Abstract
Objectives
Recent studies have shown that nano-sized carbon black is more toxic than large respirable carbon black because of its higher surface area. However, it is not clear if carbon black made larger by agglomeration demonstrates decreased toxicity. The purpose of this study was to verify if agglomeration affects the toxicity of carbon black using three differently prepared nano-sized carbon black aerosols in nose-only inhalation chambers for 13 weeks.
Methods
Printex 90 was selected as a representative nano-sized carbon black. To generate aerosols of three different types of agglomerates, Printex 90 was dispersed in distilled water by three different methods: vortex, vortex+sonication, and vortex+sonication with dispersion in a stabilizer. Then, the three differently prepared solutions were aerosolized through venturi nozzles. Male Sprague-Dawley rats were exposed to Printex 90 aerosols in a nose-only exposure chamber for 6 h/d, 5 d/wk for 13 weeks at a concentration of approximately 9 mg/m3.
Results
Numbers of total cells in the bronchoalveolar lavage (BAL) fluid, macrophages, and polymorphonuclear leukocytes were increased and carbon black masses were clearly seen in BAL cells and lung tissues of rats exposed to Printex 90. However, few differences were found between the three differently agglomerated aerosols. In addition, there were no significant differences in other parameters, such as body weight, lung function or cytokine levels in BAL fluid following carbon black exposure.
Conclusions
Only mild to moderate respiratory effects were found in rats exposed to nano-sized carbon black at 9 mg/m3 for 13 weeks. Agglomeration did not affect the toxicity of nano-sized carbon particles.
Keywords: Agglomeration, Carbon black, Nose-only inhalation exposure
INTRODUCTION
Many toxicity studies on nano-sized particles have been performed since Ferin et al. [1] and Oberdörster et al. [2] reported that ultra-fine particles smaller than 100 nm in diameter elicit a greater pulmonary effect than larger particles. Although there have been some controversial studies, it is generally accepted at present that smaller particles, such as nano-sized particles with the same physicochemical properties are more toxic than larger particles because of their greater surface area.
It has been reasonably questioned that the toxicity of well-agglomerating particles such as carbon black might be underestimated because the surface area decreases with agglomeration. Therefore, many researchers have tried to disperse well-agglomerated particles to study them in a in non-agglomerated state. Sager et al. [3] tried dipalmitoyl phosphatidylcholine and/or bronchoalveolar lavage (BAL) fluid as a dispersion stabilizer and Bihari et al. [4] studied human serum albumin (HSA) and/or bovine serum albumin as a stabilizer. For inhalation studies, a venturi-type dust feeder with a 83Kr source [5] or jet-O-mixer/screw feed generator [6] was applied to generate nano-sized aerosols. More recently, the electrospray method [7] and the chemical vapor deposition method [8] were developed to produce singlet nanoparticles. However, these methods were not widely applied because of the low generating capacity and/or high cost.
On the other hand, exposure to well-agglomerated nanoparticles commonly occurs in the workplace in the form of large agglomerates, even though they are made as nanoparticles because they agglomerate rapidly before exposure to workers occurs. Moreover, new technologies for measuring nanoparticles, such as scanning mobility particle sizers, have not yet been validated fully and are not widely applied in the workplace. New workplace threshold limit values (TLVs) for nano materials have been proposed on the basis of weight, rather than particle number or surface area. The National Institute for Occupational Safety and Health (NIOSH) [9] and the New Energy and Industrial Technology Development Organization [10] suggested a TLV of nano materials (for example titanium dioxide, carbon nanotubes, etc.) measured by weight such as mg/m3.
It is not certain if particle size increases by agglomeration, or if the toxicity of well-agglomerated particles decreases. Therefore, toxicity studies conducted according to a mass-based unit are still required for risk assessments of nanoparticles. The effect of agglomeration on the toxicity of nanoparticles could provide important information for risk assessments. In this study, to minimize the knowledge gap between the agglomeration and the toxicity of nanoparticles, the toxicities of three different agglomerated states of nano-sized carbon black were evaluated using a nose-only inhalation chamber.
MATERIALS AND METHODS
I. Animals
Animal studies were approved by the animal ethics committee to ensure appropriate animal care for research. Five-week-old male specific pathogen-free Sprague-Dawley (SD) rats were obtained from Central Lab Animal Inc. (Seoul, Korea). Rats were acclimatized for two weeks prior to exposure to carbon black. During the acclimation and experimental period, rats were housed in polycarbonate cages in a room with controlled temperature (23±2℃), humidity (55±7%) and a 12-hour light/dark cycle. Rats were fed with filtered water and a rodent diet (LabDiet 5053; PMI Nutrition, St Louis, MO, USA) ad libitum. Animals were examined daily on weekdays for any evidence of exposure-related effects, including respiratory, dermal, behavioral, nasal or genitourinary changes.
