Abstract
The house-dust mite (HDM), commonly found in human dwellings, is an important source of inhalant and contact allergens. In this report, the importance of HDM allergy in Korea and the characteristics of allergens from dust mite are reviewed with an emphasis on investigations performed in Korea. In Korea, Dermatophagoides farinae is the dominant species of HDM, followed by D. pteronyssinus. Tyrophagus putrescentiae is also found in Korea, but its role in respiratory allergic disease in Korea is controversial. The relatively low densities of mite populations and concentrations of mite major allergens in dust samples from Korean homes, compared to westernized countries, are thought to reflect not only different climatic conditions, but also cultural differences, such as the use of 'ondol' under-floor heating systems in Korean houses. HDM are found in more than 90% of Korean houses, and the level of exposure to HDM is clinically significant. About 40%-60% of Korean patients suffering from respiratory allergies, and more than 40% of patients suffering from atopic dermatitis, are sensitized to HDM. Mite allergens can be summarized according to their inherent auto-adjuvant activities and/or their binding affinities to the adjuvant-like substances: proteolytic enzymes, lipid binding proteins, chitin binding proteins, and allergens not associated with adjuvant-like activity. In general, allergens with a strong adjuvant-like activity or adjuvant-binding activity elicit potent IgE reactivity. In Korea, Der f 2 is the most potent allergen, followed by Der f 1. Immune responses are modulated by the properties of the allergen itself and by the adjuvant-like substances that are concomitantly administered with the antigens. Characterization of allergenic molecules and elucidation of mechanisms by which adjuvant-like molecules modulate allergic reactions, not only in Korea but also worldwide, will provide valuable information on allergic diseases, and are necessary for the development of diagnostic tools and therapeutic strategies.
Keywords: Allergen, allergy, house dust mite, Korea
INTRODUCTION
The house-dust mite (HDM) was first suspected as a source of allergen in 1928,1 and has been recognized as an important cause of allergic disorders since 1968.2,3 The relationship between house dust and HDM in bronchial asthmatics has been demonstrated using the skin reactivity test, bronchial provocation test, serologic test for mite-specific IgE and the basophil histamine release test.3,4 Epidemiologic studies performed with the skin prick test showed that HDM is the most common cause of allergic diseases in Korea. In Korea, the rate of sensitization to HDM has been reported to be 27.9%-68.8% in patients with atopic dermatitis5-10 and 40%-60% in patients with respiratory allergy, allergic rhinitis and asthma.11-14 In a study of 431 allergic subjects in Seoul, about 60% were sensitized to HDM, regardless of their allergic symptoms.15
The rate of sensitization to HDM has increased with industrialization and the westernization of Korean lifestyles.16,17 With the advent of molecular biological technology, proteins from various mite species responsible for the induction of allergic responses have been investigated. HDM produces various proteins that could elicit IgE-mediated immune responses, and some molecules may modulate the allergic reactions by their adjuvant-like characteristics or their affinity to adjuvant. However, it is not clear which allergenic substances of HDM are responsible for the increase in allergic disorders in Korea. Molecular characterization of these substances will provide new insight and new strategies for the development of immunotherapeutic tools to modulate the immune response.
HDMS AND ALLERGEN LEVELS IN THE ENVIRONMENT
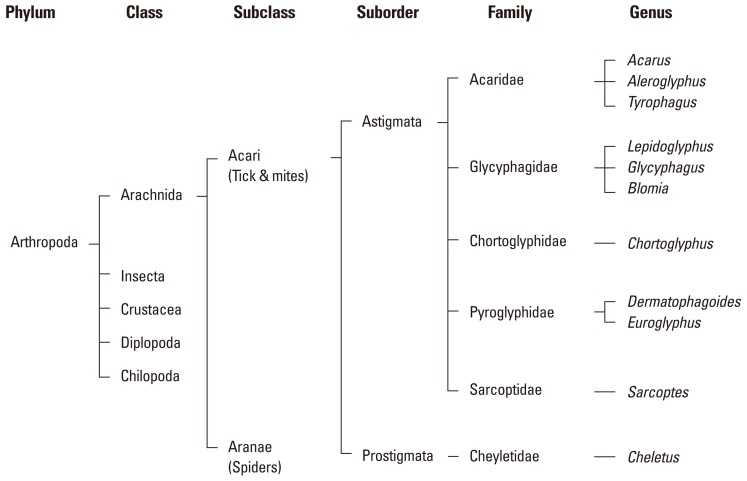
In house dust, five families of mites (Pyrogliphidae, Acaridae, Glycyphagidae, Chortoglyphidae and Chelyetidae) have been identified.18 HDM belongs to the family Phyrogliphidae, and mites of the other families are collectively called storage mites. The term 'dust mites' refers to the mite species isolated from dust samples, regardless of their family; i.e., HDM and storage mite (Figure).
Figure.
Classification of dust mites of allergenic importance.
In Korea, five species of mites (Tyrophagus dimidiatus, Chibidania tokyoensis, Rhizoglyphus echinopus, Ornithonyssus nagayoi and Dermanyssus gallinae) were reported from house dust samples for the first time in 1967.19 Dust mites of allergenic importance (Dermatophagoides fainae, D. pteronyssinus, and Tyrophagus putrescentae) were first described by Cho and Houh in 1977.20 Studies on the geographical distribution of dust mites in Korea confirmed D. farinae as the predominant species (65.3%), followed by D. pteronyssinus (20.6%) and T. putrescentiae (6.5%) among 7,257 mites collected.21,22 Marked cross-reactivity between D. farinae and D. pteronyssinus extracts has been reported.23
The storage mite, T. putrescentiae, is the third most commonly found dust mite in Korean homes. However, its role in allergic diseases is controversial. IgE-binding components of T. putrescentiae were reported to be completely inhibited by D. pteronyssinus or D. farinae extracts.24,25 A 16-kDa allergen, probably Tyr p 2, is the most potent, and is responsible for cross-reactivity with Der f 2 and Der p 2 in urban areas (Seoul).25 However, studies conducted in rural areas of Sweden showed that inhabitants were exposed to high levels of T. putrescentiae, which is an important inhalant allergen with little cross-reactivity to D. pteronyssinus.26
Nationwide studies reflecting the regional differences between urban and rural areas of Korea are necessary to evaluate the importance of allergies against storage mites. T. putrescentiae is one of the most common allergens, and some patients suffering from perennial asthma in Daejeon were reported to be sensitized only to T. putrescentiae.27 Sensitization to T. putrescentiae is also prevalent (19.5%) in allergic children in Seoul.28
Different procedures for mite collection and isolation from dust samples may result in different mite counts and allergen concentrations. Recently, a standard method for the investigation of dust mite density was suggested for use in objective and convenient field surveys.29 The number of mites per unit area (1 m2) collected in a given time (2 minutes) was measured using a house-hold vacuum cleaner equipped with a nonwoven fabrics.
Using the standard collection method, HDM allergens have been found in more than 85%-90% of houses in Korea. About one-third (31.2%) of dust samples from houses in Korea (mostly Seoul) contained more than 2 µg/g dust that consisted of mite group 1 allergen,30 a known risk factor for sensitization and development of asthma (equivalent to 100 mites/g dust).31,32 In 10% of dust samples from Korean homes, 10 µg/g of dust consisting of mite group 1 allergen was detected, which is known to be a major risk factor for the development of acute asthma (equivalent to 500 mite/g dust).33 In 100 randomly selected domestic homes in Cheonan, Korea, an average of 7.46 and 10.2 µg/g dust was measured from floors and mattresses, respectively.34 High levels of mite allergen were reported in Cheonan, which may indicate a geographical difference in mite allergen levels in Korea.
It would be interesting to perform a nationwide study to investigate the regional differences in mite allergen concentrations in house dust. A low sensitization rate to inhalant allergens including HDM (17.2% to D. farinae and 19.5% to D. pteronyssinus) in Gangwon area was reported,35 whereas a high prevalence of sensitization to HDM has been described in other areas, such as Chungbuk (more than 50%)36 and Busan (44.9% to D. farinae and 49.3% to D. pteronyssinus).37
The bedclothes and sewing dolls of Korean children were reported to have 3.24±0.50 and 3.43±0.30 µg/g dust of Der f 1.38 A median of 250 ng/g dust of Der f 1 was detected in the settled dust samples from 34 classrooms of Korean primary schools, which is higher than those (median, <200 ng/g dust) from 23 classrooms in Sweden.39 Common contamination of schools with mite allergens, along with cat and dog allergens, may contribute to sensitization of Korean pupils.
The distribution and seasonal variations of mite allergens in homes were measured by radioallergosorbent (RAST) inhibition.40 The mite allergen concentrations increased from spring (May) to autumn (September), and decreased from late autumn to early spring (October to May).30 This seasonal pattern is quite similar to a study performed in the US.41
Seasonal changes in the mite allergen concentrations in bedding, which reflect the natural exposure, can lead to concurrent changes in skin test reactivity and the presence of mite-specific IgE antibody in mite-asthmatic patients.42 A correlation between the allergen concentration and specific IgE to the HDM allergen, Der f 1, was described in 2006.30 The seasonal change in mite allergen levels in house dust can lead to concurrent changes in mite-specific IgG antibody levels, especially in the case of IgG4 in mite asthmatics;43 however, the significance of IgG changes remains unknown.
