Abstract
Objectives
5-Hydroxymethylfurfural (HMF) regularly occurs in foods and in alcoholic beverages. However, the risk of HMF associated with alcohol consumption has not been systematically studied, so that this study will provide the first quantitative risk assessment of HMF for consumers of alcoholic beverages.
Methods
Human dietary intake of HMF via alcoholic beverages in the European Union was estimated based on WHO alcohol consumption data combined with our own survey data (n=944) and literature data (n=147) about the HMF contents of different beverage groups (beer, wine, spirits and unrecorded alcohol). The risk assessment was conducted using the margin of exposure (MOE) approach.
Results
For olfactory epithelium metaplasia in female mice, a benchmark dose (BMD) of 127 mg/kg bodyweight (bw)/d and a BMD lower confidence limit (BMDL) of 79 mg/kg bw/d were calculated from National Toxicology Program oral long-term animal experiments. The average human exposure to HMF from alcoholic beverages was estimated at 6.0E-3 mg/kg bw/d, which is approximately 8.5% of the total dietary exposure. In comparison of the human exposure with BMDL, the MOE was 13,167 for average alcohol consumption scenarios, which is a value that would be generally assumed as safe for threshold based compounds.
Conclusions
The results show that the risk from HMF to the alcohol-consuming population is rather low and the priority for risk management (e.g. to reduce the contamination) is also low. Further toxicological research about HMF is required to further elucidate its mechanism.
Keywords: Alcoholic beverages, Alcohol drinking, Cancer, 5-Hydroxymethylfurfural, Risk assessment
INTRODUCTION
5-Hydroxymethylfurfural (HMF, C6H6O3, CAS No 67-47-0) is a common product of the Maillard reaction and can be found in many foods and beverages [1,2]. In particular, this compound has been shown to be a good indicator for heat processing of industrial manufactured foods [3]. The toxicological relevance of HMF for humans is not yet fully elucidated. At high concentrations that are not nutritionally relevant, HMF is cytotoxic and causes irritation to the eyes, the upper respiratory tract and the skin [4]. In the only available long-term study by the National Toxicology Program (NTP) in rats and mice, HMF did not demonstrate any neoplastic effects in the intestinal tract [5]. Nevertheless, an elevated incidence of hepatocellular adenoma and carcinoma in the liver was observed in female mice [5].
Relatively high concentrations of HMF (exceeding 1 g/kg) were found in some products such as dried fruits, caramel products or instant coffee powder [1]. However, information about the human daily dietary HMF exposure is scarce and inconsistent. For example, Ulbricht et al. [4] estimated that the HMF intake from food can reach 150 mg/person (p)/d, while recent research showed a dietary intake of 27.6 mg/p/d for the Norwegian population (95th percentile) [2] or 10 mg/p/d in the Spanish diet [6]. For Germany, the total HMF intake was estimated in the 4 to 30 mg/p/d range [7]. It should be noted that HMF consumption with alcoholic beverages was not included in the German study because of a lack of monitoring data [7]. In alcoholic beverages, HMF was found, for example, in fortified wine (maximum 840 mg/L) [1], whiskey (maximum 55.9 mg/L) [8] or rum (maximum 43.5 mg/L) [9]. Thus, the formation of HMF from sugar dehydration or due to caramel color addition in alcohol products could be a potential problem.
In contrast to other constituents of alcoholic beverages (e.g. higher alcohols or acetaldehyde), for which excellent risk assessments have been published [10,11], we found a major knowledge regarding the potential lack of public health impact of HMF, resulting in an inability to satisfactorily assess the risk for the consumers of the alcoholic beverages researched. This study will therefore give an overview about the HMF content of different kinds of alcoholic beverages and, applying the harmonised approach of the European Food Safety Authority (EFSA) [12], we will provide a reliable human exposure estimate as well as a risk assessment about HMF from alcohol consumption.
MATERIALS AND METHODS
Data on HMF were obtained by a computer-assisted literature search. Searches were carried out in the following databases: PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed), Toxnet (http://www.toxnet.nlm.nih.gov/) and ChemIDplus (http://chem.sis.nlm.nih.gov/chemidplus/), Web of Science (http://wokinfo.com/products_tools/multidisciplinary/webofscience/) and International Programme on Chemical Safety/Chemical Safety Information from Intergovernmental Organizations (http://www.inchem.org/). We specifically aimed to find long-term animal studies that would be applicable for dose-response modelling as well as studies about occurrence of HMF in alcoholic beverages.
The data about HMF concentrations in alcoholic beverages were obtained from the literature as well as from our in-house analysis by means of nuclear magnetic resonance (NMR) spectroscopy [13] and visible spectroscopy using barbituric acid and para-toluidine for derivatization [14]. The visible spectroscopic assay was linear in a working concentration range of 1.7 to 60 mg/L (R>0.99). The limit of detection (LOD) and limit of quantification (LOQ) were 0.5 mg/L and 1.7 mg/L. For authentic samples, the relative standard deviations (RSD) were below 2%. Spiked recoveries were found to be 98% (at 5 mg/L) and 97% (at 20 mg/L). The NMR method was also shown to provide adequate sensitivity with LOD of 2.0 mg/L and LOQ of 6.1 mg/L. The precision expressed as RSD was around 4%, linearity was observed from 1 to 200 mg/L (r=0.99). The correlation between the results from these two analytical techniques was significant (p<0.001), so that all results could be combined to calculate the distribution for the different beverage groups.
