Abstract
Purpose
House dust mites are the most important cause of respiratory allergy in Korea. Standardization of allergen extracts is essential for improving diagnostics and immunotherapeutics. This study was undertaken to evaluate the allergenicity of standardized house dust mite allergen extracts from Korean house dust mite isolates.
Methods
Allergen extracts were prepared from cultured Korean house dust mites (Dermatophagoides farinae and D. pteronyssinus). Allergenic activities of Korean house dust mite extracts were compared to standardized extracts from a company in the United States whose allergen concentrations were expressed as Allergy Units (AUs). Specifically, we compared group 1 and 2 major allergens using two-site enzyme-linked immunosorbent assay (ELISA) kits and an in vivo intradermal test.
Results
Major allergen concentrations were 17.0 µg/mg (5.0 µg/mg of Der f 1 and 12.0 µg/mg of Der f 2) for a D. farinae extract and 24.0 µg/mg (11.6 µg/mg of Der p 1 and 12.4 µg/mg of Der p 2) for a D. pteronyssinus extract. Using chloramphenicol (CAP) inhibition assays, AUs were 12.5 AU/µg for a D. farinae extract and 12.8 AU/µg for a D. pteronyssinus extract. Allergenic activities were 3- to 4-fold stronger when assessed by intradermal skin tests for in vivo standardization.
Conclusions
Allergen extracts were prepared from Korean house dust mites and the allergenicities of the extracts were estimated using AU measurements. House dust mite extracts prepared in this study could be utilized as a reference material, which will be useful for the development of diagnostic and immunotherapeutic reagents in Korea.
Keywords: Allergen, house dust mite, standardization
INTRODUCTION
House dust mites are an important cause of respiratory allergic disorders and atopic dermatitis.1 Standardization of house dust mite extracts is necessary for the production of diagnostic and therapeutic agents for allergic diseases. Two species of house dust mite, Dermatophagoides farinae and D. pteronyssinus, are prevalent in Korea.2 Group 1 and group 2 allergens constitute 40%-60% of the total allergenicity in house dust mites.3
Unfortunately, mite allergens from geographically different regions often show different allergenicities. For example, allergen molecules have various immunoglobulin E (IgE) reactivities in European countries.4 The differences may be due to amino acid sequence changes in the allergenic proteins. Raw material from different mite culture isolates might also have an influence on the quality of allergen extracts.5 Importantly, commercial mite extracts produced in European countries, which are generally used in Korea, have considerable variability in protein and major allergen content.6,7 Significant protein heterogeneity and a substantial variability in skin prick test reactivity have been described in the literature. In addition, manufacturers use different allergen units to label the allergenic activities of their products. This makes it difficult to compare products from different companies.8 The selection of source material for extract preparation is a critical factor affecting the quality of allergen extracts used for clinical research.
In this study, we prepared standardized house dust mite extracts from cultured mites that are indigenous to Korea. Contents of the major allergens (Der f 1, Der f 2, Der p 1, and Der p 2) were compared with standardized products from a company in the United States (US). Allergenic activities were compared with the standardized products using in vitro and in vivo methods.
MATERIALS AND METHODS
Allergen extraction
Two species of house dust mites, D. farinae and D. pteronyssinus, which were maintained by the Arthropods of Medical Importance Resource Bank, Yonsei University College of Medicine, Seoul, Korea, were used in this study. Mite bodies were collected by using saturated salt water and were then freeze dried.2 After defatting with ethyl ether, allergens were extracted with various buffers (with and without 0.2% phenol) as shown in Table 1. The extracts were dialyzed against distilled water with or without 0.2% phenol and freeze-dried. Protein concentrations were determined using Protein Assay kits (Bio-rad, Hercules, CA, USA).
Table 1.