Recently, enzyme-linked immunosorbent assays (ELISAs) locally produced in Thailand detected higher levels of Der f 1 from dust samples compared to a commercially available immunoassay kit (Indoor Biotechnology, UK).44 Commercial two-site ELISA kits do not reflect the sequence polymorphism in a region. Therefore, it is thought that the mite allergen levels reported in Korea may have underestimated the actual allergen levels. Estimation of mite allergen level based on group 1 allergens is thought to be more reliable than that based on group 2 allergens because the amino acid sequences of group 2 allergens are more highly polymorphic, which hinders the precise determination of allergen level.45
Almost all Korean houses have 'ondol' heating, an under-floor heating system. Ondol removes house dust and provides hot and dry conditions when activated. Usually, bedclothes are stored inside furniture compartments during the daytime, and a bed is laid on the bedroom floor in the evening. Traditionally, Koreans do not have the mattresses or carpets that provide ideal habitats for HDM. These cultural and lifestyle differences may partly explain the dominance of D. farinae in Korean homes. In fact, a much lower mite and mite antigen density was detected in fine dust in Seoul, Korea, compared to those from Saitama prefecture, Japan, where climatic conditions are similar.46 Since the use of mattresses and carpets is becoming more common in Korea, it will be interesting to measure the change in the mite population density and the concentration of mite allergens in Korean homes as these lifestyle changes occur. The influence of global warming on the HDM population density and its allergen level should also continue to be monitored.
Recently, it was suggested that there is a bell-shaped relationship between exposure and sensitization, rather than a linear relationship.47 It was also suspected that reduced allergen exposure results in the failure to induce and maintain immune tolerance to common environmental allergens.48
HDMS IN ALLERGIC DISEASES
More than 60% of patients in Seoul that suffer from respiratory allergic symptoms are reported to be sensitized to HDM allergens.15,49 HDMs were also found to be the most frequent cause of respiratory allergic diseases, and 40%-60% of Korean respiratory allergic subjects were reported to show positive skin test reactions to HDM extracts between 1983 and 1985.11-14 In more recent studies, a sensitization rate to HDM of 17.2%-64.7% has been reported among respiratory allergic subjects, with some geographical differences as described above.15,35-37,49 There is no significant difference in the rate of sensitization to HDM between patients suffering from rhinitis and asthma. The rate of sensitization to HDM has been reported to be 27.9%-66.7% in patients suffering from atopic dermatitis.5-10 HDM allergic subjects are known to most commonly suffer from perennial rhinitis.33 The rate of sensitization to HDM in children suffering from atopic diseases is reported to be very high. The percentage of HDM-positive asthmatic children increases with age. In particular, as a risk factor for asthma symptom, positive HDM sensitivity values were lower in children who were 0-3 years (53.5%) compared with those who were 4-7 years (68.9%) and 8-12 years (80.2%) of age.50
Immunotherapy is an important approach to the treatment of HDM allergy. However, large-scale studies of their efficacy and mechanism have not been performed. Notably, immunotherapy of monosensitized patients showed improved allergic indices compared with polysensitized patients.51 Successful immunotherapy depends not only on carefully controlled treatment, but also on the careful selection of subjects.
ENVIRONMENTAL CONTROL IN HDM STUDIES PERFORMED IN KOREA
Simple employment of allergen-impermeable covers is thought to be an effective allergen avoidance measure. Vapor-permeable water-proof fabric bedding covers did not decrease HDM allergen levels in bedding.52 Selected use of an allergen-impermeable cover was reported to significantly reduce exposure to allergens from bedding dust and had clinical benefits.53 Interventions that achieve substantial reductions in dust mite load may reduce clinical symptoms. However, isolated use of a mite-impermeable bedding cover is not thought to offer general benefits.54
HDM species were detected in air conditioner filters,55 and air cleaners with electrostatic filters were shown to remove the mite allergen effectively.56 Airborne mite allergens were also measured during normal domestic activity.57 Airborne Der f 1 levels (14.0 pg/m3) were significantly correlated with those in bedding samples. However, the role of air cleaners in the removal of airborne HDM allergen remains controversial. Regular laundry with mechanical washing machines proved effective for removing HDM allergens from bedding and clothes.58 Water temperature (>60℃) is critical for killing HDM in clothes or bedding samples during mechanical laundry.58
HDM ALLERGEN: RELEVANT ALLERGENIC COMPONENTS IN KOREAN PATIENTS
A total of 32 IgE-reactive proteins have been shown to be allergenic among the 51 antigens identified by crossed immunoelectrophoretic analysis (CIE).59-61 Of over 30 protein bands in a D. farinae extract, a 14-15-kDa protein allergen, which is probably a group 2 allergen, was found to be the most potent by electroblotting.60
Twenty-four groups of mite proteins have been officially listed as allergens by the allergen nomenclature subcommittee (available from: www.allergen.org). It is necessary to compare the allergenicity of these allergens and evaluate their clinical relevance in Korean patients.
Presentation of exogenous antigens by antigen-presenting cells (APCs) in the absence of direct Toll-like receptor (TLR) stimulation generally leads to immune tolerance or anergy. Adjuvants, of which many are derived from microbes, are thought to activate the innate immune system by displaying a pathogen-associated molecular pattern. Effector T cell responses to APCs are efficiently generated when TLR ligands are present in the phagosome. TLR ligands could be derived from commensals and pathogens within HDM as well as the host (allergic patients). Allergic responses to these allergens can be modulated by the concomitant administration of adjuvant-like substances.62 Therefore, dust mite allergens can be classified according to the presence of inherent adjuvant-like activity or their affinity to adjuvant substances in mite extract. The following sections summarize the allergens from dust mites that are of allergenic importance with respect to their adjuvant-like activities.
Allergens with proteolytic activities
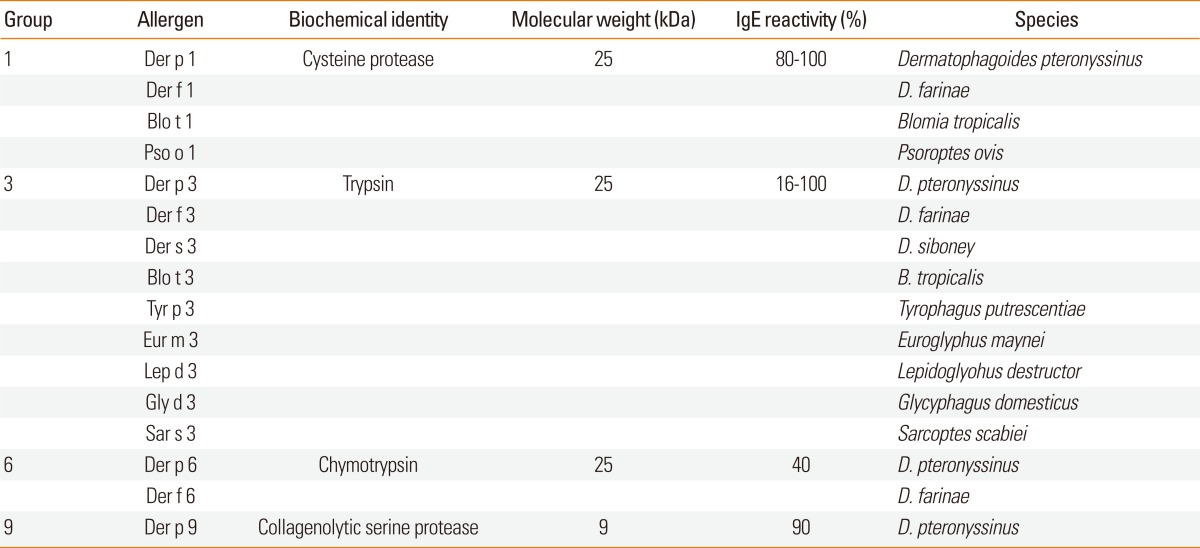
Protease activity is an important property of allergens.63 Allergens from mite groups 1, 3, 6, and 9 were identified as cysteine protease, trypsin, chymotrypsin, and collagenolytic serine protease, respectively (Table 1). Protease activities of these allergens can influence their allergenicity in various ways: (1) the proteolytic activity of mite allergens can cause disruption of the epithelium, allowing access of allergens to antigen-presenting cells64-66; (2) Der p 1 can cleave immunomodulators such as CD23 (low-affinity IgE receptor) and CD25 (α-subunit of IL-2 receptor)67; (3) trypsin-like group 3 allergens can activate complement and kallikrein, causing liberation of kinins and activated complement68,69; (4) protease allergens are known to elicit inflammatory reactions by activating protease-activated receptor-270; (5) protease activity can release inflammatory cytokines independent of PAR-2 activation71; and (6) cysteine protease, possibly Der p 1, has been shown to activate and recruit basophils to the draining lymph nodes and stimulate production of Th2-inducing cytokines, including IL-4 and thymic stromal lymphopoietin (TSLP), suggesting an important role for protease in Th2 differentiation.72 The mechanisms by which dust mite proteases might promote allergic responses were reviewed by Smith and Harper.73 Recently, it has been suggested that cysteine protease activity could be an adjuvant for Th2 responses against both themselves (Der p 1) and bystander antigens (Der p 2), even in the absence of another adjuvant.74 It is possible that there are proteolytic enzymes other than these protease allergens in mite extracts, which may affect allergic responses through induction of inflammatory reactions, regardless of IgE reactivity.75
Table 1.