II. Particles
Printex-90, a nano-sized carbon black, was obtained from Degussa (Frankfurt, Germany). The manufacturer presented the primary particle diameter of Printex 90 as 14 nm.
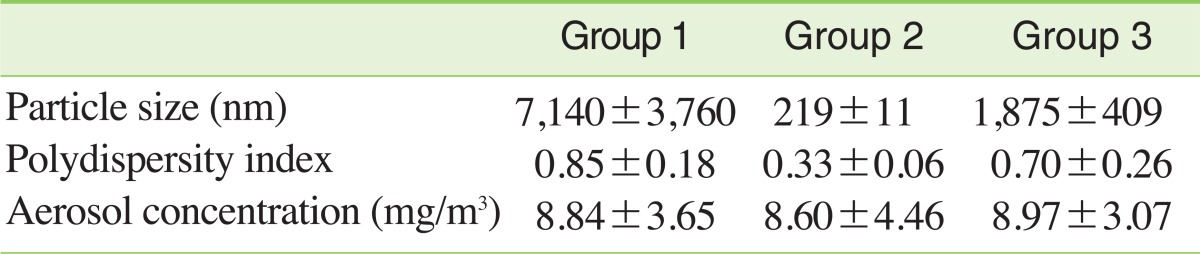
III. Generation and Exposure of Carbon Black Aerosol
Printex 90 was dispersed by three methods: (1) 1.5 g of Printex 90 was added to 300 mL of distilled water and vortexed vigorously for 1 minute; (2) Printex 90 was suspended using same method as (1) and sonicated at a power of 1,170 Joule with probe-type ultrasonicator (Vibra Cell VC-750, Newtown, CT, USA) for 3 minutes in a 3 second sonication/5 second resting cycle; (3) Printex 90 was dispersed using the same method as (2) and stabilized with 2.5 mg/mL of HSA. Particle size and dispersity in water solutions were measured with a Zetasizer (Nano Z 590, Malvern Instruments, Malvern, UK). The average diameters of Printex 90 suspensions were 7,140±3,760 nm by method (1), 219±11 nm by method (2) and 1,875±409 nm by method (3). The polydispersity indices of Printex 90 suspensions were 0.85±0.18 by method (1), 0.33±0.06 by method (2) and 0.70±0.26 by method (3) (Table 1). Carbon black suspensions prepared by the three different methods were aerosolized through venturi nozzles at 8 liters of air flow per minute in a nose-only inhalation chamber (NITC System, HCT, Incheon, Korea). Rats were exposed to Printex 90 aerosols 6 hours a day, 5 days per week for 13 weeks. Carbon black aerosol size distributions were performed using a small-sized cascade impactor (Minimoudi M135, Shoreview, MN, USA). For calculating carbon black concentrations, aerosols were sampled using membrane cellulose este filters and the weights of the filters were measured using an electric balance (Kern 770, Balingen, Germany).
Table 1.
Particle characterization in dispersion solution and concentrations in the nose-only inhalation chamber during the 13-week exposure period
Values are presented as mean±SD.
IV. Transmission Electron Microscopy (TEM)
Printex 90 aerosols in nose-only inhalation chambers were sampled with filters coated with carbon, mounted on an electron microscope grid (200 Mesh, Veco, Eerbeek, Holland) and visualized under a field emission-transmission electron microscope (Hitachi H7100FA, Tokyo, Japan).
V. Bronchoalveolar Lavage
After rats were anesthetized with isoflurane (Ilsung Seoul, Korea), the trachea was cannulated and the lungs were lavaged five times with 3 mL of calcium- and magnesium-free phosphate buffered saline (PBS), pH 7.4. BAL fluids were centrifuged at 1500 rpm for 10 minutes and the supernatants were stored at -80℃ for protein, lactate dehydrogenase (LDH) and cytokine assays. After total cell numbers were counted with a Coulter Counter (Hemarvet 850, Drew Science, Miami Lakes, FL, USA), lavaged cells were centrifuged using a Cytospin (Hanil, Gangneung, Korea) and stained by the Diff-Quick staining technique. Differential counts of macrophages, lymphocytes, and polymorphonuclear leukocytes (PMNs) were determined by counting approximately 300 cells under a microscope at 20x magnification. LDH, albumin, interleukin (IL)-6, IL-1β, IL-4, IL-10, tumor necrosis factor-α and interferon-gamma levels were determined using the first BAL fluid fraction. LDH and albumin were measured using a biochemistry analyzer (TBA 20FR, Toshiba, Tokyo, Japan) and cytokines were measured using BD Enzyme-linked immunosorbent assay kits (BD Biosciences, San Diego, CA, USA).