Risk assessment analysis was conducted according to the harmonised approach of the EFSA [12] and similar to our previous acetaldehyde risk assessment [11]. This includes an approach known as the margin of exposure (MOE). The benchmark dose (BMD), derived from animal data by mathematical dose-response modelling within the observed range of experimental data, was used as a reference point. To obtain the MOE, the benchmark dose lower confidence limit (BMDL) for a 10% effect was calculated. The BMDL is an estimate of the lowest dose that is 95% certain to cause no more than a 10% effect (e.g. cancer incidence) in rodents. The BMD and BMDL values were calculated using the US Environmental Protection Agency's BMD software version 2.2 (http://www.epa.gov/ncea/bmds/index.html).
RESULTS
I. Toxicity of Orally Ingested HMF
There is no human study available, which would be adequate for a risk assessment. There are some animal experiments on HMF available in the literature. In the study of Ulbricht et al. [4], rats were given 0, 40, 80 or 160 mg/kg bodyweight (bw)/d HMF for 6 days a week over a period of 11 months. In the 160 mg/kg bw/d group, minor changes in the clinical chemical parameters (globulin levels, hepatic tributyrase) were revealed. Corpet et al. [15] examined a tumour-inducing effect of HMF after initiation with azoxymethane in CF1 mice and Fisher 344 rats which had been given heat-treated sugar. Significantly higher number of microadenomas of the intestines were observed after 100 days. The authors therefore concluded that heat-treatment induced compounds promote colon cancer. However, the actual role of HMF in this process was unclear. Another study by the same research group revealed significantly larger aberrant crypt foci (ACF, precursors of microadenomas) in the group of rats fed with 1% HMF compared to the control group [16]. In another study, where rats were given 100 to 300 mg/kg bw/d HMF dissolved in water, a dose-related increase was also observed in the number of ACF [17].
A more reliable characterization of HMF toxicity and carcinogenicity was recently conducted by NTP [5]. Male and female F344/N rats and B6C3F1 mice were administered HMF by gavage in deionised water for 3 weeks, 3 months or 2 years. In the three-week study 0 to 1,500 mg/kg bw/d doses were administered to mice and rats. Although no chemical-related lesions were observed, the final mean body weights of 1,500 mg/kg bw/day dose males of both species were significantly less than those of the vehicle control groups. In the 3-month study in rats additional effects such as influences on oestrous cycles were observed. Finally, groups of 50 male and female rats were administered 0, 188, 375, or 750 mg/kg bw/d in deionised water by gavage for 104 weeks. Survival of the dose groups was greater than or similar to the control group. Mean body weights of dosed groups of males and females were generally similar to those of controls throughout the study. In 750 mg/kg bw/d males, incidences of olfactory epithelium degeneration were significantly increased as well as in 188 mg/kg bw/d and 375 mg/kg bw/d females. Incidences of respiratory metaplasia and squamous metaplasia were significantly increased in 750 mg/kg bw/d males and females. Incidences of suppurative inflammation of the nose and chronic active inflammation of the nasolacrimal duct were increased in 750 mg/kg bw/d females. In the 104-week study with mice with the same doses, significantly less survival of 750 mg/kg bw/d males and females in comparison with the vehicle control group was observed. In the 750 mg/kg bw/d group, males and females exhibited clinical signs indicative of neurological effects of HMF administration. Importantly, the incidences of hepatocellular adenoma were significantly increased in 188 and 375 mg/kg bw/d females. The incidences of olfactory epithelium metaplasia, degeneration in the nose as well as hyperplasia, dilatation, and chronic active inflammation of the glands were increased in 375 and 750 mg/kg bw/d males and females [5].
The overall result of the NTP studies was classified as some evidence of carcinogenic activity of HMF in female B6C3F1 mice based on elevated incidences of hepatocellular adenomas (53% and 52% of total number of animals in the 188 mg/kg bw/d and 375 mg/kg bw/d groups, respectively) [5].
