Abstract
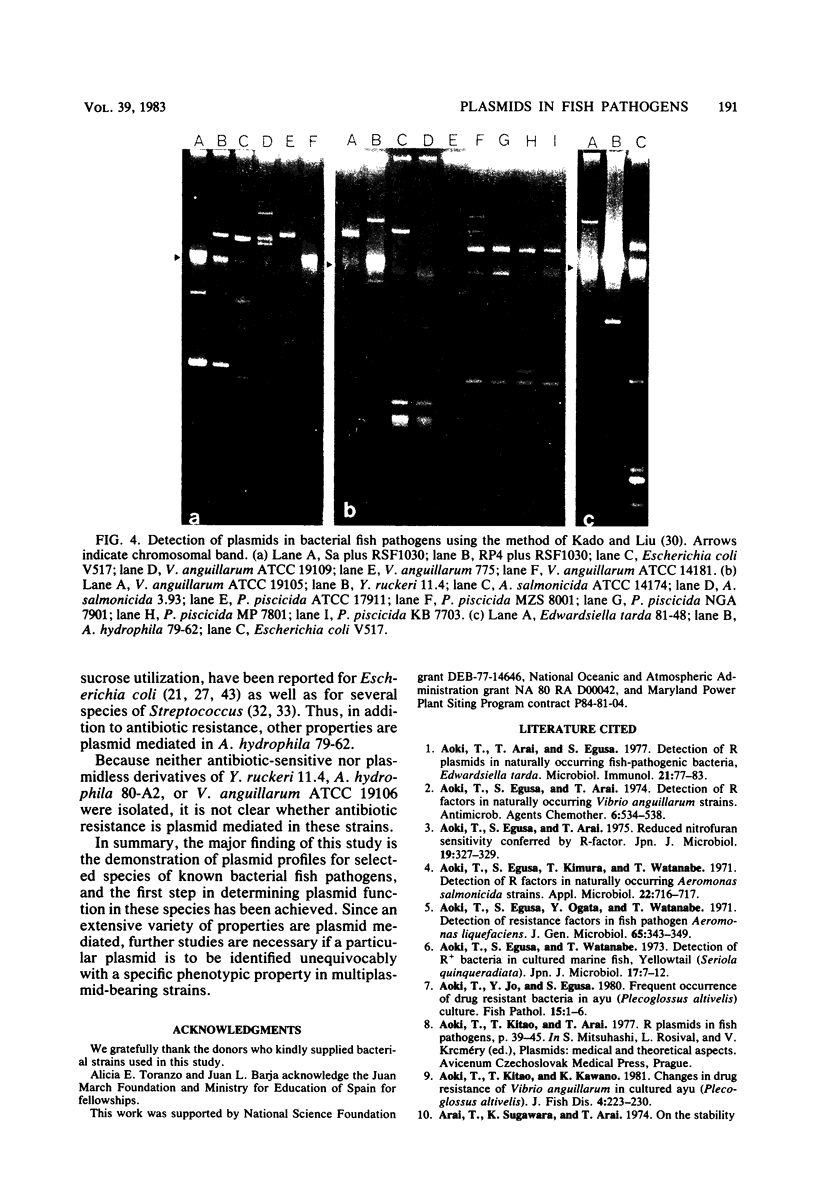
Plasmid profiles of representative fish pathogens, Aeromonas salmonicida, Aeromonas hydrophila, Vibrio anguillarum, Pasteurella piscicida, Yersinia ruckeri, Edwardsiella tarda, and Renibacterium salmoninarum, were determined by agarose gel electrophoresis with four different plasmid detection methods. A combination of two methods was required to detect the plasmids present in these strains and to calculate precisely the molecular weights of the plasmids. Of 38 strains, 28 harbored one or more plasmids, with the majority of strains demonstrating multiplasmid banding. Similarity in plasmid banding between strains was noted and related to geographic source. Five strains of A. salmonicida possessed six plasmid bands having molecular weights of 8.6 X 10(6), 8.4 X 10(6), 8.1 X 10(6), 3.6 X 10(6), 3.5 X 10(6), and 3.4 X 10(6). Four P. piscicida isolates shared three plasmid bands having molecular weights of 37 X 10(6), 15 X 10(6), and 5 X 10(6), and five A. hydrophila strains harbored a common plasmid having a molecular weight of ca. 20 X 10(6) to 30 X 10(6). The highest-molecular-weight plasmids (145 X 10(6) and 130 X 10(6) were detected in V. anguillarum. From curing experiments, it was found that in A. hydrophila strain 79-62, a loss of resistance to tetracycline was associated with loss of plasmid content in all susceptible derivatives, suggesting plasmid-mediated tetracycline resistance. Cell surface characteristics and metabolic properties were also modified in cured derivatives of A. hydrophila strain 79-62.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Arai T., Egusa S. Detection of R plasmids in naturally occurring fish-pathogenic bacteria, Edwardsiella tarda. Microbiol Immunol. 1977;21(2):77–83. doi: 10.1111/j.1348-0421.1977.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Arai T. Detection of R factors in naturally occurring Vibrio anguillarum strains. Antimicrob Agents Chemother. 1974 Nov;6(5):534–538. doi: 10.1128/aac.6.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Arai T. Reduced nitrofuran sensitivity conferred by R factors. Jpn J Microbiol. 1975 Aug;19(4):327–329. doi: 10.1111/j.1348-0421.1975.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Kimura T., Watanabe T. Detection of R factors in naturally occurring Aeromonas salmonicida strains. Appl Microbiol. 1971 Oct;22(4):716–717. doi: 10.1128/am.22.4.716-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Ogata Y., Watanabe T. Detection of resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol. 1971 Mar;65(3):343–349. doi: 10.1099/00221287-65-3-343. [DOI] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Watanabe T. Detection of R + bacteria in cultured marine fish, yellowtails (Seriola quinqueradiata). Jpn J Microbiol. 1973 Jan;17(1):7–12. doi: 10.1111/j.1348-0421.1973.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Boulanger Y., Lallier R., Cousineau G. Isolation of enterotoxigenic Aeromonas from fish. Can J Microbiol. 1977 Sep;23(9):1161–1164. doi: 10.1139/m77-174. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L. Outer membrane proteins induced under conditions of iron limitation in the marine fish pathogen Vibrio anguillarum 775. Infect Immun. 1981 Jan;31(1):223–227. doi: 10.1128/iai.31.1.223-227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer J. L., Sanders J. E. Bacterial kidney disease of salmonid fish. Annu Rev Microbiol. 1981;35:273–298. doi: 10.1146/annurev.mi.35.100181.001421. [DOI] [PubMed] [Google Scholar]