Dust mite allergens with proteolytic activity
Allergens with affinities to lipids
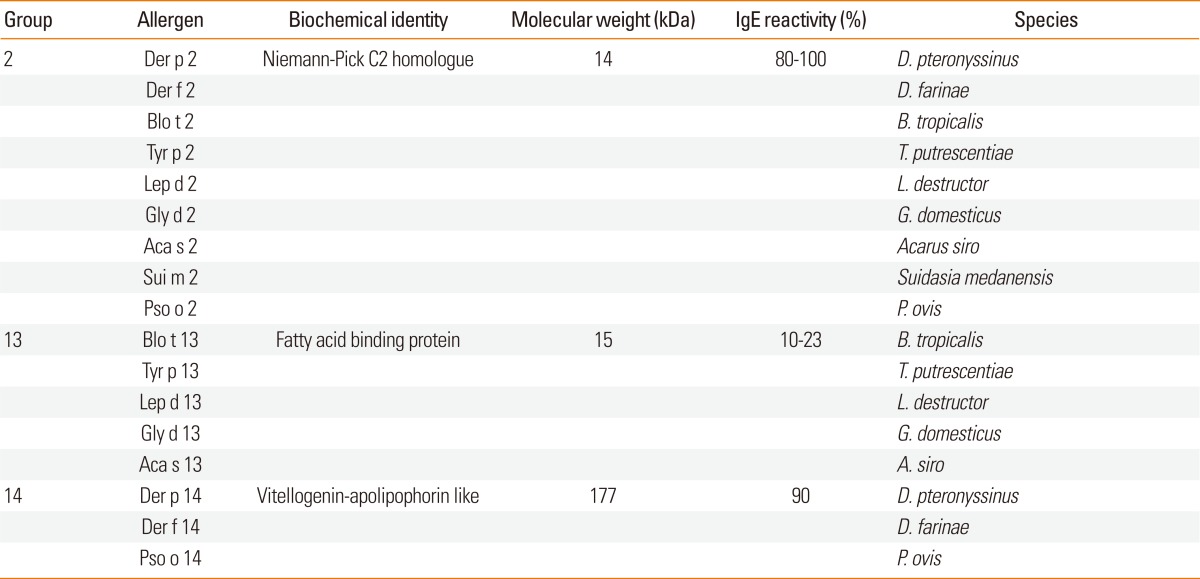
Lipid-binding properties are important characteristics of allergens.76,77 Group 2, 13, and 14 allergens are associated with lipid-binding activity (Table 2). Der f 2 showed stronger IgE reactivity than Der f 1 in Korean mite-allergy patients.78-80 Der p 2 and Der f 2 were localized in the midgut and fecal pellets of the mite, indicating that these are associated with the digestive system.81,82 Jeong et al.,82 suggested that Der f 2 plays a role in preventing microbes from penetrating the mite epithelia. However, the exact function of Der f 2 and Der p 2 in mites is not yet clear. Der p 2 can bind lipopolysaccharides, subsequently reconstituting and amplifying TLR4 signaling.83 This observation suggests that the intrinsic adjuvant-like activity of allergens can enhance their allergenicity. Der p 2 is known to activate respiratory epithelial cells and induce secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, IL-8, monocyte-chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-3α (MIP-3α), which is regulated by nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK).84 Der f 2 was shown to activate transcription factor-2, and subsequently induce activation of phospholipase D1 and expression of IL-13 in human bronchial epithelial cells.85
Table 2.
Dust mite allergens with lipid binding properties
Der f 2 and Der p 2 of Korean HDM have frequent polymorphisms in their amino acid sequences,86 which affect their affinities to human specific IgE and monoclonal antibodies.45,87-89
Lipids may play a role in triggering of immune reactions by activation of CD1+ dendritic cells and restriction of T lymphocytes.90 The size of the lipid vesicle entrapped by macrophages is reported to determine the pattern of cytokine production by T helper cells.91 Vesicles with a mean diameter of less than 155 nm were shown to induce a Th2 response, whereas vesicles with a mean diameter larger than 225 nm induced Th1 responses. These reports suggest that the lipid-binding properties of allergens affect its allergenicity.
Allergens from mite groups 13 and 14 are thought to have lipid-binding properties. Group 13 allergens are homologous to fatty-acid-binding proteins,92,93 and group 14 allergens show some homology with apolipophorin and vitellogenin. The allergenicity of Der f 14 might have been underestimated because it is not water soluble.94 Fujikawa et al.95 reported that the allergenicity of Der f 14 is greater than that of Der p 2. Furthermore, Der f 14 is highly susceptible to proteolytic degradation, and the fragments so generated could be more allergenic than the intact protein.96
Chitin-binding proteins
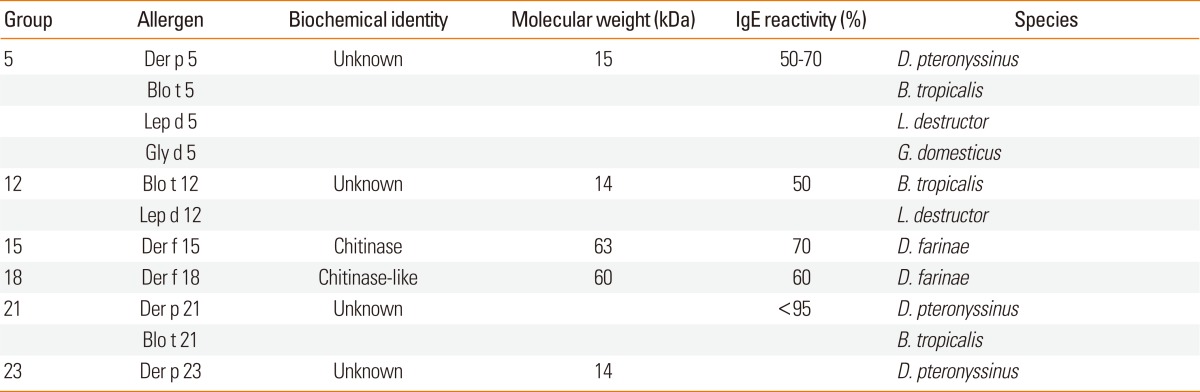
Chitin, an abundant polysaccharide, is an important exoskeleton component of arthropods. Mites shed their chitin coats for growth, and recently, chitin was identified as a common constituent of house dust samples.97 Interestingly, acidic mammalian chitinases were identified in human beings, even though humans do not metabolize chitin. Chitinase was found to play an important role in the pathogenesis of asthma.98,99 Allergens from mite groups 5, 12, 15, 18, 21, and 23 are thought to bind to chitin (Table 3). Der f 15 shows homology with insect chitinases (family 18 of the glucohydrolase superfamily).100 Group 18 allergens are also homologs of chitinase, and contain two putative chitinase catalytic domains.101,102 The function of group 5 allergens is elusive. Secondary structure prediction and NMR analysis showed a pattern of heptad repeats, which might be associated with polymerization.103,104 Blo t 21, which has a high sequence identity with group 5 allergens of Dermatophagoides spp., was localized in the midgut epithelium and fecal pellets,105 implying that mite group 5 and 21 allergens may be involved in the formation of the peritrophic membrane envelope comprised of a protein matrix and chitin fibrils. Structural analysis by molecular modeling approaches revealed that group 5 and 21 allergens could dimerize and form a large hydrophobic cavity, which could be involved in the binding of hydrophobic ligands.106 Der p 23 also shows some homology with peritrophin A, whose function is also associated with chitin binding. Group 12 allergens, Blo t 12107 and Led d 12 (AY293744), show limited homology a peritrophic membrane-binding protein from the moth cabbage looper, Trichoplusia ni, and Der f 15.
Table 3.
Dust mite allergens with chitin binding property
Allergens not associated with adjuvant-like activity
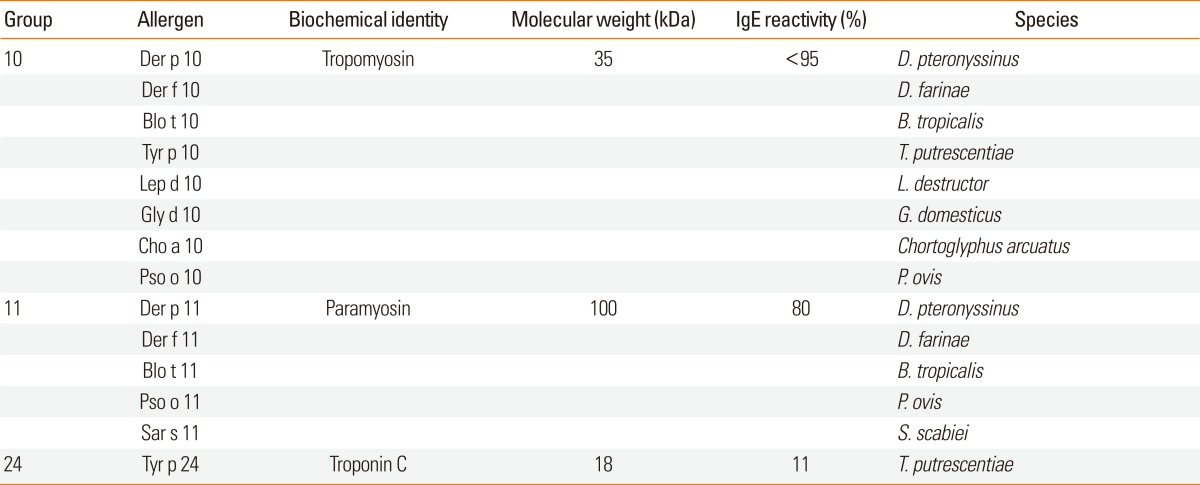
Some groups of mite allergens are not known to be associated with adjuvant-like activity. However, some of these have been described as potent allergens. These may be bystander allergens or could have an unidentified adjuvant-like function. This group of allergens is subclassified into muscle and non-muscle proteins for convenience.
Muscle proteins derived from dead debris could be inhaled and ingested with mite-contaminated food.108 Allergens from mite groups 10, 11, and 24 are muscle proteins (Table 4). Tropomyosin (mite group 10 allergens), paramyosin (mite group 11 allergens), and troponin C (mite group 24 allergens) are known to be allergenic.
Table 4.
