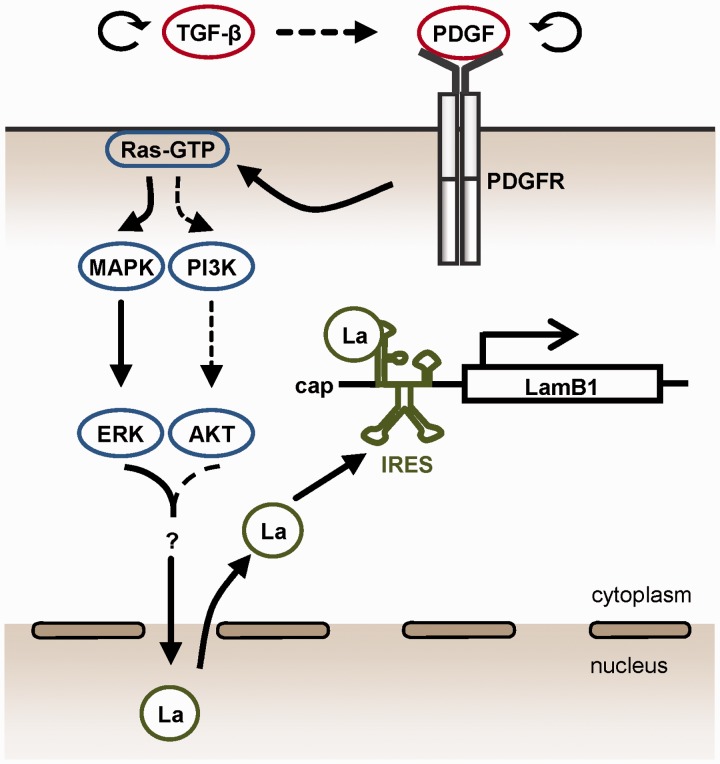
EMT is a crucial step in the metastatic cascade that provides carcinoma cells with the ability to remodel and cross the ECM of the basement membrane as well as to enter circulation (7). Hepatocellular EMT is induced by the cooperation of oncogenic Ras and TGF-β signaling and essentially depends on the activation of PDGF signaling (26). Here, we present evidence that PDGF triggers the cytoplasmic accumulation of La during EMT in a PDGF-Rα-dependent way that predominantly involves the downstream MAPK/ERK pathway. The signaling events regulating LamB1 IRES translation during EMT are summarized in Figure 7. Cytoplasmic La acts as an ITAF and enhances IRES-mediated translation of LamB1 in cells that have undergone EMT (28). To our knowledge, these are the first data describing the cytoplasmic shuttling of La during EMT and its regulation by PDGF-Rα that controls IRES activity.
Figure 7.
Model of regulating IRES-mediated LamB1 translation during hepatocellular EMT and carcinoma progression. PDGF signaling induced by TGF-β activates the MAPK/ERK pathway that predominantly regulates the cytoplasmic accumulation of La. PI3K/AKT might be additionally involved in the translocation of La to the cytoplasm (dashed lines). La binds as an ITAF to the minimal IRES motif and enhances IRES-mediated translation of LamB1 in EMT-transformed cells. Winded arrows indicate autocrine regulation of TGF-β and PDGF (top).