Allergen extraction using different buffers
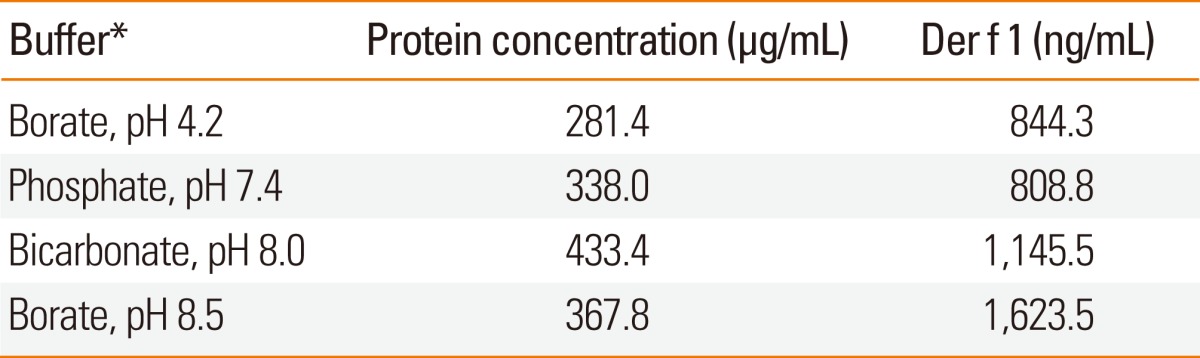
*Proteins were extracted by 1:100 (w/v) of each buffer.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses
Lyophilized allergen extracts were reconstituted in a solution containing 0.9% NaCl, 0.03% human serum albumin, and 50% glycerol. The extracts (30 µg each sample/well) were separated on a 12% SDS-PAGE gel (Tris-glycine system) under reducing conditions. Before separation, 5×SDS sample buffer was added to each extract and the mixture was heated for 5 minutes in boiling water. Gels were stained with Coomassie brilliant blue R250.
Western blot analyses were performed for the analysis of group 2 allergens with monoclonal antibody 2F38. 2F38 was raised against recombinant Der f 2 and recognizes both Der f 2 and Der p 2.9 Proteins separated by SDS-PAGE were transferred electrophoretically onto polyvinylidine difluoride (PVDF) membranes (0.45 µm, GE Water & Process Technologies, Trevose, PA, USA). PVDF membranes were reacted with hybridoma culture fluid and then incubated with 1:2,000 diluted goat anti-mouse IgG conjugated with alkaline phosphatase (Sigma-Aldrich, Sydney, Australia). Color was developed using nitro blue tetrazolium and 3-bromo-4-chloro-5-indolyl-phosphate (Promega, Madison, WI, USA).
In vitro standardization using a chloramphenicol (CAP) inhibition assay
Allergen activities in Korean extracts were compared to extracts standardized by a US company (HollisterStier Laboratories LLC, Spokane, WA, USA) using competitive inhibition CAP assays. For inhibition assays, we used pooled sera from five highly atopic patients with D. farinae-specific IgE levels higher than 100 kU/L. The serum samples (1:4 diluted) were pre-incubated with various concentrations (0.001 to 20 µg/mL) of inhibitors. Subsequently, anti-human IgE reactivity was measured by UniCAP (Phadia, Uppsala, Sweden) according to the manufacturer's instruction. The percentage of inhibition was calculated as (1-Ai/A0)×100, where Ai stands for IgE value (kU/mL) with an inhibitor, and A0 stands for IgE value without an inhibitor.
In vivo standardization using an intradermal skin test
In vivo standardization was performed using the Bioequivalent Allergy Unit (BAU) measurement.8 Briefly, allergen extracts were diluted in 0.9% NaCl, 0.4% phenol solution. Histamine hydrochloride (1 mg/mL) and physiological saline were used as positive and negative controls. A 3-fold dilution that induced a sum of erythema of 50 mm by the intradermal skin test (ED50) was calculated. A five million-fold dilution (the 14th three-fold dilution) of 100,000 BAU/mL produced a sum of erythema diameter of 50 mm by intradermal skin testing in highly reactive subjects. Allergic subjects (n=15) having D. farinae-specific IgE levels higher than 3.5 kU/L were enrolled for this in vivo study.
Measurement of major allergen content (two-site enzyme-linked immunosorbent assay [ELISA]) and endotoxin
Major allergens, Der f 1, Der p 1, Der f 2, and Der p 2, in the allergen extracts were assessed using two-site ELISA kits (Indoor Biotechnologies Inc., Charlottesville, VA, USA). Endotoxin content was estimated by the QCL-1000 (Lonza, Walkersville, MD, USA), which utilizes Limulus Amebocyte Lysate (LAL).