Dust mite allergens from muscle
Tropomyosins are the most important food allergens of shellfish and mollusks, such as shrimp, lobster, crab, oyster and squid. The amino acid sequences of invertebrate tropomyosins are highly conserved and they can cause cross-reactivities.109 However, dust mite tropomyosins are thought to contribute a limited degree of dust mite cross-reactivity due to their low IgE reactivity.110 Furthermore, tropomyosin is a major concern regarding neo-sensitization in patients with mite immunotherapy.111
Paramyosin is a ubiquitous protein found in invertebrate muscle. A high frequency of IgE binding was reported for Der f 11, Der p 11 and Blo t 11 (67%-80%).112-114 The amino acid sequences of mite paramyosins are highly conserved and might be cross-reactive. Paramyosin, an important parasite antigen, is a target for vaccine development.115 Parasite paramyosin may affect the cross-reactivity of mite paramyosin. However, mite paramyosins were found to be easily degraded and present in the allergen extract at less than 1 µg/mL.116
Troponin C, a mite group 24 allergen, was recently described as an allergen from T. putrescentiae.117 Interestingly, the IgE reactivity of Tyr p 24 was dependent on the Ca2+ concentration. It showed a limited degree of cross-reactivity with Bla g 6, the cockroach troponin C (68.2% identical). The allergenicity of troponin C from HDM has not been investigated.
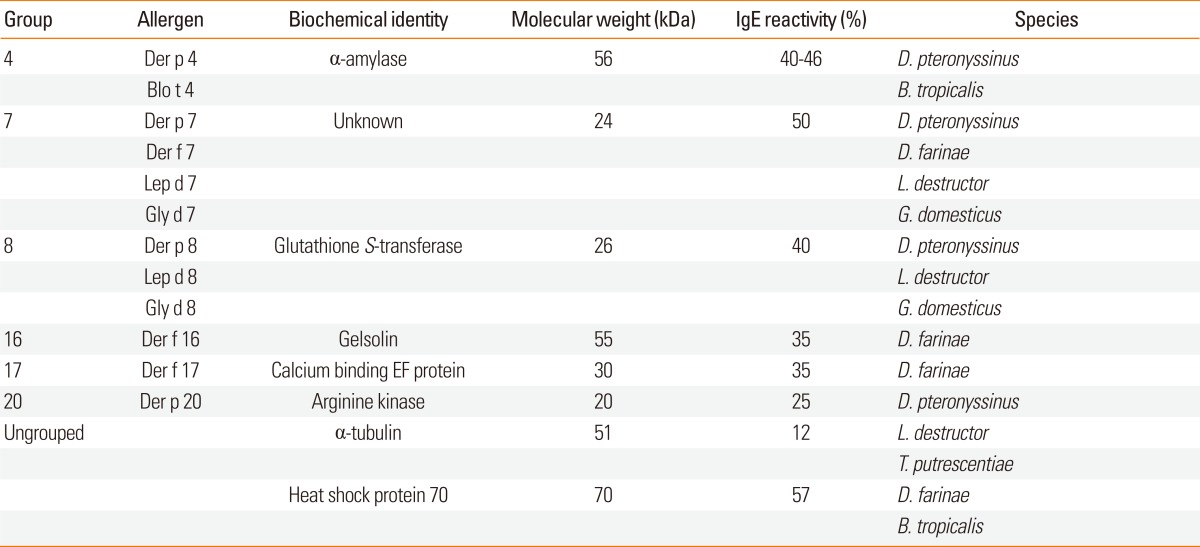
Some mite allergens, such as those from groups 4, 7, 8, 16, 17, and 20, are neither muscle proteins nor associated with any known adjuvant-like molecules (Table 5). For example, α-amylase is a group 4 allergen.118,119 Interestingly, amylase inhibitors from cereals are known as important occupational allergens.120 Insects and mites produce multiple isozymes against these dietary inhibitors.121 It is worth noting that the amino acid sequences of amylases are highly conserved across the animal kingdom. Investigations of cross-reactivities between related species should be performed.
Table 5.
Dust mite allergens without autoadjuvant or adjuvant binding activity
Little is known about the structure and function of group 7 allergens. Despite its abundance in the mite,122 the group 7 allergen concentration in mite extract was low.123 Group 7 allergen is glycosylated and easily degraded in extract.124 The IgE binding frequency of group 7 allergens was reported to be 37%-67%.125-127 Mite group 7 allergens are thought to be important molecules for the standardization of recombinant protein production.
The group 8 allergens, which are mu-class glutathione S-transferases (GSTs), play a role in detoxification.128 Huang et al.129 also described cross-reactivity between the mite and cockroach GSTs. However, Bla g 5, the cockroach GST, belongs to the sigma class. There is a limited degree of cross-reactivity between sigma- and delta-class GSTs, even within the same species.130 Cross-reactivities between the same GST class should be examined.
Der f 16, a member of the gelsolin/vollin family, and Der f 17, an EF-hand Ca2+-binding protein, were identified by screening cDNA libraries.131 Blo t 19 is known to have 76% homology with an antibacterial factor, ASABF, from Ascaris suum. However, no detailed information on these allergens has been published to-date.
Group 20 allergens consist of arginine kinase, an equivalent to the vertebrate creatine kinase. Der p 20 bound 40% of IgE antibody, although with a low titer.132 Interestingly, the group 2 allergen of shrimp is also arginine kinase.133 Invertebrate arginine kinases have a high homology (70%-80% identity), suggesting the possibility of cross-reactivity.
Der f 22, recognized in the International Union of Immunological Societies (IUIS) official list of allergens (available from: www.allergen.org), is known to have some homology with mite group 2 allergen. However, no published data on mite group 22 allergen is available at present.
β-glucan derived from fungi has also been detected in house dust samples,134 and is known to be associated with asthma symptoms.135,136 However, none of the allergens identified to-date exhibited a relationship with β-glucan. It would be interesting to investigate the role of β-glucan in HDM allergy and characterize the molecules from mite species that are associated with β-glucan-like substances.
Additional proteins with IgE reactivity from dust mites have been described, although they are not included in the IUIS list, including hydrolase,137 a heat shock protein-70 homologue,138 α-tubulin,139,140 a 39-kDa allergen of unknown function,141,142 a 244-kDa allergen of unknown function,143 and 79- and 93-kDa proteins from L. destructor.144 Of these, the IgE reactivity of recombinant α-tubulin from Korean T. putrescentiae was investigated,140 and the frequency of IgE reactivity was 29.3%. However, it is noteworthy for its highly conserved amino acid sequence among almost all known species.
Induction of various cytokines and chemokines by treatment with mite extract has been described in peripheral blood mononuclear cells and human monocytic THP-1 cells.145,146 Identification of the molecules responsible for these observations is necessary.
Allergenic relevance of dust mite allergens in Korea
IgE immunoblotting showed some differences in IgE-reactive components among individuals,60 and therefore, the development of personalized immunotherapeutic reagents might be beneficial.
A 15-kDa allergen, possibly belonging to group 2, was the most potent in terms of IgE-binding frequency and intensity in Korea.147 Serum samples from 60% and 61% of patients who showed positive skin prick test responses to HDM extract were found to contain IgE specific for Der f 1 and Der f 2, respectively.78 However, Der f 2 showed significant inhibition of specific IgE to HDM whole body extract, whereas Der f 1 did not at an inhibitory concentration (20 ng/mL). IgE responses to Der f 2 were also significantly higher than those to Der f 1 in children over 4 years of age.80 IgE reactivity to Der f 1 and Der f 2 was higher in an atopic than a non-atopic group.79 However, IgE to Der f 1 was more common in a group of asthma patients. This finding suggests a relationship between the properties of allergens and their allergic manifestation. Recombinant Der f 2 produced in E. coli is recognized by 90%-100% of serum IgE from Korean D. farinae-sensitized subjects (data not shown). Vaccination with DNA-encoding T cell epitopes of Der p 1 and Der p 2 effectively inhibits allergen-specific IgE synthesis and reduces cell infiltration in lung tissue in mice.148 A mixture of DNA vaccines encoding mite group 1, 2, and 3 allergens had a protective effect in the murine model sensitized to HDM crude extract, indicating that these allergens play important roles in HDM allergy.149 However, their effect in humans remains to be evaluated.
The recombinant mite tropomyosins, Der f 10 and Tyr p 10, showed 25% and 12.5% IgE-binding frequency to Korean mite-sensitized patients.110 However, neither allergen significantly inhibited IgE binding to crude extracts. Recombinant fatty acid-binding protein, Tyr p 13, was recognized in 6.4% of sera from T. putrescentiae-sensitized subjects.92 Recombinant Tyr p 13 was able to inhibit up to 61.9% of IgE reactivity to crude extract in serum. This suggests that minor allergens play an important role in some cases. For troponin C, Tyr p 24, a 10.6% IgE binding frequency was detected, but it did not significantly inhibit IgE reactivity to crude extract.117 The frequency of IgE reactivity to recombinant α-tubulin from T. putrescentiae was 29.3%, although its titer was very low.140 The allergenicities of the other groups of allergens in Korea have yet to be investigated.
