Abstract
Objective
To determine whether animal age impacts in vitro preantral follicle growth. Effects of hCG, stem cell factor (SCF), and/or insulin-like growth factor (IGF) supplementation in growth medium were also investigated.
Methods
Intact preantral follicles were mechanically isolated from fresh ovaries of BDF1 mice and cultured in growth medium for 9 to 11 days. Surviving follicles with antrum formation were transferred to maturation medium for 14 to 18 hours. Follicle survival, antrum formation, and retrieval of metaphase II (MII) oocytes were compared among three age categories (4-5, 7-8, and 10-11 week-old). By using 7- to 8-week-old mice, preantral follicles were cultured in growth medium supplemented with hCG (0, 5, or 10 mIU/mL), SCF (50 ng/mL), IGF-1 (50 ng/mL), and SCF+IGF-1.
Results
Seven- to eight-week-old mice showed a higher follicle survival and antrum formation and produced more MII oocytes compared to other groups. In the 7- to 8-week-old mice, supplementation of 5 mIU/mL hCG significantly enhanced the antrum formation but the percentage of MII oocytes was similar to that of the control. Supplementation of SCF+IGF-1 did not enhance follicle survival or antrum formation but the percentage of MII oocytes increased modestly (39.1%) than in the control (28.6%, statistically not significant).
Conclusion
Seven- to eight-week-old mice showed better outcomes in growth of preantral follicles in vitro than 4- to 5- or 10- to 11-week-old mice. Supplementation of hCG enhanced antrum formation and supplementation of SCF+IGF-1 yielded more mature oocytes; hence, these should be considered in the growth of preantral follicles in vitro.
Keywords: Ovarian follicle, Oocyte, Age groups, Stem cell factor, Insulin-like growth factor
Introduction
Ovarian cryopreservation is a valuable option for female fertility preservation in young patients submitted to aggressive chemotherapy and/or radiotherapy treatment [1-3]. Long-term survival of frozen-thawed tissue after transplantation has been a great concern. In addition, some cancers have an elevated risk of metastatic spread to the ovary (e.g., leukemia, neuroblastoma, breast cancer); thus ovarian autotransplantation may re-introduce aggressive cancer cells to the recent survivors of their disease. To minimize the risk of re-introducing cancer cells, an in vitro follicle culture method as an alternative to homologous transplantation of the stored ovarian tissue was developed [4].
Several culture systems have been developed to support preantral follicle growth in vitro to produce meiotically competent oocytes that can be fertilized and produce offspring in mice [5-7]. The production of live offspring from primordial and pre-antral follicles from cryopreserved/thawed ovarian tissue that were cultured to mature in vitro has been also reported from immature [8-10] and adult mice [11]. Wang et al. [11] demonstrated that preantral follicles isolated from fresh or vitrified-warmed adult mouse ovarian tissue survive and develop to the antral stage after 12 days of culture in vitro; follicle survival, oocyte maturation, fertilization rates, and subsequent developmental potential including live births were similar from fresh and vitrified-warmed ovaries.
It is unclear how patient-specific characteristics impact follicle growth and quality during follicle culture. A recent report denoted that follicles isolated from older and heavier mice contain oocytes with reduced meiotic competence and produced oocytes with greater spindle defects [12]. Here we used a mouse model to determine how animal age impacts in vitro follicle growth. In addition, impacts of hCG, stem cell factor (SCF), and/or insulin-like growth factor (IGF) supplementation into growth media were also evaluated.
Methods
1. In vitro growth of preantral follicle
Female BDF1 mice (Orient Co., Seoul, Korea) were maintained under a 12 hours light:12 hours dark cycle at 23℃ and fed ad libitum. The experimental protocols and animal handling procedures were reviewed and approved by the Animal Care and Use Committee of Seoul National University Bundang Hospital. After one week of the adaptation, the mice were sacrificed by cervical dislocation and the bilateral ovaries were collected in a 1 mL L-15 medium (WelGENE, Daegu, Korea) supplemented with 0.4% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA).