RESULTS
Allergen extraction and major allergen content
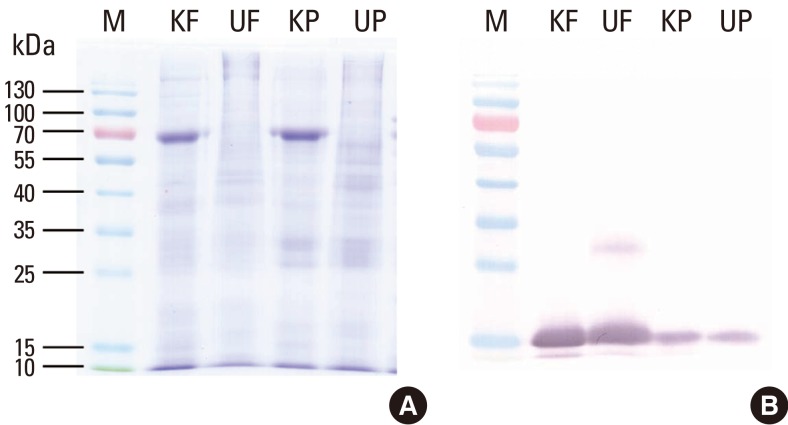
Total protein and major allergen concentrations in D. farinae extracts prepared using four different buffers (pH 4.2-8.5) were measured (Table 1). More Der f 1 was detected when buffers had a higher pH. Total protein concentration was found to be highest in bicarbonate buffer, pH 8.0. The concentration of Der f 2 was higher in Korean HDM extracts compared to the US standardized extract. In contrast, group 1 allergens (Der f 1 and Der p 1) and Der p 2 were higher in the US standardized extracts (Table 2). There was no difference in group 2 allergens based on Western blot analysis (Fig. 1B). A putative dimer band of Der f 2 was detected in D. farinae extracts from the US.
Table 2.
Major allergen contents of house dust mite extracts
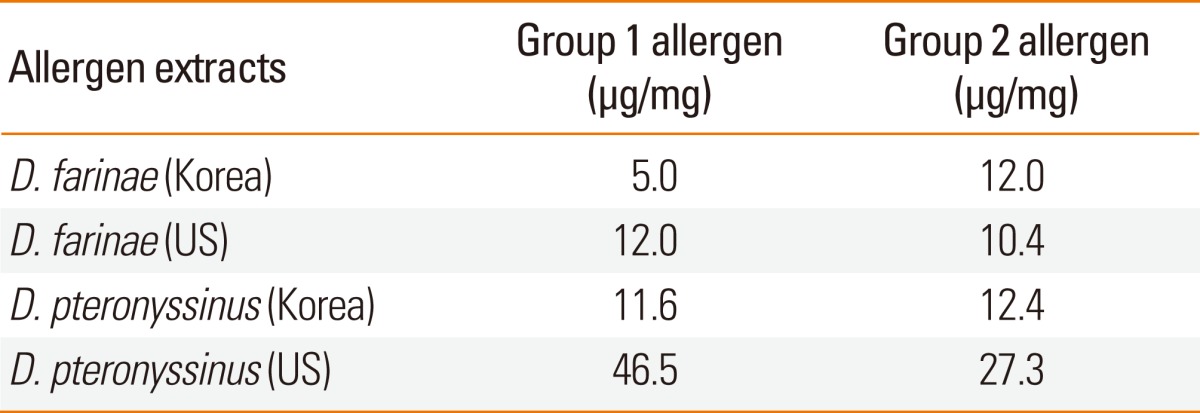
Fig. 1.
SDS-PAGE and Western blot analysis of house dust mite extracts. Allergen extracts were separated on 12% SDS-PAGE gels under reducing conditions (A) and probed with a monoclonal antibody raised against recombinant Der f 2 (B). M, molecular mass standard; KF, D. farinae extract standardized in Korea; UF, D. farinae extract standardized in USA; KP, D. pteronyssinus extract standardized in Korea; UP, D. pteronyssinus extract standardized in the US.
Allergen potency of the extracts measured by CAP inhibition
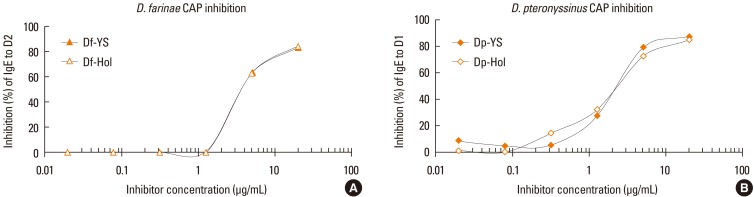
Korean and US standardized allergen extracts were analyzed by SDS-PAGE. The patterns of protein bands separated by SDS-PAGE were not identical (Fig. 1A). The thick band that resolved at 66 kDa might be human serum albumin (HSA). HSA is often included in allergen extracts in order to increase protein stabilities. Allergy Units per mL (AU/mL) were determined by in vitro comparison to the reference standard. Competitive IgE-binding CAP inhibition was performed for in vitro standardization. Korean standardized D. farinae and D. pteronyssinus extracts were able to inhibit 99.9% and 103.8% of allergenicity respectively, compared to US standardized extracts (Fig. 2). Korean standardized D. farinae and D. pteronyssinus extracts had AUs of 12.5 AU/µg and 12.8 AU/µg, respectively.