Autocrine secretion of TGF-β results in a persistent PDGF expression in EMT-transformed hepatocytes (26). Interference with TGF-β and PDGF signaling using the TGF-β receptor I/II kinase inhibitor LY02109761 or expression of dnP revealed that cytoplasmic accumulation of La and IRES-driven translation of LamB1 depends on active TGF-β signaling and downstream PDGF-Rα expression. Furthermore, we found that PDGF contributes to the activation of MAPK/ERK and PI3K/AKT pathways in mesenchymal cells. Experiments with hepatocytes expressing mutated Ras versions clearly indicate that La translocation and LamB1 IRES translation during EMT is regulated by MAPK/ERK signaling. Yet combined pharmacological inhibition of MAPK/ERK and PI3K/AKT pathways had a more significant effect than sole inhibition of MEK1/2, suggesting that active PI3K/AKT signaling contributes to La translocation. Accordingly, kinetics of TGF-β-induced EMT show a more prominent upregulation of LamB1 IRES activity at later time points correlating with the elevation of both MAPK/ERK and PI3K/AKT signaling (Figure 1D and F). Interestingly, the analysis of EMT kinetics revealed no significant change in cap-dependent translation (Figure 1D). These data suggest that there is no general switch to IRES-dependent translation in EMT-transformed cells and that elevation of IRES-mediated LamB1 translation is selectively regulated by the cytoplasmic presence of La. Importantly, an assay employing 2A protease expression showed that the effects observed upon MEK1/2 or AKT inhibition are not merely a result of decreased eIF4E availability, but rather depend on cytoplasmic accumulation of La.
PDGF signaling was not only activated in EMT-transformed cells, but also shortly after TGF-β stimulation of epithelial cells by upregulation of PDGF-Rα (Figure 1E) and PDGF-A as shown recently (26). Cap-dependent translation is reduced during 48 h post-TGF-β treatment. This reduction correlates with activation of the p38/MAPK pathway, pointing to cellular stress (Figure 1F and Supplementary Figure 4D). Indeed, a link between EMT and ER stress has been reported in thyroid cells (47). Activation of PDGF-Rα expression as well as cytoplasmic localization of La in response to cellular stress has been described recently (36,37). PDGF-Rα was found to signal via Src during cellular stress. However, experiments with pharmacological inhibitors against Src or Janus kinases (JAKs) had no effect on LamB1 IRES activity (data not shown). Therefore, we exclude the involvement of the Src or JAK/Stat pathway in regulating cytoplasmic accumulation of La and the increase of LamB1 IRES translation during TGF-β-induced EMT. Our data rather indicate that MAPK/ERK signaling regulates the immediate translocation of La upon TGF-β stimulation. The switch to PDGF-Rα signaling and IRES-mediated translation during the induction of EMT suggests that PDGF signaling could be involved in the regulation of further ITAFs in addition to La. This opens the question whether other ITAFs are regulated upon EMT and possibly contribute to the enhanced IRES translation of LamB1.
The significance of these findings is underlined by recent findings demonstrating that cytoplasmic La contributes to the translation of oncogenes. Aberrant regulation of La has been particularly described in chronic myeloid leukemia, where BCR/ABL-mediated activation of La supports tumorigenesis (48,49). Phosphorylation-dependent shuttling of La and translational activation of target genes that include tumor promoting factors was found in glioma cells (39). However, La modulates translation in diverse ways including binding to the 3′-UTR or to the cap of oligopyrimidine containing mRNAs depending on the phosphorylation status (39,50). Cytoplasmic La in EMT-transformed cells might therefore affect translation of target genes on multiple levels. So far, La-mediated modulation of IRES translation during apoptosis and cell cycle progression has only been described for XIAP, BIP and Cyclin D1 (35,40,41). Truncation as well as phosphorylation was found to trigger the cytoplasmic translocation of La during apoptosis or cellular stress, suggesting that multiple signaling events are involved in regulating subcellular La distribution (38,42,43). Recently, Brenet et al. published a PDGF-B induced, AKT- dependent phosphorylation of La. In contrast to these findings, PDGF-induced translocation of La rather depends on the MAPK/ERK pathway during hepatocellular EMT.