II. Dose-response Analysis
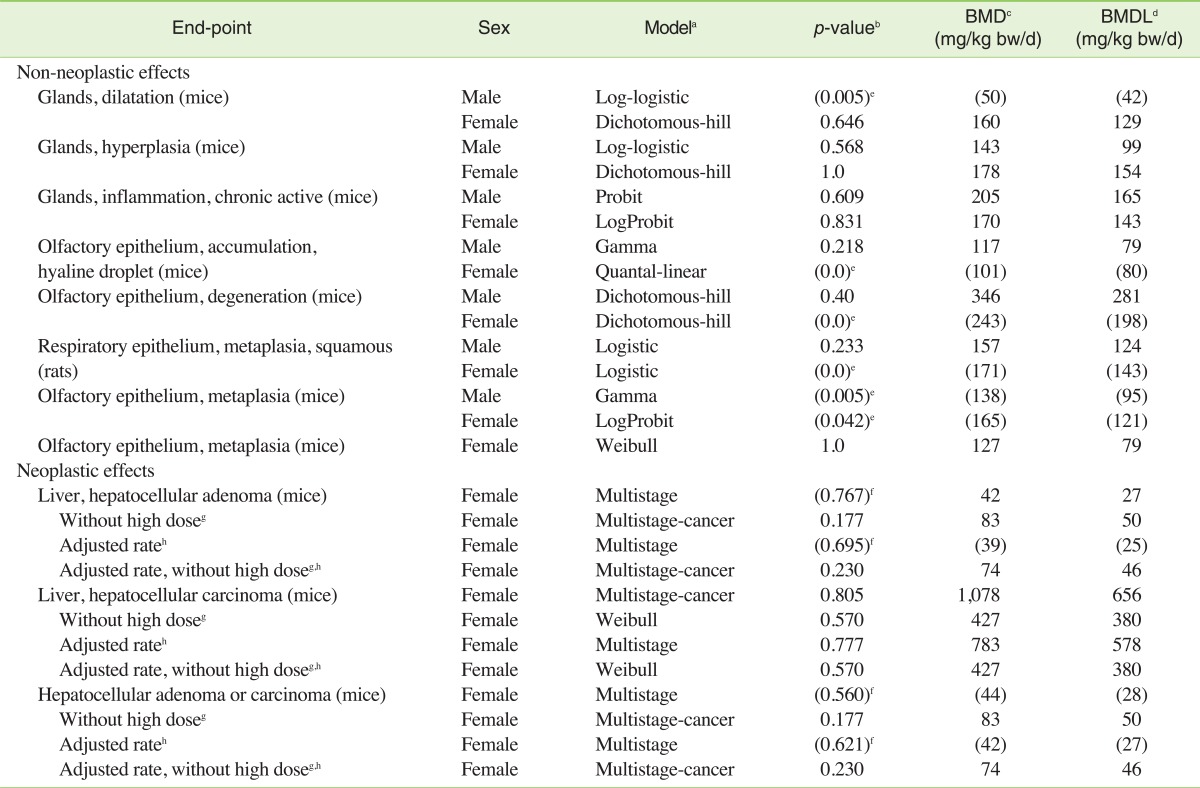
From the animal experiments mentioned in the previous section, only the two NTP long-term studies researching the oral route of exposure to HMF appear to be suitable for dose-response modelling for risk assessment purposes [5]. All end-points considered by NTP as related to the administration of HMF [5] were selected for modelling. The best-fitting significant models (own calculations) are listed in Table 1. It can be seen that the values for different non-neoplastic end-points calculated from the NTP study with rats and mice are consistent with each other: the BMD and BMDL values are in the range of 50 to 346 and 79 to 281 mg/kg bw/d, respectively. Overall the BMDs and BMDLs of the models are in the same order of magnitude, which is indicative of an overall adequacy of the calculated values, as even between different models of the same end-point, differences up to factors of 4 are accepted as typical, so that an averaging of the values would be adequate [18]. The modelling of neoplastic effects is more problematic as the highest dose-group had lower incidence rate than the control groups. For improved models for liver hepatocellular adenoma and carcinoma, the high dose group may be excluded from the calculation and the poly-3 estimated neoplasm incidence after adjustment for intercurrent mortality can be used (Table 1). Dropping the high dose could be appropriate, because the lower response at the higher dose could be due to a combination of early mortality and a plateau in the level of a toxic metabolite due to saturation of a metabolic process. According to NTP [5], above 13 mg/kg bw/d, a significant increase in the furoic acid metabolite of HMF relative to the glycine conjugate (which is the predominant metabolite at lower concentrations) was reported. At the highest dose administered (330 mg/kg bw/d near the dose at which the tumour response plateaus), the initial concentration of the furoic acid metabolite was 20- to 30-fold greater than the glycine conjugate. This may explain the lower tumour response at very high doses if (a) the glycine conjugate is a carcinogenic moiety whose body burden has "levelled off" at high doses of HMF and/or (b) the furoic acid metabolite is cytotoxic to the tumour cells (i.e., acting as a chemotherapy agent) at high doses of HMF.
Table 1.
Summary of own dose response modelling results for 5-hydroxymethylfurfural in different animal experiments conducted by NTP [5]
aData from best-fitting models selected with BMD are presented.
bA p-value greater than 0.1 indicates that the model fits the data (p-value 1.0 = perfect fit).
cBMD: benchmark dose for a 10% incidence of health effect.
dBMDL: lower one-sided confidence limit of the BMD.
eNot significant dose-response. Values are shown in brackets for information.
fNo clear dose-response as the highest dose-group had lower incidence than the control group
gCalculation excluding 750 mg/kg bw/d group.
hThe adjusted rate was modelled based on poly-3 estimated neoplasm incidence after adjustment for intercurrent mortality according to Table D2 of NTP [6].
While this interpretation of the NTP data offers some plausibility for neoplastic effects, the German Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung, BfR) remarked that it had doubts that HMF has any carcinogenic potential [7]. The BfR based its assessment on the fact that the tumours were only observed in female mice, that B6C3F1 mice have a high spontaneous tumour rate, and that no dose-response was proven. It is also noteworthy that while the WHO International Agency for Research on Cancer (IARC) does currently not provide an evaluation of HMF, according to IARC criteria the evidence for carcinogenicity of HMF appears to be inadequate (sufficient evidence for animal experiments would require a causal relationship in (a) two or more species of animals or (b) two or more independent studies in one species. The NTP itself judged that only "some evidence of carcinogenic activity" was demonstrated, which means that the strength of the response is less than that required for clear evidence [5].