Intact preantral follicles were mechanically isolated from fresh ovaries. Preantral follicle culture medium was composed of α-Minimum Essential Medium (αMEM, WelGENE), 10% fetal bovine serum (FBS, Gibco, Paisley, UK), and 10 mIU/mL recombinant FSH (rFSH, Serono, Geneva, Switzerland). Individual follicles were cultured in embryo culture dishes (GPS, Life global, Guilford, CT, USA) and maintained at 37℃ in 5% CO2 for a maximum of 11 days. Each droplet contained a single follicle with 100 µL of medium under mineral oil (Sigma). Every three days, the medium was exchanged and follicle survival and formation of the antrum were assessed using an inverted Leica DM IRB microscope with transmitted light and phase objectives (Leica, Bannockburn, IL, USA). Follicles were designated as dead if the oocyte was no longer surrounded by a granulosa cell layer or if the granulosa cells had become dark and fragmented and the follicle decreased in size. The diameter of the follicle containing the oocyte was measured in duplicate from the outer layer of the theca cells using a calibrated ocular micrometer.
2. Maturation of antral follicle and oocyte retrieval
After 9-11 days' culture, the surviving follicles with antrum formation were transferred to maturation media composed of αMEM, 20% FBS, 10 mIU/mL rFSH, 1.5 IU/mL hCG (Serono), and 10 ng/mL recombinant epidermal growth factor (rEGF, Invitrogen, Carlsbad, CA, USA) for 14 to 18 hours at 37℃ in 5% CO2. The oocytes were then released from the follicles and denuded from the surrounding cumulus cells by treatment with 0.3% hyaluronidase (Sigma) and gentle aspiration through a polished drawn glass pipette. The oocytes were considered to have reached the germinal vesicle (GV) stage if the GV was visible; if not, they were recorded as having GV breakdown (GVBD). If a polar body was present in the perivitelline space, the oocytes were classified as metaphase II (MII). Fragmented or shrunken oocytes were classified as degenerated. If GV or GVBD oocytes were obtained, they were matured in vitro by additional culture for 13 to 14 hours using IVM medium which was composed of αMEM, 20% FBS, 75 mIU/mL rFSH, 0.5 IU/mL rhCG, and 10 ng/mL rEGF.
3. Study design
First, in vitro growth outcomes of preantral follicle were compared among three age categories: 4- to 5-week-old, 7- to 8-week-old and 10- to 11-week-old. Four mice were used for each group and the number of isolated preantral follicles in each group was 294, 174, and 202, respectively. Second, preantral follicles were cultured in growth medium supplemented with 0, 5, or 10 mIU/mL hCG: 7- to 8-week-old mice were