CONCLUDING REMARKS
HDM is the most important inhalant allergen in Korea, and its exposure is also significant. More than 31 allergens have been reported to-date in HDM extracts, and IgE reactivity profiles to purified allergens vary in subjects from different countries.150 In Korea, investigations of the sensitization profile of the entire panel of mite allergens, and comparisons among the relevant single allergens, are required. Additionally, future investigations will need to examine the mechanisms by which adjuvant-like activities modulate the innate immune response, subsequent to the allergic manifestation. Component-resolved diagnosis (CRD) is of growing importance for the diagnosis of allergic diseases,151 and future studies will investigate the relationship between adjuvant-like activity and allergy symptoms. Development and production of therapeutic recombinant HDM allergens will facilitate CRD and immunotherapeutic approaches using selected molecules.152
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R & D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092076).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Decker H. Asthma und milben. Munch Med Wochenschr. 1928;75:515–516. [Google Scholar]
- 2.Voorhorst R, Spieksma FT, Varekamp H, Leupen MJ, Lyklema AW. The house-dust mite (Dermatophagoides pteronyssinus) and the allergens it produces. Identity with the house-dust allergen. J Allergy. 1967;39:325–339. [Google Scholar]
- 3.Miyamoto T, Oshima S, Ishizaki T, Sato SH. Allergenic identity between the common floor mite (Dermatophagoides farinae Hughes, 1961) and house dust as a causative antigen in bronchial asthma. J Allergy. 1968;42:14–28. doi: 10.1016/0021-8707(68)90128-7. [DOI] [PubMed] [Google Scholar]
- 4.Voorhorst R, Spieksma-Boezeman MI, Spieksma FT. Is a mite (Dermatophagoides sp.) the producer of the house-dust allergen? Allerg Asthma (Leipz) 1964;10:329–334. [PubMed] [Google Scholar]
- 5.Min TH, Hong CK, Ro BI, Chang CY. Allergen prick test reactivity in the patients with urticaria and atopic dermatitis. Korean J Dermatol. 1987;25:587–598. [Google Scholar]
- 6.Kang SJ, Kim KH, Kim SH. A study on type I allergy to house dust mite in patients with atopic dermatitis. Korean J Dermatol. 1991;29:285–291. [Google Scholar]
- 7.Cho HJ, Choi HJ, Kim DK, Lee KH. Dermatophagoides farinae-specific IgE and IgG4 antibodies in atopic dermatitis patients. Korean J Dermatol. 1998;36:16–22. [Google Scholar]
- 8.Yim YS, Park CW, Lee CH. A comparative study of atopy patch test using house dust mite antigens with skin prick test and specific serum IgE level in atopic dermatitis. Korean J Dermatol. 2001;39:1072–1079. [Google Scholar]
- 9.Kim SS, Bang HD, Kim KH, Kim KJ. Evaluation of Pharmacia CAP system FEIA for house dust mites in patients with atopic dermatitis. Korean J Dermatol. 2003;41:1455–1462. [Google Scholar]
- 10.Shin JW, Jin SP, Lee JH, Cho S. Analysis of MAST-CLA results as a diagnostic tool in allergic skin diseases. Ann Dermatol. 2010;22:35–40. doi: 10.5021/ad.2010.22.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahu SD, Kim HJ. The allergen skin test and the effect of specific desensitising vaccination therapy in allergic rhinitis and bronchial asthma. Allergy. 1983;3:159–167. [Google Scholar]
- 12.Kang SY, Choi BW, Moon HB, Min KU, Kim YY. The prevalence of immediate skin reactions in patients with respiratory allergies. Allergy. 1984;4:49–56. [Google Scholar]
- 13.Cho HS, Lee KH, Choi YK, Cho DK, Kim NS. Result of allergen skin tests in type I hypersensitivity. Allergy. 1985;5:14–22. [Google Scholar]
- 14.Rhee YG, Oh YI. Results of skin test, peripheral eosinophil count, total and specific IgE in allergic patients in Chonbuk area. Allergy. 1985;5:147–155. [Google Scholar]
- 15.Jeong KY, Hong CS, Yong TS. Domestic arthropods and their allergens. Protein Pept Lett. 2007;14:934–942. doi: 10.2174/092986607782541114. [DOI] [PubMed] [Google Scholar]
- 16.Park HS, Choi GS, Cho JS, Kim YY. Epidemiology and current status of allergic rhinitis, asthma, and associated allergic diseases in Korea: ARIA Asia-Pacific workshop report. Asian Pac J Allergy Immunol. 2009;27:167–171. [PubMed] [Google Scholar]
- 17.Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, Park KS, Kim H, Kim EJ, Lee JS, Hong SJ. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012;158:168–174. doi: 10.1159/000330820. [DOI] [PubMed] [Google Scholar]
- 18.Cho BK, Lee WK. House dust mites. Allergy. 1981;1:138–144. [Google Scholar]
- 19.Chu JK, Song SB, Kim DK, Kim YK. Epidemiological studies on the acaroid mite. Korean J Parasitol. 1967;5:69–75. [PubMed] [Google Scholar]
- 20.Cho BK, Houh W. The mite fauna of Korean house dust (I) Korean J Dermatol. 1977;15:133–140. [Google Scholar]
- 21.Lee WK, Cho BK. An ecological study on the house dust mite. Korean J Dermatol. 1984;22:286–294. [Google Scholar]
- 22.Ree HI, Jeon SH, Lee IY, Hong CS, Lee DK. Fauna and geographical distribution of house dust mites in Korea. Korean J Parasitol. 1997;35:9–17. doi: 10.3347/kjp.1997.35.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Martínez J, Eraso E, Palacios R, Guisantes JA. Cross-reactions between Dermatophagoides pteronyssinus and Dermatophagoides farinae (Acari: Pyroglyphidae) related to the different growth phases of cultures. J Med Entomol. 2000;37:35–39. doi: 10.1603/0022-2585-37.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Munhbayarlah S, Park JW, Ko SH, Ree HI, Hong CS. Identification of Tyrophagus putrescentiae allergens and evaluation of cross-reactivity with Dermatophagoides pteronyssinus. Yonsei Med J. 1998;39:109–115. doi: 10.3349/ymj.1998.39.2.109. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Ko SH, Yong TS, Ree HI, Jeoung BJ, Hong CS. Cross-reactivity of Tyrophagus putrescentiae with Dermatophagoides farinae and Dermatophagoides pteronyssinus in urban areas. Ann Allergy Asthma Immunol. 1999;83:533–539. doi: 10.1016/S1081-1206(10)62865-7. [DOI] [PubMed] [Google Scholar]
- 26.van Hage-Hamsten M, Johansson SG, Johansson E, Wiren A. Lack of allergenic cross-reactivity between storage mites and Dermatophagoides pteronyssinus. Clin Allergy. 1987;17:23–31. doi: 10.1111/j.1365-2222.1987.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY. Tyrophagus putrescentiae: an important allergen in Daejeon. J Asthma Allergy Clin Immunol. 2002;22:703–710. [Google Scholar]
- 28.Choi BS, Lee YJ, Baek JY, Kim KW, Sohn MH, Kim KE. Prevalence of sensitization to Tyrophagus putrescentiae in children with allergic diseases. Pediatr Allergy Respir Dis. 2010;20:107–113. [Google Scholar]
- 29.Yong TS, Jeong KY. Review on ecology of house dust mites in Korea and suggestion of a standard survey method. Pediatr Allergy Respir Dis. 2011;21:4–16. [Google Scholar]
- 30.Kim JH, Choi SY, Lee IY, Lee YW, Yong TS, Kim CW, Song YS, Park JW, Kim YS, Park JW, Hong CS. Seasonal variation of house dust mite and its influence on the inhabitant health. Korean J Asthma Allergy Clin Immunol. 2006;26:27–34. [Google Scholar]
- 31.Dust mite allergens and asthma--a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 32.Hong CS. House dust mite and bronchial asthma - a worldwide problem. J Korean Med Assoc. 1989;32:1078–1083. [Google Scholar]
- 33.Hong CS. House dust mite and clinical allergy. Allergy. 1991;11:297–308. [Google Scholar]
- 34.Nam HS, Siebers R, Lee SH, Park JS, Kim YB, Choi YJ, Lee SH, Crane J. House dust mite allergens in domestic homes in Cheonan, Korea. Korean J Parasitol. 2008;46:187–189. doi: 10.3347/kjp.2008.46.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MK, Lee WY, Yong SJ, Shin KC, Lee SN, Lee SJ, Lee JH, Jung S, Jung YR, Kim SH. Sensitization rates to inhalant allergens in patients visiting a University Hospital in Gangwon region. Korean J Asthma Allergy Clin Immunol. 2011;31:27–32. [Google Scholar]
- 36.Kim MK, Oh SW. Change of causative inhalant allergens in respiratory allergic patients in Chungbuk district. J Asthma Allergy Clin Immunol. 1999;19:696–702. [Google Scholar]
- 37.Seo HR, Lee SM, Koo TH, Shin BC, Kim BK, Heo JH, Yang DK, Lee SK, Son CH. Sensitization rate to Tetranychus urticae and house dust mite in patients who visited the allergy clinic with allergic diseases in Busan area. Korean J Asthma Allergy Clin Immunol. 2007;27:263–267. [Google Scholar]
- 38.Choi JY, Sohn MH, Jang GC, Lee KE, Kim KE. Concentrations of dust mite in the dust of childhood bedclothing, cloth wrappers, and sewing dolls. Pediatr Allergy Respir Dis. 2002;12:185–191. [Google Scholar]
- 39.Kim JL, Elfman L, Norbäck D. Respiratory symptoms, asthma and allergen levels in schools--comparison between Korea and Sweden. Indoor Air. 2007;17:122–129. doi: 10.1111/j.1600-0668.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim SK, Park HS, Oh SH, Hong CS. Distribution of house dust mites allergen in houses measured by RAST inhibition test. Korean J Intern Med. 1988;35:65–75. [Google Scholar]