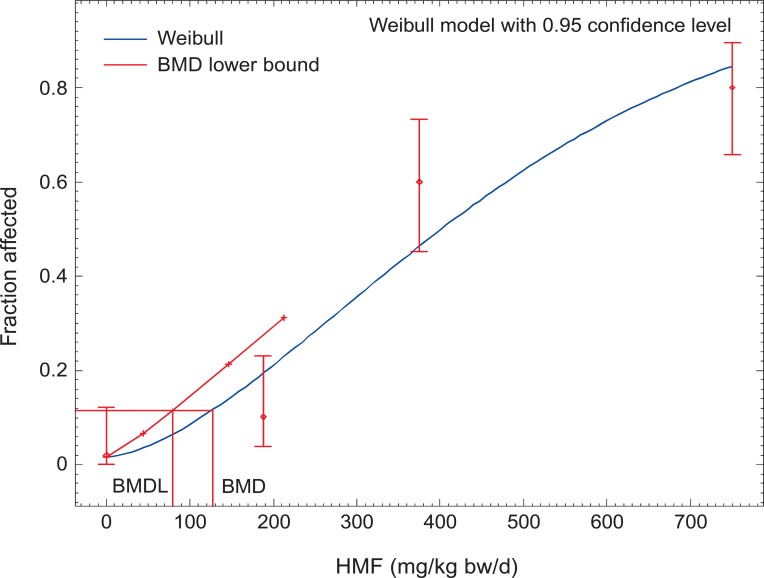
We therefore judge the evidence about carcinogenicity as too weak to be the basis for our risk assessment and therefore use the non-neoplastic effects. To be conservative, we decided to take the model with the lowest BMDL of all significant non-neoplastic models for our further calculations, which is the model for the olfactory epithelium metaplasia in female mice with a BMD of 127 mg/kg bw/d and a BMDL of 79 mg/kg bw/d (Figure 1). This BMDL is also in the same order of magnitude to the BMDL tentatively calculated for the neoplastic effects. Our values are well in line with the assumption of the BfR that the threshold for HMF effects in animals is iterating around 80 to 100 mg/kg bw/d [7]. Notably, the values for HMF are less toxic than what we calculated for acetaldehyde (BMD, 114 mg/kg bw/d; BMDL, 56 mg/kg bw/d) [11].
Figure 1.
BMD modelling for HMF. Weibull model with 0.95 confidence level. BMD for a 10% incidence of health effect (olfactory epithelium metaplasia, female mice, 104-week study); BMDL: lower one-side confidence limit of the BMD. BMD, benchmark dose; BMDL, benchmark dose lower confidence limit; HMF, 5-hydroxymethylfurfural. Calculated with data from NTP [5].
III. HMF Exposure from Alcohol Consumption
In this study we used the EFSA guidelines [12], which recommend that risk assessments provide different exposure scenarios (e.g. for entire, or specific groups of populations) along with their inherent uncertainties. Other than the mean and median, intakes from highly exposed individuals (due to high consumption or to average consumption of highly contaminated foods as represented by the 90th, 95th, 97.5th and 99th percentiles) should be considered.
To provide estimates on the dietary intake of HMF, data on the consumption of alcoholic beverages as well as their content of HMF is needed. Currently systematic data are lacking regarding HMF content of alcoholic beverages. Although HMF is a typical component of heat-processed foods [1], with the highest concentrations in balsamic vinegar [19], coffee [20] and cereals [21], monitoring has generally been sporadic and not consistent, especially regarding alcoholic beverages.
Nevertheless, there are some studies where the actual HMF content in different alcoholic beverages was determined [1-3,6,8,9,22-36]. The investigation of alcoholic beverages for HMF content started as early as 1948 with measurement of cherry wines in which a maximum content of 300 mg/L was observed [22]. In the following decades HMF has been also detected in whiskey, rum, tequila and other types of alcoholic beverages. However, in most of the studies only limited numbers of samples were evaluated.
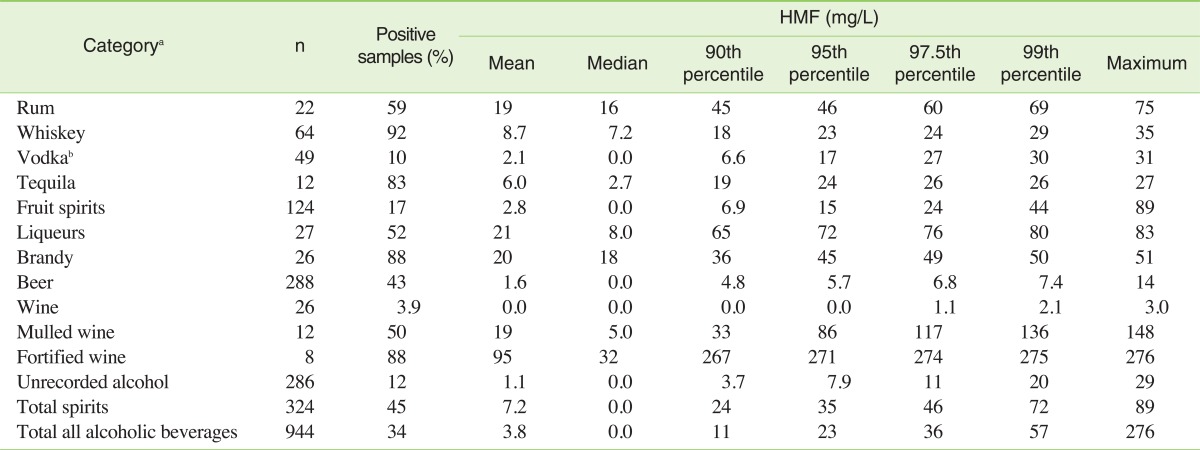
Therefore, we provided our own HMF analysis of different types of alcoholic beverages. The results for 944 samples are shown in Table 2. The highest HMF concentration was observed within fortified wines, which is also in agreement with the literature [1,25,26]. Expectedly low concentrations were obtained for beer (mean 1.6 mg/L), vodka (mean 2.1 mg/L) and fruit spirits (mean 2.8 mg/L).