Fig. 2.
In vitro standardization of house dust mite allergen extracts by CAP inhibition. CAP inhibition of standardized Korean and US D. farinae (A) and D. pteronyssinus (B) extracts.
Allergen potency of the extracts measured by in vivo intradermal tests
Intradermal skin tests were performed for in vivo standardization. Bioequivalent Allergy Units per mL (BAU/mL) are based on the quantitative skin test. We calculated activities of 39.1 BAU/µg for D. farinae (ED50=13.99±0.75) and 41.9 BAU/µg for D. pteronyssinus (ED50=13.63±1.32). Allergenicity determined by in vivo methods exhibited 3- to 4-fold stronger activities compared to the activity measured in vitro.
Endotoxin content of house dust mite extracts
Endotoxin levels were determined to be 1,320 EU/mL and 64 EU/mL in Korean D. farinae and D. pteronyssinus extracts when phenol was added during the allergen extraction process (Table 3). Much lower levels of endotoxin (68.8% for D. farinae and 3.1% for D. pteronyssinus) were detected compared to those of HollisterStier extracts per protein concentration when 0.2% phenol was added during allergen extraction. However, lower levels of total protein were detected when phenol was added during dialysis due to protein precipitation.
Table 3.
Endotoxin levels in allergen extracts
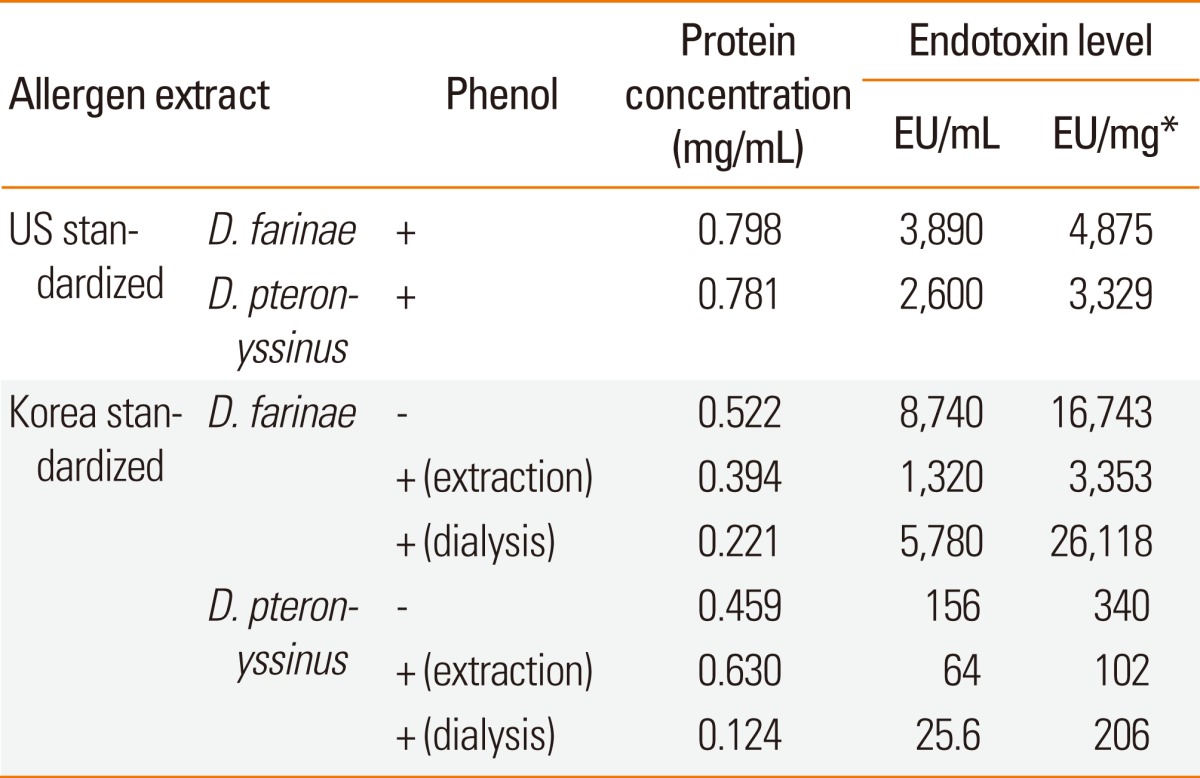
*EU/mg was calculated for endotoxin levels per protein concentration of allergen extract.