used and sixty follicles were obtained from three mice for each group. Third, preantral follicles were cultured in growth medium supplemented with 50 ng/mL SCF (Sigma), 50 ng/mL IGF-1 (Sigma), or 50 ng/mL SCF+50 ng/mL IGF-1: 7- to 8-week-old mice were used and sixty follicles were obtained from three mice for each group. In the present study, the primary outcome measures were follicle survival, antrum formation, and retrieval of MII oocytes. All variables were also presented as the rate per initiated follicle.
4. Statistical analysis
Each parameter was pooled from three or four replicates. The chi-squared test was used to compare the proportions among the groups. Continuous parameters in different groups were compared by the analysis of variance test. The statistical software package SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used and p-values less than 0.05 were always considered significant.
Results
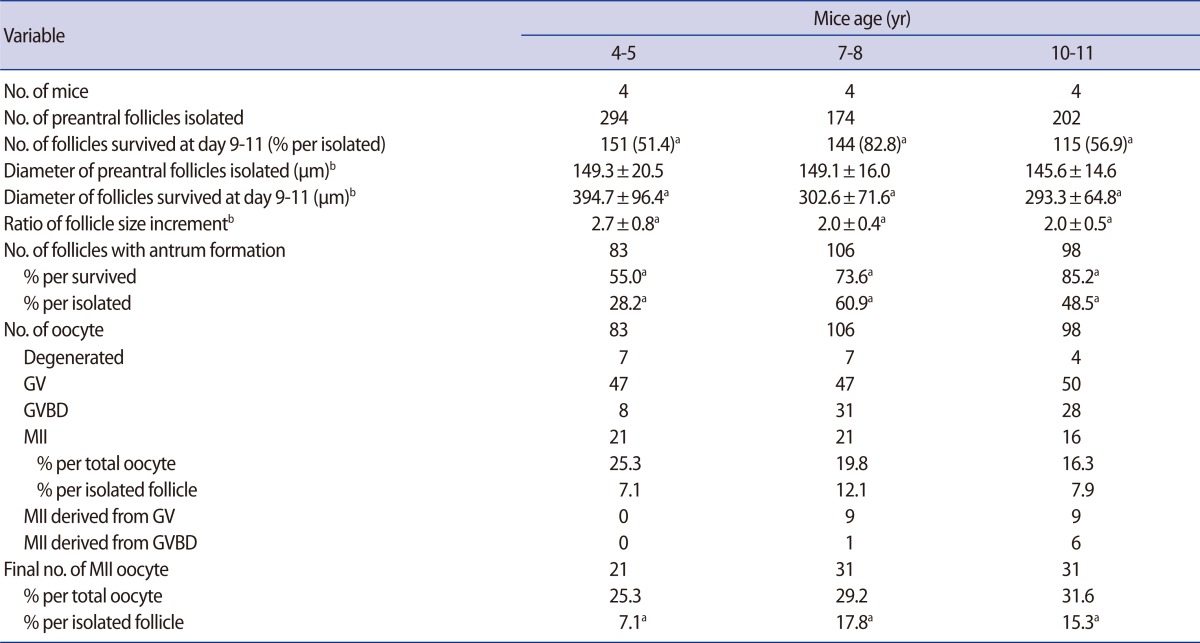
After in vitro culture of preantral follicles for 9 to 11 days, the surviving follicles showed a marked increment in their size (Figure 1). The increment of follicle size was remarkable in 4- to 5-week-old mice, and this was significantly greater than that of the 7- to 8-week or 10- to 11-week-old mice (Table 1).
Figure 1.
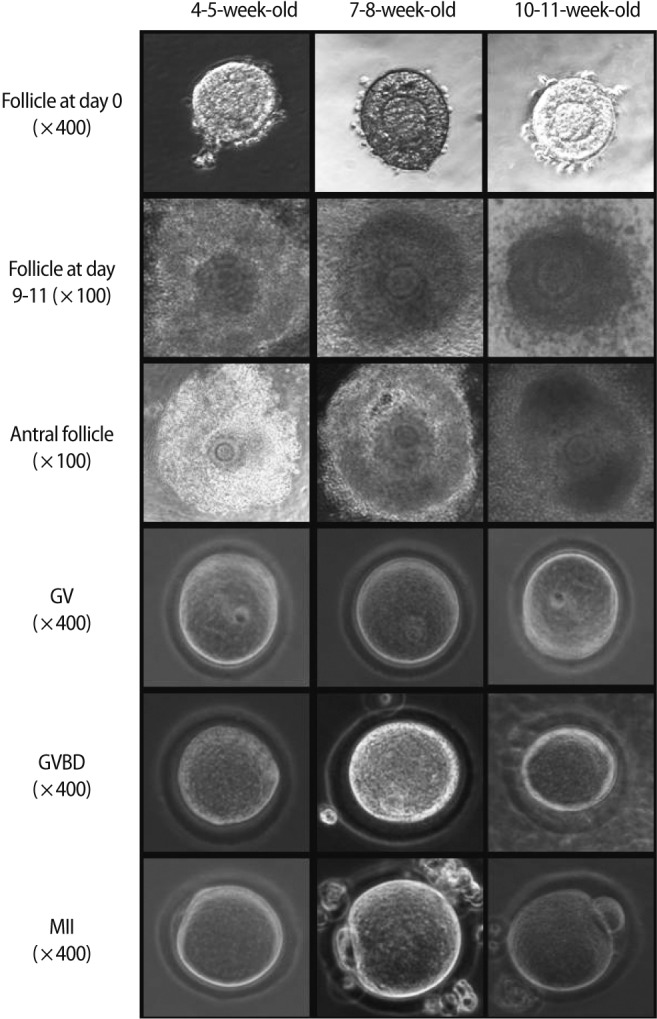
Microphotographs showing in vitro growth of mouse preantral follicles and the resultant oocytes in three age groups. GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
Table 1.
In vitro growth outcomes of preantral follicles according to different mouse age
Data are pooled from four replicates.
GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
aDifferent superscript means a statistical significance within same row (p<0.05); bPresented as mean±SD; the data were compared by analysis of variance test.
The follicle survival rate was significantly higher in the 7- to 8-week-old mice, but antrum formation per surviving follicle was significantly higher in the 10- to 11-week-old mice. However, antrum formation per initiated follicle was significantly higher in the 7- to 8-week-old mice (Table 1). All antral follicles produced oocytes, but the majority were GV stage oocytes. Some of the GV or GVBD oocytes had matured in vitro from the 7- to 8- and 10- to 11-week-old mice; thus, the final number of MII oocytes was enhanced in these groups. Although the proportion of MII oocytes was similar among the three groups, the acquisition of MII oocytes per initiated follicle was significantly higher in the 7- to 8-week- and 10- to 11-week-old groups than the 4- to 5-week-old group.