Table 2.
5-Hydroxymethylfurfural (HMF) concentration in alcoholic beverages from 2009 to 2011
aThe categories were primarily chosen based on available consumption data (see Lachenmeier et al. [11] for details).
bIncluding flavoured vodka.
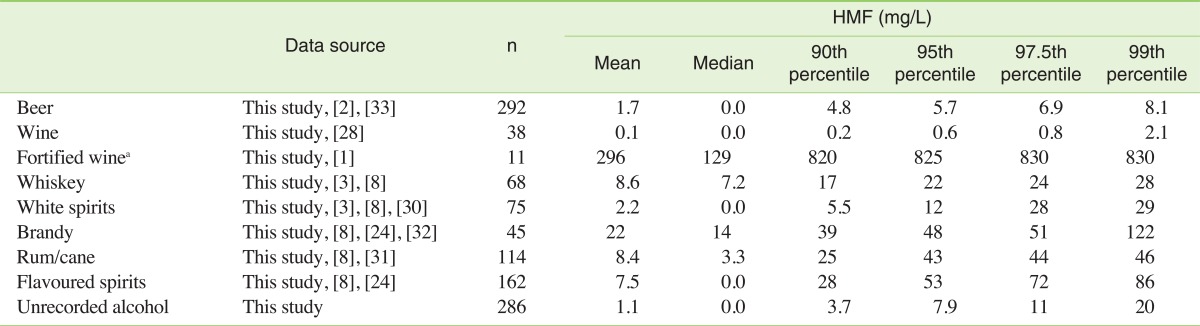
Furthermore, we combined our results of HMF occurrence in alcoholic beverages with previously reported data (Table 3). Table 3 shows the meta analysis as mean, median as well as 90th, 95th, 97.5th and 99th percentiles. When the HMF values came from different studies, the values were obtained by combination of all data available after proper transformation into the same unit (mg/L). Again, highly variable concentrations were observed: mean concentrations of HMF in a variety of alcoholic beverages ranged from 0.1 mg/L in wines to 296 mg/L in fortified wine.
Table 3.
5-Hydroxymethylfurfural (HMF) concentration in alcoholic beverages
The values are presented as meta-analysis combining own results from Table 2 with literature data.
aNot used for exposure estimation.
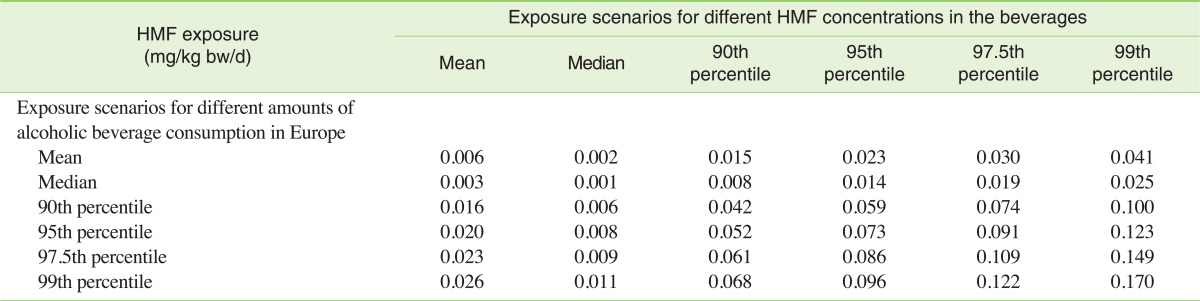
The annual consumption of different types of alcoholic beverages for the population older than 15 can be easily obtained from the WHO databases. This can be done for the majority of the countries around the world. However, as studies about HMF concentrations in alcoholic beverages other than European-style beverages are lacking, we decided to limit the whole population dietary intake estimate to the European Union (EU). The HMF exposure due to alcoholic beverage consumption was calculated from Table 3 combined with values of annual per capita consumption of alcoholic beverages in the EU (see Lachenmeier et al. [11] for details on annual consumption of the different beverage groups). The average exposure was found to be about 0.006 mg/kg bw/d, while in a worst case of very high consumption combined with very high concentrations in the beverages, the exposure may reach up to 0.170 mg/kg bw/d. Table 4 summarizes the exposure for the different scenarios.
Table 4.
Exposure with 5-hydroxymethylfurfural (HMF) from alcoholic beverages
The table shows the sum of all alcoholic beverages (beer, wine, spirits, unrecorded) calculated as mg/kg bw/d (calculated for a 60 kg person)
IV. Risk Characterization for the Alcohol-drinking Population
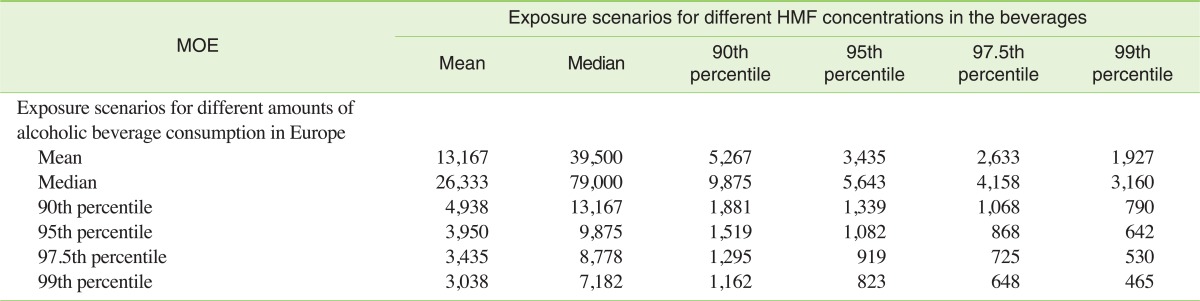
The exposure data from Table 4 was used to characterize risk using the MOE calculated from BMDL (Table 5). By risk managers, MOEs can be used for setting priority, with a large MOE representing a lower risk than a smaller one. Species differences and human variability in the basic process of toxicokinetics and toxicodynamics are inherent in the use of data from studies in animals for human risk assessment. A factor of 100-fold is usually used to allow for these uncertainties in the risk assessment of non-genotoxic substances [12].