DISCUSSION
Standardization of house dust mite allergen extracts is essential for the development and improvement of diagnostic and therapeutic agents for house dust mite allergy patients. In Korea, almost all allergy diagnostics and immunotherapeutics are imported. This study may encourage researchers to initiate the manufacturing of diagnostic and therapeutic reagents in Korea.
A previous study produced house dust mite extracts using phosphate buffered saline. Its allergenic activity was evaluated using skin prick tests and intradermal skin tests.11 In this study, they achieved 81.0%-84.6% allergenic activity from Korean D. farinae and D. pteronyssinus extracts compared to commercially available skin test reagent extracts. Major allergen concentrations were measured to be 34.6 µg/mg for Der f 1, 17.0 µg/mg for Der f 2, 21.0 µg/mg for Der p 1, and 18.6 µg/mg for Der p 2.10
In the present study, bicarbonate buffer was utilized. Bicarbonate buffer led to enhanced extraction of allergenic molecules, especially Der f 1 (Table 1). Major allergen concentrations were also compared to US standardized extracts (Table 2). Although lower concentrations of major allergens were measured by two-site ELISA, the results were not found to be directly proportional to the in vitro allergenic activity assayed by CAP inhibition tests or in vivo assays. Sequence polymorphisms may partially explain the differences between the allergen content and skin test reactivities.12 It is necessary to investigate the sequence polymorphisms of major allergens. The difference in extraction procedures may also affect the concentrations of major allergens in the extracts. Notably, addition of phenol affected the protein concentration of allergen extracts (Table 3).
Recently, we studied sequence polymorphisms of Der f 1, Der p 1, Der f 2, and Der p 2, which may influence quantification, by using monoclonal antibody-based two-site ELISA kits.13 In a polymorphism study, Der p 2 variants with Asn114, which is responsible for the strong affinity to the monoclonal antibodies 1D8 (coating antibody in Indoor two-site ELISA kit) and 4G7 (detection antibody in indoor two-site ELISA kit) (currently 7A1 is being used), constitute 76.7% (46/60), and variants with Ser at position 47, which may have strong IgE affinity, accounted for 83.3% (50/60) from Korean D. pteronyssinus isolate. The higher concentration of Der f 2 may be partly explained by decreased polymorphisms in antibody binding regions. For example, no sequence variation was found at position 114 of Der f 2 from the Korean house dust mite isolate. The lower levels of detected Der p 1 may have been influenced by the amino acid substitution at positions 124 and 182. This amino acid substitution may influence antibody binding, subsequently leading to decreased detection.
The allergenic activity of Korean and US allergen extracts was determined to be similar based on in vitro standardization. This observation is interesting because we observed some differences in major allergen content as determined by a commercial two-site ELISA kit (Table 2). These results imply that the actual concentration of group 1 and 2 allergens may be similar in these extracts. Western blot analysis of group 2 allergen using the monoclonal antibody 2F38 showed that there are similar concentrations of Der f 2 and Der p 2 in each mite extract, although there was stronger reactivity to Der f 2 than Der p 2 (Fig. 1B). It is also possible that the allergenic components that do not belong to group 1 or 2 allergens could contribute to the overall allergenicity of the extracts.