LamB1 signaling includes binding to LamR and β1 integrin receptors that were both found to be overexpressed in HCC (21,22). Rearrangement of integrin receptor allocation and abundance is an essential step during EMT that leads to enhanced cell motility and survival (9,14,51). Consistently, LamB1 was found to be involved in the regulation of cell migration, suggesting that increased IRES translation of LamB1 during EMT may contribute to tumor cell migration and metastasis (18,52). Furthermore, EMT is associated with the activation of cell programs that regulate dedifferentiation during embryogenesis and has been suggested to be involved in the generation of tumor stem cells (8,53). Interestingly, LamB1 is the first laminin subunit to be expressed during early embryogenesis and is essentially involved in cell differentiation (54). While embryonic cells expressing LamB1 alone have a mesenchymal phenotype, additional activation of Laminin α expression during E4 stage induces epithelial cell polarization via α6β1 integrin signaling. Accordingly, a recent publication reported the cultivation of embryonic stem cells on laminin 10, which contains the LamB1 subunit, without the need of feeder cells (55). These data indicate that the context of laminin expression might be important for the generation of stem cell niches. Finally, Laminin 1 as well as LamB1 peptides was found to regulate tube formation and sprouting during tumor vascularization (56,57). Angiogenesis is induced by hypoxic conditions and is driven by the increased IRES translation of VEGF-A, PDGF and bFGF mRNAs in response to HIF1α activation (58,59). Thus, it is promising to investigate the involvement of IRES-driven LamB1 translation during tumor angiogenesis and metastasis.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1, Supplementary Figures 1–8 and Supplementary Methods.
FUNDING
Austrian Science Fund (FWF) [P20905, SFB F28]. Funding for open access charge: FWF [P20905, SFB F28].
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun VC, Sarna L. Symptom management in hepatocellular carcinoma. Clin. J. Oncol. Nurs. 2008;12:759–766. doi: 10.1188/08.CJON.759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taub R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 6.van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H, Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169–1179. doi: 10.2217/fon.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat. Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 10.Kumar NM, Sigurdson SL, Sheppard D, Lwebuga-Mukasa JS. Differential modulation of integrin receptors and extracellular matrix laminin by transforming growth factor-beta 1 in rat alveolar epithelial cells. Exp Cell. Res. 1995;221:385–394. doi: 10.1006/excr.1995.1389. [DOI] [PubMed] [Google Scholar]
- 11.Fransvea E, Mazzocca A, Antonaci S, Giannelli G. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49:839–850. doi: 10.1002/hep.22731. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J. Cell Sci. 2002;115:4641–4648. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- 13.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem. J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 14.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 16.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev. Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 18.Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells: the role of the M(r) 67,000 laminin receptor. Cancer Res. 2004;64:3572–3579. doi: 10.1158/0008-5472.CAN-03-3424. [DOI] [PubMed] [Google Scholar]
- 19.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 20.Lim SO, Park SJ, Kim W, Park SG, Kim HJ, Kim YI, Sohn TS, Noh JH, Jung G. Proteome analysis of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2002;291:1031–1037. doi: 10.1006/bbrc.2002.6547. [DOI] [PubMed] [Google Scholar]