- 41.Chew GL, Higgins KM, Gold DR, Muilenberg ML, Burge HA. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy. 1999;54:1058–1066. doi: 10.1034/j.1398-9995.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 42.Nahm DH, Park HS, Kang SS, Hong CS. Seasonal variation of skin reactivity and specific IgE antibody to house dust mite. Ann Allergy Asthma Immunol. 1997;78:589–593. doi: 10.1016/S1081-1206(10)63221-8. [DOI] [PubMed] [Google Scholar]
- 43.Nahm DH, Park HS, Kim CW, Park JW, Hong CS. Seasonal variation of IgG subclass antibodies to house dust mite in sera from mite-sensitive asthmatic patients. Ann Allergy Asthma Immunol. 1998;80:411–415. doi: 10.1016/s1081-1206(10)62993-6. [DOI] [PubMed] [Google Scholar]
- 44.Sookrung N, Kamlanghan T, Indrawattana N, Tungtrongchitr A, Tantilipikorn P, Bunnag C, Pattanapanyasat K, Chaicumpa W. Quantification of Der f 1 in houses of patients allergic to house dust mite, Dermatophagoides farinae, using a locally produced detection reagents. Asian Pac J Allergy Immunol. 2011;29:78–85. [PubMed] [Google Scholar]
- 45.Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, Hong CS, Park JW. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. doi: 10.1007/s10493-012-9553-x. Forthcoming 2012. [DOI] [PubMed] [Google Scholar]
- 46.Paik YH, Takaoka M, Matsuoka H, Ishii A. Mite fauna and mite antigen in house dust from houses in Seoul, Korea. Jpn J Sanit Zool. 1992;43:29–35. [Google Scholar]
- 47.van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007;119:270–277. doi: 10.1016/j.jaci.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 48.Linneberg A. Are we getting enough allergens? Int Arch Allergy Immunol. 2008;147:93–100. doi: 10.1159/000135695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong KY, Yum HY, Lee IY, Ree HI, Hong CS, Kim DS, Yong TS. Molecular cloning and characterization of tropomyosin, a major allergen of Chironomus kiiensis, a dominant species of nonbiting midges in Korea. Clin Diagn Lab Immunol. 2004;11:320–324. doi: 10.1128/CDLI.11.2.320-324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin JW, Sue JH, Song TW, Kim KW, Kim ES, Sohn MH, Kim KE. Atopy and house dust mite sensitization as risk factors for asthma in children. Yonsei Med J. 2005;46:629–634. doi: 10.3349/ymj.2005.46.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KW, Kim EA, Kwon BC, Kim ES, Song TW, Sohn MH, Kim KE. Comparison of allergic indices in monosensitized and polysensitized patients with childhood asthma. J Korean Med Sci. 2006;21:1012–1016. doi: 10.3346/jkms.2006.21.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang SS, Nahm DH, Kim CW, Park JW, Hong CS. The effect of the bedding cover made of a vapour-permeable water-proof fabric on allergic immune responses and clinical symptoms in house dust mite-sensitive asthmatics. Korean J Allergy. 1996;16:26–37. [Google Scholar]
- 53.Kim C, Hong C, Choi S, Kim D. Simple application of allergen-impermeable bed covers for adult patients with allergic rhinitis in Korea. J Allergy Clin Immunol. 2005;115:S268. [Google Scholar]
- 54.Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–165. doi: 10.1111/j.1398-9995.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Bai Y, Ji K, Liu X, Cai C, Yu H, Li M, Bao Y, Lian Y, Gao B. Detection of Dermatophagoides farinae in the dust of air conditioning filters. Int Arch Allergy Immunol. 2007;144:85–90. doi: 10.1159/000102619. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal SR, Kim HJ, Lee YW, Sohn JH, Lee JH, Kim YJ, Lee SH, Hong CS, Park JW. Effect of an air cleaner with electrostatic filter on the removal of airborne house dust mite allergens. Yonsei Med J. 2010;51:918–923. doi: 10.3349/ymj.2010.51.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JW, Kim CW, Kang DB, Lee IY, Choi SY, Yong TS, Shin DC, Kim KE, Hong CS. Low-flow, long-term air sampling under normal domestic activity to measure house dust mite and cockroach allergens. J Investig Allergol Clin Immunol. 2002;12:293–298. [PubMed] [Google Scholar]
- 58.Choi SY, Lee IY, Sohn JH, Lee YW, Shin YS, Yong TS, Hong CS, Park JW. Optimal conditions for the removal of house dust mite, dog dander, and pollen allergens using mechanical laundry. Ann Allergy Asthma Immunol. 2008;100:583–588. doi: 10.1016/S1081-1206(10)60060-9. [DOI] [PubMed] [Google Scholar]
- 59.Baldo BA, Ford SA, Tovey ER. Toward a definition of the 'complete' spectrum and rank order of importance of the allergens from house dust mite: Dermatophagoides pteronyssinus. Adv Biosci. 1989;74:13–31. [Google Scholar]
- 60.Hong CS, Lee MK, Oh SH. Identification of major allergens from the house dust mites, Dermatophagoides farinae and Dermatophagoides pteronyssinus, by electroblotting. Yonsei Med J. 1991;32:24–32. doi: 10.3349/ymj.1991.32.1.24. [DOI] [PubMed] [Google Scholar]
- 61.Arlian LG, Bernstein IL, Geis DP, Vyszenski-Moher DL, Gallagher JS, Martin B. Investigations of culture medium-free house dust mites. III. Antigens and allergens of body and fecal extract of Dermatophagoides farinae. J Allergy Clin Immunol. 1987;79:457–466. doi: 10.1016/0091-6749(87)90363-0. [DOI] [PubMed] [Google Scholar]
- 62.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121:847–852.e7. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 64.Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–378. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- 65.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–294. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 67.Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi K, Aoki T, Kohmoto S, Nishimura H, Kodera Y, Matsushima A, Inada Y. Activation of kallikrein-kinin system in human plasma with purified serine protease from Dermatophagoides farinae. Int Arch Allergy Appl Immunol. 1990;91:80–85. doi: 10.1159/000235094. [DOI] [PubMed] [Google Scholar]
- 69.Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J Allergy Clin Immunol. 1997;100:253–260. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- 70.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51:808–822. doi: 10.3349/ymj.2010.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 72.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith PK, Harper JI. Serine proteases, their inhibitors and allergy. Allergy. 2006;61:1441–1447. doi: 10.1111/j.1398-9995.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- 74.Cunningham PT, Elliot CE, Lenzo JC, Jarnicki AG, Larcombe AN, Zosky GR, Holt PG, Thomas WR. Sensitizing and Th2 adjuvant activity of cysteine protease allergens. Int Arch Allergy Immunol. 2012;158:347–358. doi: 10.1159/000334280. [DOI] [PubMed] [Google Scholar]
- 75.Jeong KY, Kim C, Yong TS. Enzymatic activities of allergen extracts from three species of dust mites and cockroaches commonly found in Korean home. Korean J Parasitol. 2010;48:151–155. doi: 10.3347/kjp.2010.48.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas WR, Hales BJ, Smith WA. Structural biology of allergens. Curr Allergy Asthma Rep. 2005;5:388–393. doi: 10.1007/s11882-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 77.Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Park JW, Nahm DH, Hong CS. Determination of specific IgE to two major allergens (Der f I and Der f II) of house dust mite (D. farinae) in Korean adult respiratory allergy patients. Allergy. 1993;13:476–486. [Google Scholar]
- 79.Hong CS, Park JW, Nahm DH. Measurement of IgE and IgG subclass antibodies to whole body antigen and two major allergens (Der fI & Der fII) of Dermatophagoides farinae in normal subjects and asthmatics. Yonsei Med J. 1994;35:453–463. doi: 10.3349/ymj.1994.35.4.453. [DOI] [PubMed] [Google Scholar]
- 80.Nahm DH, Park JW, Hong CS, Lee SY, Lee KY. Specific IgE antibodies to D. farinae whole body extract, Der f I and Der f II in child age groups. Pediatr Allergy Respir Dis. 1995;5:117–124. [Google Scholar]
- 81.Park GM, Lee SM, Lee IY, Ree HI, Kim KS, Hong CS, Yong TS. Localization of a major allergen, Der p 2, in the gut and faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2000;30:1293–1297. doi: 10.1046/j.1365-2222.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- 82.Jeong KY, Lee IY, Ree HI, Hong CS, Yong TS. Localization of Der f 2 in the gut and fecal pellets of Dermatophagoides farinae. Allergy. 2002;57:729–731. doi: 10.1034/j.1398-9995.2002.23623.x. [DOI] [PubMed] [Google Scholar]
- 83.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Osterlund C, Grönlund H, Polovic N, Sundström S, Gafvelin G, Bucht A. The non-proteolytic house dust mite allergen Der p 2 induce NF-κB and MAPK dependent activation of bronchial epithelial cells. Clin Exp Allergy. 2009;39:1199–1208. doi: 10.1111/j.1365-2222.2009.03284.x. [DOI] [PubMed] [Google Scholar]