Table 5.
Margin of Exposure (MOE) for 5-hydroxymethylfurfural (HMF) in different exposure scenarios
Values are calculated with benchmark dose lower confidence limit (BMDL) of 79 mg/kg bw/d (MOE = BMDL/exposure).
In the case of HMF, the MOEs were in some scenarios below 1,000 but never below 100, demonstrating that in general HMF in alcoholic beverages is not a public health concern not even in worst-case scenarios. In comparison to other constituents of alcoholic beverages, HMF also shows lower potential risk. For example, the calculated MOE value was 465 for HMF in the worst-case scenario (99th percentile), which is of same order of magnitude as the MOE value for average exposure to acetaldehyde from alcoholic beverages [11,37]. A previous risk assessment of HMF in food in general also showed a comparably low risk of HMF for the average population [38]. It was estimated that even in a worst case where up to 4.3 mg/kg bw of HMF is consumed every day with beverages made from dried plums, the margin of safety would still be of around 20 [38].
DISCUSSION
I. Limitations of the Approach
In contrast to the risk assessment of another toxic aldehyde - acetaldehyde - for which a considerably larger database about human toxicity and genetic epidemiology exists [11,39], our HMF assessment contains certain limitations:
The assessment is based on only one oral animal study. Additionally, there are no other estimates for BMDL and BMD values in the literature. However, as the values obtained for different end-points corresponded well to each other, we believe that the chosen BMDL value is certainly in the correct order of magnitude and could be used for quantitative risk assessment. Uncertainty remains if a carcinogenic effect exists and if it is non-threshold-based. In that case, higher safety factors than 100 also would have to be applied (but the average exposure would still be below an MOE threshold of 10,000, which is typically applied to carcinogens). Additionally, uncertainty remains regarding the possibility of chronic toxic effects not studied by NTP (such as some biochemical and haematological parameters) which may show lower BMD than the neoplastic and non-neoplastic effects. Finally, the absence of studies on reproductive toxicity has to be considered [7].
We did not include fortified wine in our exposure estimations at all because of the limited number of samples we have. Therefore, the whole evaluation may be underestimated because this group usually contains higher amount of HMF than other types of alcoholic beverages (Tables 2 and 3). However, the population-based consumption of fortified wine is very low.
The data on exposure are still incomplete; especially data on levels in fortified wine is scarce. Therefore, analytical data on the concentrations of HMF in this beverage group would be required for more reliable risk assessment, especially for individual drinkers that prefer this beverage group.
II. Estimation of Total Dietary Exposure
Besides alcoholic beverages, humans could be exposed to HMF from other sources. However, the current data only allow rough estimations. HMF occurs in almost all heat-processed foods in the concentrations ranging from trace levels in juices to approximately 4,000 mg/kg in coffee. In a study conducted in Spain, HMF exposure of the Spanish population from heat processed food was estimated [6]. An average HMF intake of 10 mg/p/d was obtained with coffee and bread as the most important food items that contribute to nearly 85% of daily exposure. In another study by Husoy et al. [2], the 95th percentile of the estimated daily intake was 27.6 mg/p/d (0.46 mg/kg bw/d) for the Norwegian population [2]. For Germany, HMF consumption with food was estimated at 4.04 mg/p/d (mean exposure) and 12.87 mg/p/d (95th percentile exposure) [7]. Based on these and our data we can estimate the total exposure (from food and alcoholic beverages) at 4.40 mg/p/d (mean exposure) and 14.7 mg/p/d (95th percentile exposure) for the German population. Thus, the average exposure via alcoholic beverages does not play an important role and makes up only approximately 8.5% of the total exposure according to our calculations. Nevertheless, data on cumulative HMF exposure (especially for food and beverages containing caramel colour) are sparse and should be updated in the future.
III. Suggestions for Risk Management
For regulatory toxicology, it is important to know if an agent has a threshold-based mechanism, which would allow to define tolerable daily intakes. Although no-observed adverse effect levels around 80 to 100 mg/kg bw/d were suggested for HMF, it is currently impossible to estimate a tolerable daily intake because of the limited mechanistic data and especially the concerns for carcinogenicity [38].
Our conclusion is that the occurrence of HMF in alcoholic beverages does not constitute an additional risk for average drinkers. Our data showed that in typical scenarios, the HMF exposure in alcoholic beverages (mean 0.006 mg/kg bw/d) is very low relative to the hazard (BMDL 79 mg/kg bw/d) and is therefore not a public health concern. For other compounds of alcoholic beverages such as acetaldehyde or ethyl carbamate, MOEs were in considerably lower ranges [11,37] as our calculations for HMF. The major risk, however, certainly comes from ethanol with a MOE of 1 or even smaller [40]. Therefore, for the regulation of alcoholic beverages, we do not consider HMF to be a high priority for risk management measures (such as mitigative efforts to reduce its content).