After culture in growth medium with or without hCG supplementation, follicle survival was similar (Table 2). However, the antrum formation rate per initiated follicle was significantly higher in the group supplemented with 5 mIU/mL of hCG when compared to the non-supplemented control. All of the antral follicles produced oocytes but none of the GV or GVBD oocytes matured in vitro in this experiment. Acquisition of MII oocytes per initiated follicle was higher in the two hCG-treatment groups but not significantly different than that of the control.
Table 2.
Effect of supplementation of hCG in preantral follicle culture in vitro
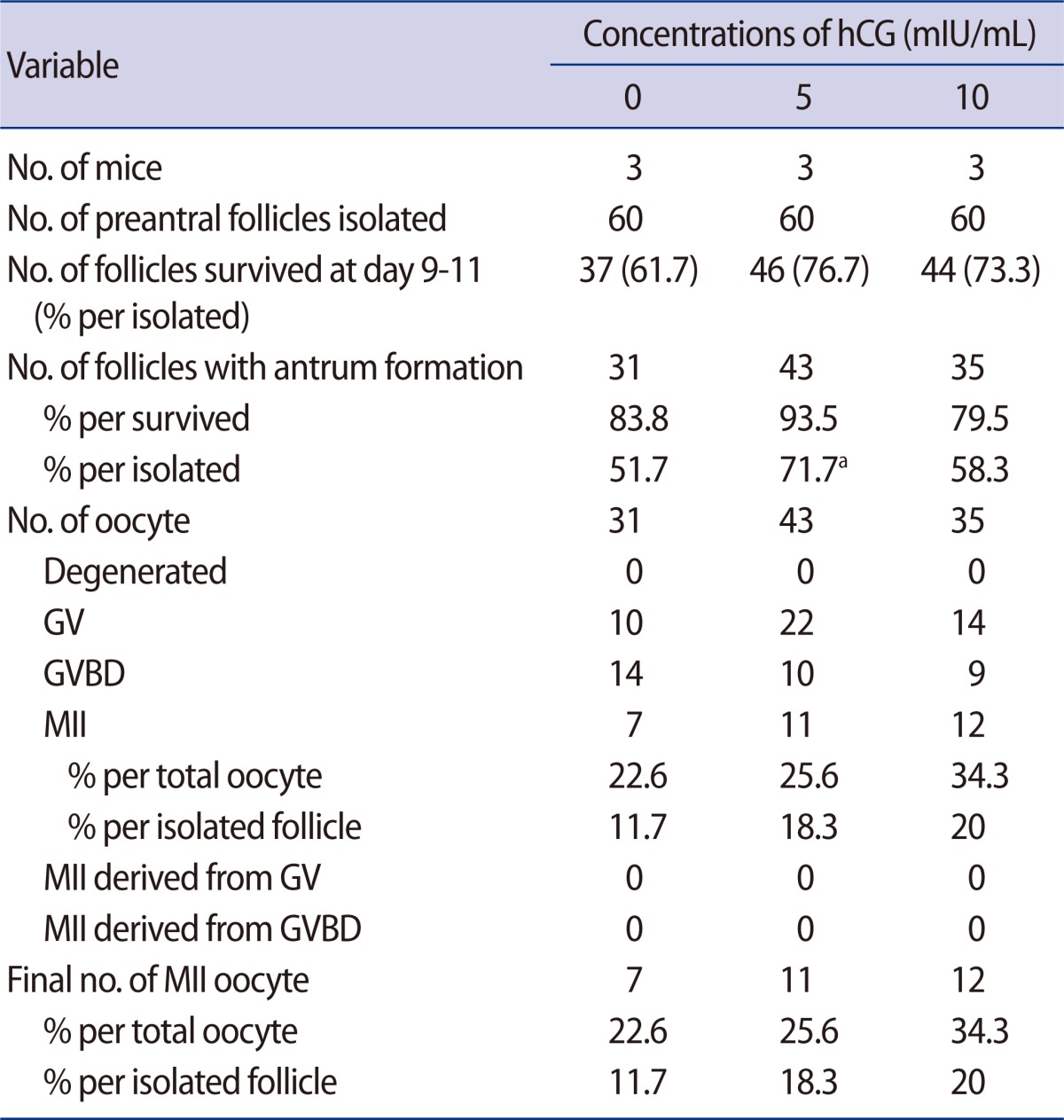
Data are pooled from three replicates.
GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
aStatistically significant (p<0.05) when compared to control (no hCG).
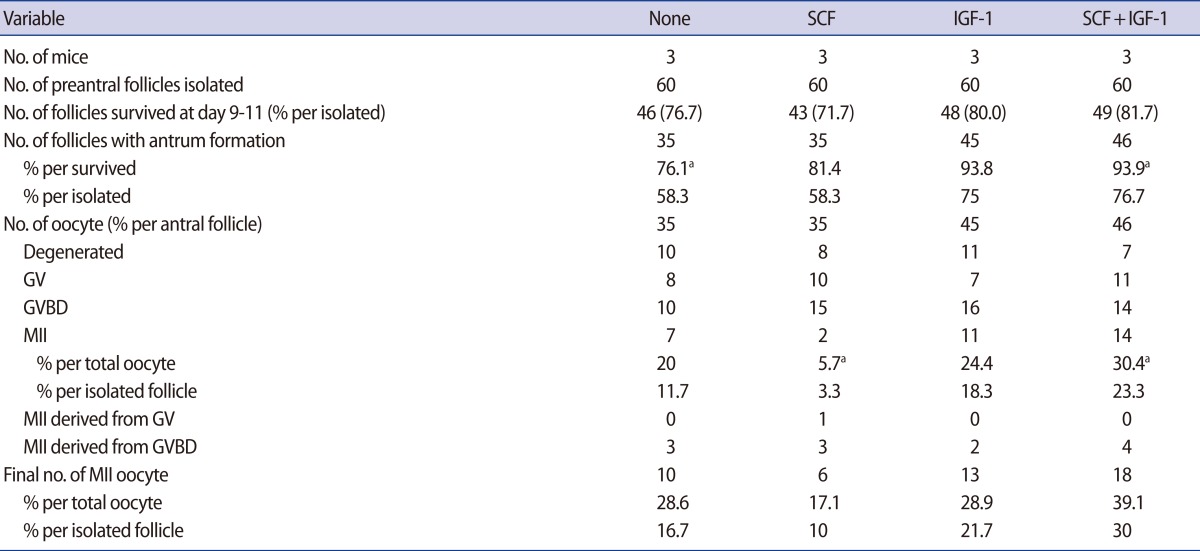
Supplementation of SCF (50 ng/mL) and/or IGF-1 (50 ng/mL) did not enhance follicle survival or antrum formation (Table 3). Acquisition of MII oocytes was highest in the group supplemented with SCF+IGF-1 but not significant compared to the non-supplemented control.
Table 3.
Effect of supplementation of SCF and/or IGF-1 in preantral follicle culture in vitro
Data are pooled from three replicates.
SCF, stem cell factor (50 ng/mL); IGF-1, insulin-like growth factor (50 ng/mL); GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
aDifferent superscript means a statistical significance within same row (p<0.05).
Discussion
In the present study, we attempted to determine the postpubertal mouse age with better efficiency for in vitro culture of preantral follicles. In our setting, 7- to 8-week-old mice showed better outcomes in terms of follicle survival, antrum formation, and acquisition of mature oocytes. Contrary to our expectation, preantral follicles isolated from very young mice (i.e., 4- to 5-week-old mice) did not retain the highest developmental competence.
Prepubertal mice (i.e., 8 to 14 days old) were used in the majority of studies with regard preantral follicle culture experiments [7,9,13-19]. However, for the translation into humans, experiments with regard to fertility preservation should be focused on postpubertal mice with full reproductive potential. In general, 8- to 11-week-old mice are considered to be adults with active sexual activity. In the present study, as representatives of mice with full reproductive potential, we choose 4- to 5-week-, 7- to 8-week-, and 10- to 11-week-old mice, respectively. As a result, acquisition of mature oocytes per initiated preantral follicle was similar in 7- to 8-week- and 10- to 11-week-old mice, and both groups showed better production of mature oocytes than did 4- to 5-week-old mice.