- 85.Park SY, Cho JH, Oh DY, Park JW, Ahn MJ, Han JS, Oh JW. House dust mite allergen Der f 2-induced phospholipase D1 activation is critical for the production of interleukin-13 through activating transcription factor-2 activation in human bronchial epithelial cells. J Biol Chem. 2009;284:20099–20110. doi: 10.1074/jbc.M109.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piboonpocanun S, Malainual N, Jirapongsananuruk O, Vichyanond P, Thomas WR. Genetic polymorphisms of major house dust mite allergens. Clin Exp Allergy. 2006;36:510–516. doi: 10.1111/j.1365-2222.2006.02464.x. [DOI] [PubMed] [Google Scholar]
- 87.Jeong KY, Jin HS, Oh SH, Hong CS, Lee IY, Ree HI, Yong TS. Monoclonal antibodies to recombinant Der f 2 and development of a two-site ELISA sensitive to major Der f 2 isoallergen in Korea. Allergy. 2002;57:29–34. [PubMed] [Google Scholar]
- 88.Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, Yong TS, Oh SH, Hong CS. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002;32:1042–1047. doi: 10.1046/j.1365-2222.2002.01421.x. [DOI] [PubMed] [Google Scholar]
- 89.Jin HS, Yong TS, Park JW, Hong CS, Oh SH. Immune reactivity of recombinant group 2 allergens of house dust mite, Dermatophagoides pteronyssinus, and Dermatophagoides farinae. J Investig Allergol Clin Immunol. 2003;13:36–42. [PubMed] [Google Scholar]
- 90.Russano AM, Agea E, Casciari C, de Benedictis FM, Spinozzi F. Complementary roles for lipid and protein allergens in triggering innate and adaptive immune systems. Allergy. 2008;63:1428–1437. doi: 10.1111/j.1398-9995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 91.Brewer JM, Tetley L, Richmond J, Liew FY, Alexander J. Lipid vesicle size determines the Th1 or Th2 response to entrapped antigen. J Immunol. 1998;161:4000–4007. [PubMed] [Google Scholar]
- 92.Jeong KY, Kim WK, Lee JS, Lee J, Lee IY, Kim KE, Park JW, Hong CS, Ree HI, Yong TS. Immunoglobulin E reactivity of recombinant allergen Tyr p 13 from Tyrophagus putrescentiae homologous to fatty acid binding protein. Clin Diagn Lab Immunol. 2005;12:581–585. doi: 10.1128/CDLI.12.5.581-585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan SL, Ong ST, Ong SY, Chew FT, Mok YK. Nuclear magnetic resonance structure-based epitope mapping and modulation of dust mite group 13 allergen as a hypoallergen. J Immunol. 2006;176:4852–4860. doi: 10.4049/jimmunol.176.8.4852. [DOI] [PubMed] [Google Scholar]
- 94.Epton MJ, Dilworth RJ, Smith W, Hart BJ, Thomas WR. High-molecular-weight allergens of the house dust mite: an apolipophorin-like cDNA has sequence identity with the major M-177 allergen and the IgE-binding peptide fragments Mag1 and Mag3. Int Arch Allergy Immunol. 1999;120:185–191. doi: 10.1159/000024266. [DOI] [PubMed] [Google Scholar]
- 95.Fujikawa A, Ishimaru N, Seto A, Yamada H, Aki T, Shigeta S, Wada T, Jyo T, Murooka Y, Oka S, Ono K. Cloning and characterization of a new allergen, Mag 3, from the house dust mite, Dermatophagoides farinae: cross-reactivity with high-molecular-weight allergen. Mol Immunol. 1996;33:311–319. doi: 10.1016/0161-5890(95)00127-1. [DOI] [PubMed] [Google Scholar]
- 96.Fujikawa A, Uchida K, Yanagidani A, Kawamoto S, Aki T, Shigeta S, Wada T, Suzuki O, Jyo T, Ono K. Altered antigenicity of M-177, a 177-kDa allergen from the house dust mite Dermatophagoides farinae, in stored extract. Clin Exp Allergy. 1998;28:1549–1558. doi: 10.1046/j.1365-2222.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 97.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol. 2011;187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 99.Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149:369–377. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 100.McCall C, Hunter S, Stedman K, Weber E, Hillier A, Bozic C, Rivoire B, Olivry T. Characterization and cloning of a major high molecular weight house dust mite allergen (Der f 15) for dogs. Vet Immunol Immunopathol. 2001;78:231–247. doi: 10.1016/s0165-2427(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 101.Weber E, Hunter S, Stedman K, Dreitz S, Olivry T, Hillier A, McCall C. Identification, characterization, and cloning of a complementary DNA encoding a 60-kd house dust mite allergen (Der f 18) for human beings and dogs. J Allergy Clin Immunol. 2003;112:79–86. doi: 10.1067/mai.2003.1602. [DOI] [PubMed] [Google Scholar]
- 102.O'Neil SE, Heinrich TK, Hales BJ, Hazell LA, Holt DC, Fischer K, Thomas WR. The chitinase allergens Der p 15 and Der p 18 from Dermatophagoides pteronyssinus. Clin Exp Allergy. 2006;36:831–839. doi: 10.1111/j.1365-2222.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 103.Liaw SH, Chen HZ, Liu GG, Chua KY. Acid-induced polymerization of the group 5 mite allergen from Dermatophagoides pteronyssinus. Biochem Biophys Res Commun. 2001;285:308–312. doi: 10.1006/bbrc.2001.5184. [DOI] [PubMed] [Google Scholar]
- 104.Naik MT, Chang CF, Kuo IC, Kung CC, Yi FC, Chua KY, Huang TH. Roles of structure and structural dynamics in the antibody recognition of the allergen proteins: an NMR study on Blomia tropicalis major allergen. Structure. 2008;16:125–136. doi: 10.1016/j.str.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 105.Gao YF, Wang de Y, Ong TC, Tay SL, Yap KH, Chew FT. Identification and characterization of a novel allergen from Blomia tropicalis: Blo t 21. J Allergy Clin Immunol. 2007;120:105–112. doi: 10.1016/j.jaci.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 106.Khemili S, Kwasigroch JM, Hamadouche T, Gilis D. Modelling and bioinformatics analysis of the dimeric structure of house dust mite allergens from families 5 and 21: Der f 5 could dimerize as Der p 5. J Biomol Struct Dyn. 2012;29:663–675. doi: 10.1080/073911012010525018. [DOI] [PubMed] [Google Scholar]
- 107.Puerta L, Caraballo L, Fernández-Caldas E, Avjioglu A, Marsh DG, Lockey RF, Dao ML. Nucleotide sequence analysis of a complementary DNA coding for a Blomia tropicalis allergen. J Allergy Clin Immunol. 1996;98:932–937. doi: 10.1016/s0091-6749(96)80009-1. [DOI] [PubMed] [Google Scholar]
- 108.Yi FC, Chen JY, Chee KK, Chua KY, Lee BW. Dust mite infestation of flour samples. Allergy. 2009;64:1788–1789. doi: 10.1111/j.1398-9995.2009.02116.x. [DOI] [PubMed] [Google Scholar]
- 109.Jeong KY, Hong CS, Yong TS. Allergenic tropomyosins and their cross-reactivities. Protein Pept Lett. 2006;13:835–845. doi: 10.2174/092986606777841244. [DOI] [PubMed] [Google Scholar]
- 110.Jeong KY, Lee H, Lee JS, Lee J, Lee IY, Ree HI, Hong CS, Yong TS. Molecular cloning and the allergenic characterization of tropomyosin from Tyrophagus putrescentiae. Protein Pept Lett. 2007;14:431–436. doi: 10.2174/092986607780782777. [DOI] [PubMed] [Google Scholar]
- 111.Rossi RE, Monasterolo G, Incorvaia C, Moingeon P, Frati F, Passalacqua G, Rossi L, Canonica GW. Lack of neo-sensitization to Pen a 1 in patients treated with mite sublingual immunotherapy. Clin Mol Allergy. 2010;8:4. doi: 10.1186/1476-7961-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai LC, Chao PL, Shen HD, Tang RB, Chang TC, Chang ZN, Hung MW, Lee BL, Chua KY. Isolation and characterization of a novel 98-kd Dermatophagoides farinae mite allergen. J Allergy Clin Immunol. 1998;102:295–303. doi: 10.1016/s0091-6749(98)70099-5. [DOI] [PubMed] [Google Scholar]
- 113.Tsai LC, Peng HJ, Lee CS, Chao PL, Tang RB, Tsai JJ, Shen HD, Hung MW, Han SH. Molecular cloning and characterization of full-length cDNAs encoding a novel high-molecular-weight Dermatophagoides pteronyssinus mite allergen, Der p 11. Allergy. 2005;60:927–937. doi: 10.1111/j.1398-9995.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 114.Ramos JD, Cheong N, Lee BW, Chua KY. cDNA cloning and expression of Blo t 11, the Blomia tropicalis allergen homologous to paramyosin. Int Arch Allergy Immunol. 2001;126:286–293. doi: 10.1159/000049525. [DOI] [PubMed] [Google Scholar]
- 115.Kojima S. Overview: from the horse experimentation by Prof. Akira Fujinami to paramyosin. Parasitol Int. 2004;53:151–162. doi: 10.1016/j.parint.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 116.Ramos JD, Teo AS, Ou KL, Tsai LC, Lee BW, Cheong N, Chua KY. Comparative allergenicity studies of native and recombinant Blomia tropicalis Paramyosin (Blo t 11) Allergy. 2003;58:412–419. doi: 10.1034/j.1398-9995.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 117.Jeong KY, Kim CR, Un S, Yi MH, Lee IY, Park JW, Hong CS, Yong TS. Allergenicity of recombinant troponin C from Tyrophagus putrescentiae. Int Arch Allergy Immunol. 2010;151:207–213. doi: 10.1159/000242358. [DOI] [PubMed] [Google Scholar]
- 118.Lake FR, Ward LD, Simpson RJ, Thompson PJ, Stewart GA. House dust mite-derived amylase: allergenicity and physicochemical characterization. J Allergy Clin Immunol. 1991;87:1035–1042. doi: 10.1016/0091-6749(91)92147-s. [DOI] [PubMed] [Google Scholar]
- 119.Mills KL, Hart BJ, Lynch NR, Thomas WR, Smith W. Molecular characterization of the group 4 house dust mite allergen from Dermatophagoides pteronyssinus and its amylase homologue from Euroglyphus maynei. Int Arch Allergy Immunol. 1999;120:100–107. doi: 10.1159/000024227. [DOI] [PubMed] [Google Scholar]