- 21.Liu LX, Jiang HC, Liu ZH, Zhou J, Zhang WH, Zhu AL, Wang XQ, Wu M. Integrin gene expression profiles of human hepatocellular carcinoma. World J. Gastroenterol. 2002;8:631–637. doi: 10.3748/wjg.v8.i4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozaki I, Yamamoto K, Mizuta T, Kajihara S, Fukushima N, Setoguchi Y, Morito F, Sakai T. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut. 1998;43:837–842. doi: 10.1136/gut.43.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannelli G, Bergamini C, Fransvea E, Marinosci F, Quaranta V, Antonaci S. Human hepatocellular carcinoma (HCC) cells require both alpha3beta1 integrin and matrix metalloproteinases activity for migration and invasion. Lab. Invest. 2001;81:613–627. doi: 10.1038/labinvest.3780270. [DOI] [PubMed] [Google Scholar]
- 24.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 25.Fischer AN, Herrera B, Mikula M, Proell V, Fuchs E, Gotzmann J, Schulte-Hermann R, Beug H, Mikulits W. Integration of Ras subeffector signaling in TGF-beta mediated late stage hepatocarcinogenesis. Carcinogenesis. 2005;26:931–942. doi: 10.1093/carcin/bgi043. [DOI] [PubMed] [Google Scholar]
- 26.Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–3185. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 27.Petz M, Kozina D, Huber H, Siwiec T, Seipelt J, Sommergruber W, Mikulits W. The leader region of Laminin B1 mRNA confers cap-independent translation. Nucleic Acids Res. 2007;35:2473–2482. doi: 10.1093/nar/gkm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petz M, Them N, Huber H, Beug H, Mikulits W. La enhances IRES-mediated translation of laminin B1 during malignant epithelial to mesenchymal transition. Nucleic Acids Res. 2012;40:290–302. doi: 10.1093/nar/gkr717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graff JR, Konicek BW, Lynch RL, Dumstorf CA, Dowless MS, McNulty AM, Parsons SH, Brail LH, Colligan BM, Koop JW, et al. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 2009;69:3866–3873. doi: 10.1158/0008-5472.CAN-08-3472. [DOI] [PubMed] [Google Scholar]
- 30.Mikula M, Fuchs E, Huber H, Beug H, Schulte-Hermann R, Mikulits W. Immortalized p19ARF null hepatocytes restore liver injury and generate hepatic progenitors after transplantation. Hepatology. 2004;39:628–634. doi: 10.1002/hep.20084. [DOI] [PubMed] [Google Scholar]
- 31.Fischer AN, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395–3405. doi: 10.1038/sj.onc.1210121. [DOI] [PubMed] [Google Scholar]
- 32.Lacher MD, Tiirikainen MI, Saunier EF, Christian C, Anders M, Oft M, Balmain A, Akhurst RJ, Korn WM. Transforming growth factor-beta receptor inhibition enhances adenoviral infectability of carcinoma cells via up-regulation of Coxsackie and Adenovirus Receptor in conjunction with reversal of epithelial-mesenchymal transition. Cancer Res. 2006;66:1648–1657. doi: 10.1158/0008-5472.CAN-05-2328. [DOI] [PubMed] [Google Scholar]
- 33.Yan R, Rychlik W, Etchison D, Rhoads RE. Amino acid sequence of the human protein synthesis initiation factor eIF-4 gamma. J. Biol. Chem. 1992;267:23226–23231. [PubMed] [Google Scholar]
- 34.Holcik M, Korneluk RG. Functional characterization of the X-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: role of La autoantigen in XIAP translation. Mol. Cell. Biol. 2000;20:4648–4657. doi: 10.1128/mcb.20.13.4648-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YK, Back SH, Rho J, Lee SH, Jang SK. La autoantigen enhances translation of BiP mRNA. Nucleic Acids Res. 2001;29:5009–5016. doi: 10.1093/nar/29.24.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin. Exp. Immunol. 1989;78:454–459. [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann M, Chang S, Slor H, Kukulies J, Muller WE. Shuttling of the autoantigen La between nucleus and cell surface after uv irradiation of human keratinocytes. Exp. Cell Res. 1990;191:171–180. doi: 10.1016/0014-4827(90)90002-r. [DOI] [PubMed] [Google Scholar]
- 38.Ayukawa K, Taniguchi S, Masumoto J, Hashimoto S, Sarvotham H, Hara A, Aoyama T, Sagara J. La autoantigen is cleaved in the COOH terminus and loses the nuclear localization signal during apoptosis. J. Biol. Chem. 2000;275:34465–34470. doi: 10.1074/jbc.M003673200. [DOI] [PubMed] [Google Scholar]