Some components of the allergen extracts are known to influence immunologic and/or adjuvant activity.14 Chitin and β-glucan in house dust extracts may aggravate house dust mite allergen-induced allergic inflammation.15 Endotoxin is one of the most well characterized immunomodulatory substances contained in mite extracts. Due to the microflora residents in the mite gut, endotoxin is present in house dust mite extracts. Experimental studies have shown that exposure to low levels of endotoxin may exacerbate an allergic response; however, exposure to high levels might be protective.16-19 Epidemiologic studies have also shown that high endotoxin levels prevented the development of allergic disease in rural areas populated with stable animals. We compared endotoxin levels in Korean and US extracts (Table 3). Endotoxin contents measured in the extracts are within the range of those found in various US standardized dust mite extracts.20 D. farinae extracts showed relatively high endotoxin concentrations, whereas D. pteronyssinus extracts contained little endotoxin. This observation is in line with a previous report by Trivedi et al., in 2003.20 The difference in endotoxin concentration may reflect the different microflora in the mite gut. 16S rDNA fragments from multiple bacterial species were detected from house dust mite cultures.21 Bartonella species are thought to be the main source of endotoxins in house dust mite extracts.
These results suggest that researchers should choose the appropriate strain of house dust mite for their research aim. Further, removal of endotoxin from extracts may be necessary to accurately interpret results. Standardized allergen extracts produced in this study will be useful for the development of allergy diagnostics and immunotherapeutics in Korea.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092076).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Jeong KY, Hong CS, Yong TS. Domestic arthropods and their allergens. Protein Pept Lett. 2007;14:934–942. doi: 10.2174/092986607782541114. [DOI] [PubMed] [Google Scholar]
- 2.Ree HI, Jeon SH, Lee IY, Hong CS, Lee DK. Fauna and geographical distribution of house dust mites in Korea. Korean J Parasitol. 1997;35:9–17. doi: 10.3347/kjp.1997.35.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Thomas WR, Heinrich TK, Smith WA, Hales BJ. Pyroglyphid house dust mite allergens. Protein Pept Lett. 2007;14:943–953. doi: 10.2174/092986607782541169. [DOI] [PubMed] [Google Scholar]
- 4.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, Horak F, Grönlund H, van Hage M, Valenta R, Vrtala S. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;38:959–965. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 5.Burazer L, Milovanovic K, Milovanovic M, Vuckovic O, Velickovic TC, Gavrovic-Jankulovic M. Impact of Dermatophagoides pteronyssinus mite body raw material on house dust mite allergy diagnosis in a Serbian population. Med Vet Entomol. 2011;25:77–83. doi: 10.1111/j.1365-2915.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 6.Park HS, Nahm DH, Chae BW. Allergen standardization of Dermatophagoides farinae (D. farinae) extracts. Korean J Allergy. 1996;16:19–25. [Google Scholar]
- 7.Brunetto B, Tinghino R, Braschi MC, Antonicelli L, Pini C, Iacovacci P. Characterization and comparison of commercially available mite extracts for in vivo diagnosis. Allergy. 2010;65:184–190. doi: 10.1111/j.1398-9995.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 8.Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011;52:393–400. doi: 10.3349/ymj.2011.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong KY, Jin HS, Oh SH, Hong CS, Lee IY, Ree HI, Yong TS. Monoclonal antibodies to recombinant Der f 2 and development of a two-site ELISA sensitive to major Der f 2 isoallergen in Korea. Allergy. 2002;57:29–34. [PubMed] [Google Scholar]
- 10.Kim CW, Park JW, Hong CS. Allergen standardization of the whole body extracts of the Korean house dust mites by in vitro method. J Asthma Allergy Clin Immunol. 1998;18:28–39. [Google Scholar]
- 11.Kim CW, Park JW, Hong CS. Allergen standardization of whole body extract of Korean house dust mite by in vivo method. J Asthma Allergy Clin Immunol. 1998;18:232–242. [Google Scholar]
- 12.Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, Yong TS, Oh SH, Hong CS. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002;32:1042–1047. doi: 10.1046/j.1365-2222.2002.01421.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, Hong CS, Park JW. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. doi: 10.1007/s10493-012-9553-x. Forthcoming 2012. [DOI] [PubMed] [Google Scholar]
- 14.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011;17:604–611. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000;30:426–432. doi: 10.1046/j.1365-2222.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- 17.Tulic MK, Holt PG, Sly PD. Modification of acute and late-phase allergic responses to ovalbumin with lipopolysaccharide. Int Arch Allergy Immunol. 2002;129:119–128. doi: 10.1159/000065881. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E Allergy and Endotoxin Study Team. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–783. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- 21.Valerio CR, Murray P, Arlian LG, Slater JE. Bacterial 16S ribosomal DNA in house dust mite cultures. J Allergy Clin Immunol. 2005;116:1296–1300. doi: 10.1016/j.jaci.2005.09.046. [DOI] [PubMed] [Google Scholar]