Footnotes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/
References
- 1.Bachmann S, Meier M, Känzig A. 5-Hydroxymethyl-2-furfural (HMF) in Lebensmitteln. Lebensmittelchemie. 1997;51(3):49–50. (German) [Google Scholar]
- 2.Husøy T, Haugen M, Murkovic M, Jöbstl D, Stølen LH, Bjellaas T, et al. Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. Food Chem Toxicol. 2008;46(12):3697–3702. doi: 10.1016/j.fct.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 3.de la Iglesia F, Lazaro F, Puchades R, Maquieira A. Automatic determination of 5-hydroxymethylfurfural (5-HMF) by a flow injection method. Food Chem. 1997;60(2):245–250. [Google Scholar]
- 4.Ulbricht RJ, Northup SJ, Thomas JA. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Fundam Appl Toxicol. 1984;4(5):843–853. doi: 10.1016/0272-0590(84)90106-4. [DOI] [PubMed] [Google Scholar]
- 5.National Toxicology Program. NTP toxicology and carcinogenesis studies of 5-(hydroxymethyl)-2-furfural (CAS No. 67-47-0) in F344/N rats and B6C3F1 mice (gavage studies) [cited 2012 Mar 10]. Available from: http://ntp.niehs.nih.gov/?objectid=793A39F7-F1F6-975E-761D9DAC33E41B3F. [PubMed]
- 6.Rufían-Henares JA, de la Cueva SP. Assessment of hydroxymethylfurfural intake in the Spanish diet. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25(11):1306–1312. doi: 10.1080/02652030802163406. [DOI] [PubMed] [Google Scholar]
- 7.Bundesinstitut fur Risikobewertung (BfR) According to the current state of scientific knowledge 5-HMF concentrations occurring in foods do not give rise to safety concerns. [cited 2012 May 15]. Available from: http://www.bfr.bund.de/cm/349/according-to-the-current-state-of-scientific-knowledge-5-hmf-concentrations-occuring-in-foods-do-not-give-rise-to-safety.pdf.
- 8.Frischkorn HE, Wanderley-Casado M, Frischkorn CGB. Rapid determination of furfural and hydroxymethylfurfural in alcoholic beverages by reverse-phase-chromatography. Z Lebensm Unters Forsch. 1982;174(2):117–121. (German) [Google Scholar]
- 9.Villalon MM, Montilla GJ, Garcia-Villanova R. Spectrophotometric determination of phenolic and furanic aldehydes in rum. Anal Bromatol. 1987;39(2):281–289. (Spanish) [Google Scholar]
- 10.Lachenmeier DW, Haupt S, Schulz K. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regul Toxicol Pharmacol. 2008;50(3):313–321. doi: 10.1016/j.yrtph.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Lachenmeier DW, Kanteres F, Rehm J. Carcinogenicity of acetaldehyde in alcoholic beverages: risk assessment outside ethanol metabolism. Addiction. 2009;104(4):533–550. doi: 10.1111/j.1360-0443.2009.02516.x. [DOI] [PubMed] [Google Scholar]
- 12.European Food Safety Authority (EFSA) Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005;282:1–31. [Google Scholar]
- 13.Monakhova YB, Schäfer H, Humpfer E, Spraul M, Kuballa T, Lachenmeier DW. Application of automated eightfold suppression of water and ethanol signals in 1H NMR to provide sensitivity for analyzing alcoholic beverages. Magn Reson Chem. 2011;49(11):734–739. doi: 10.1002/mrc.2823. [DOI] [PubMed] [Google Scholar]
- 14.International Organisation of Vine and Wine. Compendium of international methods of wine and must analysis. [cited 2012 May 15]. Available from: http://www.scribd.com/doc/80906777/OIV-Methods-Vol-1-en-2012.
- 15.Corpet DE, Stamp D, Medline A, Minkin S, Archer MC, Bruce WR. Promotion of colonic microadenoma growth in mice and rats fed cooked sugar or cooked casein and fat. Cancer Res. 1990;50(21):6955–6958. [PubMed] [Google Scholar]
- 16.Archer MC, Bruce WR, Chan CC, Corpet DE, Medline A, Roncucci L, et al. Aberrant crypt foci and microadenoma as markers for colon cancer. Environ Health Perspect. 1992;98:195–197. doi: 10.1289/ehp.9298195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XM, Chan CC, Stamp D, Minkin S, Archer MC, Bruce WR. Initiation and promotion of colonic aberrant crypt foci in rats by 5-hydroxymethyl-2-furaldehyde in thermolyzed sucrose. Carcinogenesis. 1993;14(4):773–775. doi: 10.1093/carcin/14.4.773. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Environmental Protection Agency. The use of the benchmark dose approach in health risk assessment. [cited 2012 May 15]. Available from: http://www.epa.gov/raf/publications/pdfs/BENCHMARK.PDF.