According to the observations of Hirshfeld-Cytron et al. [12], follicles isolated from 7- and 14-week-old mice appeared to have similar in vitro growth outcomes; however, follicles from 38-week-old mice showed a significantly lower survival rate than those from 7- or 14-week-old mice. They also observed a significantly lower percentage of oocytes that reached MII in 38-week-old mice (20% vs. 38.2%, and 39%) than in the other groups. Greater spindle defects of MII oocytes were also observed in 38-week-old mice. Interestingly, they did not observe any statistically significant differences in meiotic progression in oocytes obtained from mice at different stages of the estrous cycle.
Wang et al. [11] used 6- to 7-week-old mice , as similar in our study, and obtained a 79.4% survival rate and 80% antrum formation rate; a total of 172 oocytes were recovered and 107 (62.2%) matured to the MII stage. We obtained an 82.8% survival rate and 73.6% antrum formation rate; a total of 106 oocytes were recovered and 31 (29.2%) matured to the MII stage. Despite a similar survival and antrum formation rate, a relatively lower percentage of mature oocytes might be attributed to our culture system in which several growth-promoting additives were not included. Our experiments were undertaken without insulin, transferrin, selenium, or fetuin to explore the pure effects of hCG or SCF/IGF-1 supplementation.
A recent report indicated that mature oocytes, cleaving embryos, and blastocysts could also be obtained from cultured preantral follicles retrieved from highly aged (63- to 67-week-old) mice but general retardation of follicle development and oocyte maturation and development after IVF was observed [20].
In general, FSH supplementation is essential for in vitro growth and survival of preantral follicles [17,21,22]. The follicles cultured in fetal calf serum alone, without FSH supplementation, did not form an antral cavity [23]. Whereas FSH promotes the differentiation of granulosa cells from preantral follicles in vitro, LH can stimulate GVBD and cumulus expansion [24]. Nonetheless, LH supplementation in growth medium was reported to be helpful for in vitro growth of preantral follicles [25]. Liu et al. [19] found that an initially high ratio of LH/FSH (1:1) followed by a low ratio (1:10) could be optimal for obtaining mature oocytes [13]. A recent report also indicates that oocyte maturation was significantly higher in FSH+LH treatment (48.9%) than FSH only (30.1%) or LH only (22.2%) [15]. In the experiments with monkeys, the addition of LH to FSH was detrimental to follicle survival and growth and resulted in reduced progesterone production [26]. Therefore, it remains to be determined whether synergistic action of two gonadotropins is required for full competence of follicular maturation.
In the present study, the addition of hCG, instead of LH, in the growth medium was not helpful for follicle survival or retrieval of MII oocytes. However, antrum formation was significantly improved in the group supplemented with 5 mIU/mL of hCG when compared to the non-supplemented control. The resultant MII oocytes were higher in the two hCG-treatment groups (18.3% and 20%) but the difference was not significant when compared to the control (11.7%).
IGF-1 is involved in the regulation of ovarian folliculogenesis in mammals; it stimulates the proliferation and steroidogenesis of granulosa cells cultured in vitro [27]. When added during in vitro culture of mouse preantral follicles, IGF-1 promotes follicular growth in synergy with FSH [28]. SCF, alternately known as c-kit ligand, plays important roles in mammalian oogenesis and folliculogenesis [29-33]. In the present study, supplementation with SCF (50 ng/mL) and/or IGF-1 (50 ng/mL) did not enhance follicle survival or antrum formation; however, oocyte maturation was much improved by the addition of SCF/IGF-1. Hence, supplementation with hCG or SCF/IGF-1 appears to be helpful for in vitro growth of preantral follicles.
Here, we used the conventional, two-layered culture system for in vitro growth; however, an alginate-based three-dimensional culture system has recently been developed to support the growth and maturation of multilayered secondary follicles [34,35]. This system has been reported to produce oocytes that successfully completed meiosis, fertilization, and development to the blastocyst stage [13]. Other researchers have reported that the rate of meiotically competent oocytes produced by culture in fibrin-alginate matrices was significantly greater than in alginate alone [36]. In a subsequent study, isolated secondary follicles cultured for 12 days showed larger increases in oocyte diameter and more frequent antrum formation and theca cell differentiation in the fibrin-alginate matrices compared with those in the alginate alone [16].
Extensive efforts are still being undertaken to derive developmentally competent oocytes from cultured preantral follicles. The current challenges are to understand better the role of gonadotropins, steroids, and peptide hormones in follicle growth and the integration of hormones with the physical environment. These fundamental studies will be of great impact for the development of a robust system for human follicle development and oocyte maturation. The future optimization of these factors will be needed to fine tune application for in vitro growth of human preantral follicles.