- 39.Brenet F, Socci ND, Sonenberg N, Holland EC. Akt phosphorylation of La regulates specific mRNA translation in glial progenitors. Oncogene. 2009;28:128–139. doi: 10.1038/onc.2008.376. [DOI] [PubMed] [Google Scholar]
- 40.Holcik M, Gordon BW, Korneluk RG. The internal ribosome entry site-mediated translation of antiapoptotic protein XIAP is modulated by the heterogeneous nuclear ribonucleoproteins C1 and C2. Mol. Cell. Biol. 2003;23:280–288. doi: 10.1128/MCB.23.1.280-288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommer G, Dittmann J, Kuehnert J, Reumann K, Schwartz PE, Will H, Coulter BL, Smith MT, Heise T. The RNA-binding protein La contributes to cell proliferation and CCND1 expression. Oncogene. 2011;30:434–444. doi: 10.1038/onc.2010.425. [DOI] [PubMed] [Google Scholar]
- 42.Intine RV, Tenenbaum SA, Sakulich AL, Keene JD, Maraia RJ. Differential phosphorylation and subcellular localization of La RNPs associated with precursor tRNAs and translation-related mRNAs. Mol. Cell. 2003;12:1301–1307. doi: 10.1016/s1097-2765(03)00429-5. [DOI] [PubMed] [Google Scholar]
- 43.Romero V, Fellows E, Jenne DE, Andrade F. Cleavage of La protein by granzyme H induces cytoplasmic translocation and interferes with La-mediated HCV-IRES translational activity. Cell Death Differ. 2009;16:340–348. doi: 10.1038/cdd.2008.165. [DOI] [PubMed] [Google Scholar]
- 44.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henis-Korenblit S, Shani G, Sines T, Marash L, Shohat G, Kimchi A. The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl Acad. Sci. USA. 2002;99:5400–5405. doi: 10.1073/pnas.082102499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spriggs KA, Stoneley M, Bushell M, Willis AE. Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biol. Cell. 2008;100:27–38. doi: 10.1042/BC20070098. [DOI] [PubMed] [Google Scholar]
- 47.Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J. Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 48.Trotta R, Vignudelli T, Candini O, Intine RV, Pecorari L, Guerzoni C, Santilli G, Byrom MW, Goldoni S, Ford LP, et al. BCR/ABL activates mdm2 mRNA translation via the La antigen. Cancer Cell. 2003;3:145–160. doi: 10.1016/s1535-6108(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 49.Eiring AM, Neviani P, Santhanam R, Oaks JJ, Chang JS, Notari M, Willis W, Gambacorti-Passerini C, Volinia S, Marcucci G, et al. Identification of novel posttranscriptional targets of the BCR/ABL oncoprotein by ribonomics: requirement of E2F3 for BCR/ABL leukemogenesis. Blood. 2008;111:816–828. doi: 10.1182/blood-2007-05-090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Intine RV, Dundr M, Vassilev A, Schwartz E, Zhao Y, Depamphilis ML, Maraia RJ. Nonphosphorylated human La antigen interacts with nucleolin at nucleolar sites involved in rRNA biogenesis. Mol. Cell. Biol. 2004;24:10894–10904. doi: 10.1128/MCB.24.24.10894-10904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. The J. Biol. Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 52.Vande Broek I, Vanderkerken K, De Greef C, Asosingh K, Straetmans N, Van Camp B, Van Riet I. Laminin-1-induced migration of multiple myeloma cells involves the high-affinity 67 kD laminin receptor. Br. J. Cancer. 2001;85:1387–1395. doi: 10.1054/bjoc.2001.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper AR, MacQueen HA. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev. Biol. 1983;96:467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- 55.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 56.Dixelius J, Jakobsson L, Genersch E, Bohman S, Ekblom P, Claesson-Welsh L. Laminin-1 promotes angiogenesis in synergy with fibroblast growth factor by distinct regulation of the gene and protein expression profile in endothelial cells. J Biol. Chem. 2004;279:23766–23772. doi: 10.1074/jbc.M311675200. [DOI] [PubMed] [Google Scholar]
- 57.Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999;13:53–62. [PubMed] [Google Scholar]
- 58.Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46:31–36. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]
- 59.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol. Cell. Biol. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.