- 19.Theobald A, Müller A, Anklam E. Determination of 5-hydroxymethylfurfural in vinegar samples by HPLC. J Agric Food Chem. 1998;46(5):1850–1854. [Google Scholar]
- 20.Murkovic M, Pichler N. Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Mol Nutr Food Res. 2006;50(9):842–846. doi: 10.1002/mnfr.200500262. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Villanova B, Guerra-Hernandez E, Martinez-Gomez E, Montilla J. Liquid-chromatography for the determination of 5-(hydroxymethyl)-2-furaldehyde in breakfast cereals. J Agric Food Chem. 1993;41(8):1254–1255. [Google Scholar]
- 22.Amerine MA. Hydroxymethylfurfural in California wines. Food Res. 1948;13(3):264–269. doi: 10.1111/j.1365-2621.1948.tb16621.x. [DOI] [PubMed] [Google Scholar]
- 23.Meidell EF. Quantitative determination of hydroxymethylfurfural in sherries and grape concentrate. Am J Enol Vitic. 1969;20(3):164–168. [Google Scholar]
- 24.Alfonso FC, Martin GE, Dyer RH. High-pressure liquid-chromatographic determination of 5-(hydroxymethyl)-2-furaldehyde in caramel solution. J Assoc Off Anal Chem. 1980;63(6):1310–1313. [Google Scholar]
- 25.Pereira V, Albuquerque F, Ferreira A, Cacho J, Marques J. Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Res Int. 2011;44(1):71–76. [Google Scholar]
- 26.Kahoun D, Rezková S, Veskrnová K, Královský J, Holcapek M. Determination of phenolic compounds and hydroxymethylfurfural in meads using high performance liquid chromatography with coulometric-array and UV detection. J Chromatogr A. 2008;1202(1):19–33. doi: 10.1016/j.chroma.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Huang X. Simultaneous determination of 5-hydroxymethylfurfural and nine kinds of phenolic compounds in rice wine using high performance liquid chromatography. Chinese J Analyt Chem. 2010;38(1):133–137. (Chinese) [Google Scholar]
- 28.Cartoni GP, Coccioli F, Spagnoli M. Analysis of ethereal extracts of wines and other alcoholic beverages by high-performance liquid chromatography with microbore columns. J Chromatogr A. 1997;782(2):219–226. doi: 10.1016/s0021-9673(97)00505-0. [DOI] [PubMed] [Google Scholar]
- 29.Akillioglu HG, Mogol BA, Gökmen V. Degradation of 5-hydroxymethylfurfural during yeast fermentation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(12):1629–1635. doi: 10.1080/19440049.2011.609491. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez DM. Wrobel K, Wrobel K. Determination of aldehydes in tequila by high-performance liquid chromatography with 2,4-dinitrophenylhydrazine derivatization. Eur Food Res Technol. 2005;221(6):798–802. [Google Scholar]
- 31.Nascimento RF, Marques JC, Lima Neto BS, De Keukeleire D, Franco DW. Qualitative and quantitative high-performance liquid chromatographic analysis of aldehydes in Brazilian sugar cane spirits and other distilled alcoholic beverages. J Chromatogr A. 1997;782(1):13–23. doi: 10.1016/s0021-9673(97)00425-1. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz M, Rodríguez MC, Guillén DA, Barroso CG. Development and validation of UPLC for the determination of phenolic compounds and furanic derivatives in Brandy de Jerez. J Sep Sci. 2009;32(11):1782–1790. doi: 10.1002/jssc.200800706. [DOI] [PubMed] [Google Scholar]
- 33.Wu JY, Shi ZG, Feng YQ. Determination of 5-hydroxymethylfurfural using derivatization combined with polymer monolith microextraction by high-performance liquid chromatography. J Agric Food Chem. 2009;57(10):3981–3988. doi: 10.1021/jf900434n. [DOI] [PubMed] [Google Scholar]
- 34.Castellari M, Sartini E, Spinabelli U, Riponi C, Galassi S. Determination of carboxylic acids, carbohydrates, glycerol, ethanol, and 5-HMF in beer by high-performance liquid chromatography and UV-refractive index double detection. J Chromatogr Sci. 2001;39(6):235–238. doi: 10.1093/chromsci/39.6.235. [DOI] [PubMed] [Google Scholar]
- 35.Kurtbay HM, Kaynak I, Bozkurt SS, Merdivan M. Densitometric HPTLC analysis of the 5-hydroxymethylfurfural content of Turkish fruit wines and vinegars. J Planar Chromat. 2009;22(5):363–366. [Google Scholar]
- 36.Saison D, De Schutter DP, Delvaux F, Delvaux FR. Determination of carbonyl compounds in beer by derivatisation and headspace solid-phase microextraction in combination with gas chromatography and mass spectrometry. J Chromatogr A. 2009;1216(26):5061–5068. doi: 10.1016/j.chroma.2009.04.077. [DOI] [PubMed] [Google Scholar]
- 37.Lachenmeier DW, Przybylski MC, Rehm J. Comparative risk assessment of carcinogens in alcoholic beverages using the margin of exposure approach. Int J Cancer. 2012;131(6):E995–E1003. doi: 10.1002/ijc.27553. [DOI] [PubMed] [Google Scholar]
- 38.Abraham K, Gürtler R, Berg K, Heinemeyer G, Lampen A, Appel KE. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Mol Nutr Food Res. 2011;55(5):667–678. doi: 10.1002/mnfr.201000564. [DOI] [PubMed] [Google Scholar]
- 39.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. [cited 2012 May 15]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf.
- 40.Lachenmeier DW, Kanteres F, Rehm J. Epidemiology-based risk assessment using the benchmark dose/margin of exposure approach: the example of ethanol and liver cirrhosis. Int J Epidemiol. 2011;40(1):210–218. doi: 10.1093/ije/dyq150. [DOI] [PubMed] [Google Scholar]