Footnotes
This work was supported by grant no. 02-2010-019 from the Seoul National University Bundang Hospital Research Fund.
No potential conflict of interest relevant to this article was reported.
References
- 1.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Wallberg KA, Oktay K. Fertility preservation medicine: options for young adults and children with cancer. J Pediatr Hematol Oncol. 2010;32:390–396. doi: 10.1097/MPH.0b013e3181dce339. [DOI] [PubMed] [Google Scholar]
- 4.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 5.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 6.Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- 7.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–178. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa A, Hamada Y, Mehandjiev T, Koyama K. In vitro growth and maturation as well as fertilization of mouse preantral oocytes from vitrified ovaries. Fertil Steril. 2004;81(Suppl 1):824–830. doi: 10.1016/j.fertnstert.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction. 2011;141:183–191. doi: 10.1530/REP-10-0383. [DOI] [PubMed] [Google Scholar]
- 12.Hirshfeld-Cytron JE, Duncan FE, Xu M, Jozefik JK, Shea LD, Woodruff TK. Animal age, weight and estrus cycle stage impact the quality of in vitro grown follicles. Hum Reprod. 2011;26:2473–2485. doi: 10.1093/humrep/der183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi SW, Kang JS, et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod Sci. 2010;17:1081–1089. doi: 10.1177/1933719110377663. [DOI] [PubMed] [Google Scholar]
- 16.Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–2639. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–2700. doi: 10.1016/j.fertnstert.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Motohashi HH, Sankai T, Kada H. Live offspring from cryopreserved embryos following in vitro growth, maturation and fertilization of oocytes derived from preantral follicles in mice. J Reprod Dev. 2011;57:715–722. doi: 10.1262/jrd.10-152h. [DOI] [PubMed] [Google Scholar]
- 19.Liu HC, He Z, Rosenwaks Z. In vitro culture and in vitro maturation of mouse preantral follicles with recombinant gonadotropins. Fertil Steril. 2002;77:373–383. doi: 10.1016/s0015-0282(01)02977-6. [DOI] [PubMed] [Google Scholar]
- 20.Choi JK, Ahn JI, Park JH, Lim JM. Derivation of developmentally competent oocytes by in vitro culture of preantral follicles retrieved from aged mice. Fertil Steril. 2011;95:1487–1489. doi: 10.1016/j.fertnstert.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 21.Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 22.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- 23.Nottola SA, Cecconi S, Bianchi S, Motta C, Rossi G, Continenza MA, et al. Ultrastructure of isolated mouse ovarian follicles cultured in vitro. Reprod Biol Endocrinol. 2011;9:3. doi: 10.1186/1477-7827-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45:824–830. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- 25.Cortvrindt R, Hu Y, Smitz J. Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone-supplemented mouse preantral follicle culture. Hum Reprod. 1998;13:1292–1302. doi: 10.1093/humrep/13.5.1292. [DOI] [PubMed] [Google Scholar]
- 26.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthrie HD, Garrett WM, Cooper BS. Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol Reprod. 1998;58:390–396. doi: 10.1095/biolreprod58.2.390. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Andoh K, Yokota H, Kobayashi J, Abe Y, Yamada K, et al. Effects of growth hormone, activin, and follistatin on the development of preantral follicle from immature female mice. Endocrinology. 1998;139:2342–2347. doi: 10.1210/endo.139.5.5987. [DOI] [PubMed] [Google Scholar]
- 29.Klinger FG, De Felici M. In vitro development of growing oocytes from fetal mouse oocytes: stage-specific regulation by stem cell factor and granulosa cells. Dev Biol. 2002;244:85–95. doi: 10.1006/dbio.2002.0592. [DOI] [PubMed] [Google Scholar]
- 30.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 31.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–69. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- 32.Hunter MG, Paradis F. Intra-follicular regulatory mechanisms in the porcine ovary. Soc Reprod Fertil Suppl. 2009;66:149–164. [PubMed] [Google Scholar]
- 33.Celestino JJ, Bruno JB, Lima-Verde IB, Matos MH, Saraiva MV, Chaves RN, et al. Steady-state level of kit ligand mRNA in goat ovaries and the role of kit ligand in preantral follicle survival and growth in vitro. Mol Reprod Dev. 2010;77:231–240. doi: 10.1002/mrd.21138. [DOI] [PubMed] [Google Scholar]
- 34.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 35.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]