- Gavini F., Izard D., Trinel P. A., Lefebvre B., Leclerc H. Etude taxonomique d'entérobactéroes a]]artemamt pi a]]aremtées à l'espèce Escherichia coli. Can J Microbiol. 1981 Jan;27(1):98–106. [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- Hazen T. C., Fliermans C. B., Hirsch R. P., Esch G. W. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl Environ Microbiol. 1978 Nov;36(5):731–738. doi: 10.1128/aem.36.5.731-738.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ishiguro N., Sato G. Properties of a transmissible plasmid conferring citrate-utilizing ability in Escherichia coli of human origin. J Gen Microbiol. 1980 Feb;116(2):553–556. doi: 10.1099/00221287-116-2-553. [DOI] [PubMed] [Google Scholar]
- Jordan G. W., Hadley W. K. Human infection with Edwardsiella tarda. Ann Intern Med. 1969 Feb;70(2):283–288. doi: 10.7326/0003-4819-70-2-283. [DOI] [PubMed] [Google Scholar]
- Joseph S. W., Daily O. P., Hunt W. S., Seidler R. J., Allen D. A., Colwell R. R. Aeromonas primary wound infection of a diver in polluted waters. J Clin Microbiol. 1979 Jul;10(1):46–49. doi: 10.1128/jcm.10.1.46-49.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Colwell R. R., Joseph S. W. Aeromonas hydrophila: ecology and toxigenicity of isolates from an estuary. J Appl Bacteriol. 1981 Apr;50(2):359–377. doi: 10.1111/j.1365-2672.1981.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaitis A. J., Maas R., Maas W. K. Structure of a naturally occurring plasmid with genes for enterotoxin production and drug resistance. J Bacteriol. 1981 Jan;145(1):97–105. doi: 10.1128/jb.145.1.97-105.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F. P., Bullock G. L. Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl Microbiol. 1973 Jan;25(1):155–156. doi: 10.1128/am.25.1.155-156.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Lalonde G., Leblanc D., Olivier G., Lallier R. Aeromonas hydrophila in rainbow trout: relation between virulence and surface characteristics. Can J Microbiol. 1980 Dec;26(12):1501–1503. doi: 10.1139/m80-248. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotts E. B., Jr, Gaines J. L., Jr, Martin L., Prestwood A. K. Aeromonas-induced deaths among fish and reptiles in an eutrophic inland lake. J Am Vet Med Assoc. 1972 Sep 15;161(6):603–607. [PubMed] [Google Scholar]
- Trust T. J., Sparrow R. A. The bacterial flora in the alimentary tract of freshwater salmonid fishes. Can J Microbiol. 1974 Sep;20(9):1219–1228. doi: 10.1139/m74-188. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Davis B. R., Allen S. D. Ureolytic Escherichia coli of human origin: serological, epidemiological, and genetic analysis. J Clin Microbiol. 1979 Dec;10(6):897–902. doi: 10.1128/jcm.10.6.897-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantanabe T., Aoki T., Ogata Y., Egusa S. Anbtibiotics and drug resistance in animals. R factors related to fish culturing. Ann N Y Acad Sci. 1971 Jun 11;182:383–410. doi: 10.1111/j.1749-6632.1971.tb30674.x. [DOI] [PubMed] [Google Scholar]
- White F. H., Simpson C. F., Williams L. E., Jr Isolation of Edwardsiella tarda from aquatic animal species and surface waters in Florida. J Wildl Dis. 1973 Jul;9(3):204–208. doi: 10.7589/0090-3558-9.3.204. [DOI] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]