- 120.Sánchez-Monge R, García-Casado G, Barber D, Salcedo G. Interaction of allergens from house-dust mite and from cereal flours: Dermatophagoides pteronyssinus α-amylase (Der p 4) and wheat and rye α-amylase inhibitors. Allergy. 1996;51:176–180. doi: 10.1111/j.1398-9995.1996.tb04583.x. [DOI] [PubMed] [Google Scholar]
- 121.Bowman CE, Lessiter MJ. Amylase and esterase polymorphisms in economically important stored product mites (Acari: Astigmata) Comp Biochem Physiol B. 1985;81:353–360. [Google Scholar]
- 122.Batard T, Hrabina A, Bi XZ, Chabre H, Lemoine P, Couret MN, Faccenda D, Villet B, Harzic P, André F, Goh SY, André C, Chew FT, Moingeon P. Production and proteomic characterization of pharmaceutical-grade Dermatophagoides pteronyssinus and Dermatophagoides farinae extracts for allergy vaccines. Int Arch Allergy Immunol. 2006;140:295–305. doi: 10.1159/000093707. [DOI] [PubMed] [Google Scholar]
- 123.Shen HD, Lin WL, Tsai LC, Tam MF, Chua KY, Chen HL, Hsieh KH, Li CS, Thomas WR. Characterization of the allergen Der f 7 from house dust mite extracts by species-specific and crossreactive monoclonal antibodies. Clin Exp Allergy. 1997;27:824–832. [PubMed] [Google Scholar]
- 124.Shen HD, Chua KY, Lin WL, Hsieh KH, Thomas WR. Characterization of the house dust mite allergen Der p 7 by monoclonal antibodies. Clin Exp Allergy. 1995;25:416–422. doi: 10.1111/j.1365-2222.1995.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 125.Shen HD, Chua KY, Lin WL, Hsieh KH, Thomas WR. Molecular cloning and immunological characterization of the house dust mite allergen Der f 7. Clin Exp Allergy. 1995;25:1000–1006. doi: 10.1111/j.1365-2222.1995.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 126.Shen HD, Chua KY, Lin WL, Chen HL, Hsieh KH, Thomas WR. IgE and monoclonal antibody binding by the mite allergen Der p 7. Clin Exp Allergy. 1996;26:308–315. [PubMed] [Google Scholar]
- 127.Eriksson TL, Rasool O, Huecas S, Whitley P, Crameri R, Appenzeller U, Gafvelin G, van Hage-Hamsten M. Cloning of three new allergens from the dust mite Lepidoglyphus destructor using phage surface display technology. Eur J Biochem. 2001;268:287–294. doi: 10.1046/j.1432-1327.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 128.Armstrong RN. Glutathione S-transferases: structure and mechanism of an archetypical detoxication enzyme. Adv Enzymol Relat Areas Mol Biol. 1994;69:1–44. doi: 10.1002/9780470123157.ch1. [DOI] [PubMed] [Google Scholar]
- 129.Huang CH, Liew LM, Mah KW, Kuo IC, Lee BW, Chua KY. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin Exp Allergy. 2006;36:369–376. doi: 10.1111/j.1365-2222.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 130.Jeong KY, Jeong KJ, Yi MH, Lee H, Hong CS, Yong TS. Allergenicity of sigma and delta class glutathione S-transferases from the German cockroach. Int Arch Allergy Immunol. 2009;148:59–64. doi: 10.1159/000151506. [DOI] [PubMed] [Google Scholar]
- 131.Kawamoto S, Aki T, Yamashita M, Tategaki A, Fujimura T, Tsuboi S, Katsutani T, Suzuki O, Shigeta S, Murooka Y, Ono K. Toward elucidating the full spectrum of mite allergens--state of the art. J Biosci Bioeng. 2002;94:285–298. doi: 10.1263/jbb.94.285. [DOI] [PubMed] [Google Scholar]
- 132.Hales BJ, Martin AC, Pearce LJ, Laing IA, Hayden CM, Goldblatt J, Le Souëf PN, Thomas WR. IgE and IgG anti-house dust mite specificities in allergic disease. J Allergy Clin Immunol. 2006;118:361–367. doi: 10.1016/j.jaci.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170:445–453. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 134.Noss I, Wouters IM, Bezemer G, Metwali N, Sander I, Raulf-Heimsoth M, Heederik DJ, Thorne PS, Doekes G. β-(1,3)-Glucan exposure assessment by passive airborne dust sampling and new sensitive immunoassays. Appl Environ Microbiol. 2010;76:1158–1167. doi: 10.1128/AEM.01486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Douwes J, Zuidhof A, Doekes G, van der Zee SC, Wouters I, Boezen MH, Brunekreef B. (1→3)-β-D-glucan and endotoxin in house dust and peak flow variability in children. Am J Respir Crit Care Med. 2000;162:1348–1354. doi: 10.1164/ajrccm.162.4.9909118. [DOI] [PubMed] [Google Scholar]
- 136.Rylander R. Organic dust induced pulmonary disease - the role of mould derived β-glucan. Ann Agric Environ Med. 2010;17:9–13. [PubMed] [Google Scholar]
- 137.Mathaba LT, Pope CH, Lenzo J, Hartofillis M, Peake H, Moritz RL, Simpson RJ, Bubert A, Thompson PJ, Stewart GA. Isolation and characterisation of a 13.8-kDa bacteriolytic enzyme from house dust mite extracts: homology with prokaryotic proteins suggests that the enzyme could be bacterially derived. FEMS Immunol Med Microbiol. 2002;33:77–88. doi: 10.1111/j.1574-695X.2002.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 138.Aki T, Fujikawa A, Wada T, Jyo T, Shigeta S, Murooka Y, Oka S, Ono K. Cloning and expression of cDNA coding for a new allergen from the house dust mite, Dermatophagoides farinae: homology with human heat shock cognate proteins in the heat shock protein 70 family. J Biochem. 1994;115:435–440. doi: 10.1093/oxfordjournals.jbchem.a124356. [DOI] [PubMed] [Google Scholar]
- 139.Saarne T, Kaiser L, Rasool O, Huecas S, van Hage-Hamsten M, Gafvelin G. Cloning and characterisation of two IgE-binding proteins, homologous to tropomyosin and α-tubulin, from the mite Lepidoglyphus destructor. Int Arch Allergy Immunol. 2003;130:258–265. doi: 10.1159/000070212. [DOI] [PubMed] [Google Scholar]
- 140.Jeong KY, Lee H, Lee JS, Lee J, Lee IY, Ree HI, Hong CS, Park JW, Yong TS. Immunoglobulin E binding reactivity of a recombinant allergen homologous to α-tubulin from Tyrophagus putrescentiae. Clin Diagn Lab Immunol. 2005;12:1451–1454. doi: 10.1128/CDLI.12.12.1451-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aki T, Ono K, Paik SY, Wada T, Jyo T, Shigeta S, Murooka Y, Oka S. Cloning and characterization of cDNA coding for a new allergen from the house dust mite, Dermatophagoides farinae. Int Arch Allergy Immunol. 1994;103:349–356. doi: 10.1159/000236653. [DOI] [PubMed] [Google Scholar]
- 142.Ansotegui IJ, Härfast B, Jeddi-Tehrani M, Johansson E, Johansson SG, van Hage-Hamsten M, Wigzell H. Identification of a new major allergen of 39 kilodaltons of the storage mite Lepidoglyphus destructor. Immunol Lett. 1991;27:127–130. doi: 10.1016/0165-2478(91)90139-2. [DOI] [PubMed] [Google Scholar]
- 143.Stewart GA, Turner KJ. Physicochemical and immunochemical characterization of the high molecular weight allergens from Dermatophagoides pteronysisinus with particular reference to the tridacnin, Con-A and S107 reactive components. Aust J Exp Biol Med Sci. 1980;58:275–288. doi: 10.1038/icb.1980.27. [DOI] [PubMed] [Google Scholar]
- 144.Olsson S, Harfast B, Johansson SG, van Hage-Hamsten M. Detection of at least one high-molecular-mass, IgE-binding component of the dust mite Lepidoglyphus destructor. Allergy. 1994;49:620–625. doi: 10.1111/j.1398-9995.1994.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 145.Park JW, Hong CS, Ko SH, Kim CW. IL-5 and IL-10 production of peripheral blood mononuclear cells by stimulation of D. farinae antigen in atopic asthmatics. J Asthma Allergy Clin Immunol. 1999;19:557–565. [Google Scholar]
- 146.Lee JS, Kim IS, Ryu JS, Yun CY. House dust mite, Dermatophagoides pteronyssinus increases expression of MCP-1, IL-6, and IL-8 in human monocytic THP-1 cells. Cytokine. 2008;42:365–371. doi: 10.1016/j.cyto.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 147.Park JW, Park SW, Koe SW, Kim CW, Ree HI, Oh SH, Hong CS. Allergic immune responses to the fractionated antigen of Dermatophagoides pteronyssinus. Korean J Allergy. 1997;17:151–164. [Google Scholar]
- 148.Kwon SS, Kim N, Yoo TJ. The effect of vaccination with DNA encoding murine T-cell epitopes on the Der p 1 and 2 induced immunoglobulin E synthesis. Allergy. 2001;56:741–748. doi: 10.1034/j.1398-9995.2001.056008741.x. [DOI] [PubMed] [Google Scholar]
- 149.Kim N, Kwon SS, Lee J, Kim S, Yoo TJ. Protective effect of the DNA vaccine encoding the major house dust mite allergens on allergic inflammation in the murine model of house dust mite allergy. Clin Mol Allergy. 2006;4:4. doi: 10.1186/1476-7961-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, Horak F, Grönlund H, van Hage M, Valenta R, Vrtala S. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;38:959–965. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 151.Treudler R. Update on in vitro allergy diagnostics. J Dtsch Dermatol Ges. 2012;10:89–99. doi: 10.1111/j.1610-0387.2011.07860.x. [DOI] [PubMed] [Google Scholar]
- 152.Jeong KY, Hong CS, Yong TS. Recombinant allergens for diagnosis and immunotherapy of allergic disorders, with emphasis on cockroach allergy. Curr Protein Pept Sci. 2006;7:57–71. doi: 10.2174/138920306775474112. [DOI] [PubMed] [Google Scholar]