VI. Measurement of Lung Burden of Carbon Black
Printex 90 concentrations in lung tissue were measured according to the method of Rudd and Storm with some modifications [11]. Lung tissues were minced and digested in 25% KOH/methanol (w/v) at 60℃ overnight. Then, the mixture was spun at 10,000 rpm for 30 minutes. After the supernatants were removed, pellets were re-suspended in 0.1% Brij-35 (v/v) and sonicated for 5 seconds with a probe-type sonicator, (Sonics Inc., Milpitas, CA, USA). Turbidity was measured at a wavelength of 620 nm with a UV/visible spectrometer (Beckman DU-650, Fullerton, CA, USA). Numbers of carbon black masses in the lung were counted by light microscopy at 20x magnification. Pulmonary function parameters (tidal volume, minute volume and frequency) were measured three times (after 2 weeks, 4 weeks and 12 weeks exposure of to carbon black) by ventilated bias flow whole-body plethysmograph (SFT3816, Buxco Electronics, Wilmington, NC, USA) consisting of a reference chamber and an animal chamber interconnected with a pressure transducer (MAX1320, Buxco Electronics, Wilmington, NC, USA). After allowing the rats to stabilize in the animal chamber for 40 minutes, pulmonary function was measured for 5 minutes.
VII. Histopathology
Lungs were fixed in a 10% formalin solution containing neutral PBS and embedded in paraffin. After staining with hematoxylin and eosin, lungs were examined by light microscopy at 20x magnification.
VIII. Statistical Analysis
Results are represented as mean±standard deviation. Data were analyzed by ANOVA followed by post hoc analysis based on Duncan's t-test to determine the differences between the control group and the three groups exposed to the agglomerated state of carbon black. Statistical analyses were performed using SigmaStat (Systat Software Inc., San Jose, CA, USA). Differences were considered significant when the p-value was < 0.05.
RESULTS
I. Particle Size Distribution and Concentrations of Carbon Black Aerosol in Inhalation Chambers
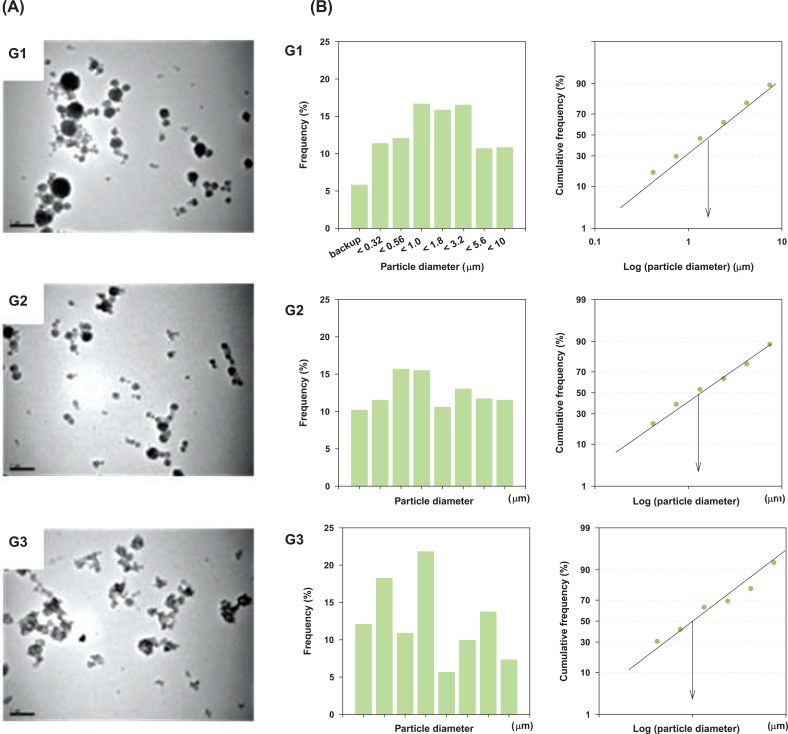
TEM was used to obtain information on the morphologies of agglomerated Printex 90 aerosols (Figure 1A). As shown in Figure 1, large agglomerates were obtained in the nose-only inhalation chambers. Agglomerates of group 1 were the largest (Figure 1A, G1), group 2 was intermediate (Figure 1A, G2) and group 3 was the smallest (Figure 1A, G3). The mass median aerodynamic diameters of particles were 1.52 µm for group 1, 1.30 µm for group 2 and 0.97 µm for group 3 (Figure 1B). During the 13-week exposure period, average concentrations were 8.8±3.7 mg/m3, 8.6±4.5 mg/m3 and 9.0±3.1 mg/m3 for group 1, group 2 and group 3, respectively (Table 1).
Figure 1.
Particle size distributions and transmission electron microscopy (TEM) morphologies of three different agglomerate states of carbon black. (A) TEM morphologies (Images were taken at 10,000x magnification and 100 kV). (B) Particle size distributions. G1, carbon black was suspended in distilled water, vortexed and aerosolized; G2, carbon black was dispersed in distilled water, vortexed+sonicated and aerosolized; G3, carbon black was dispersed in distilled water, vortexed+sonicated+stabilized with human serum albumin and aerosolized.
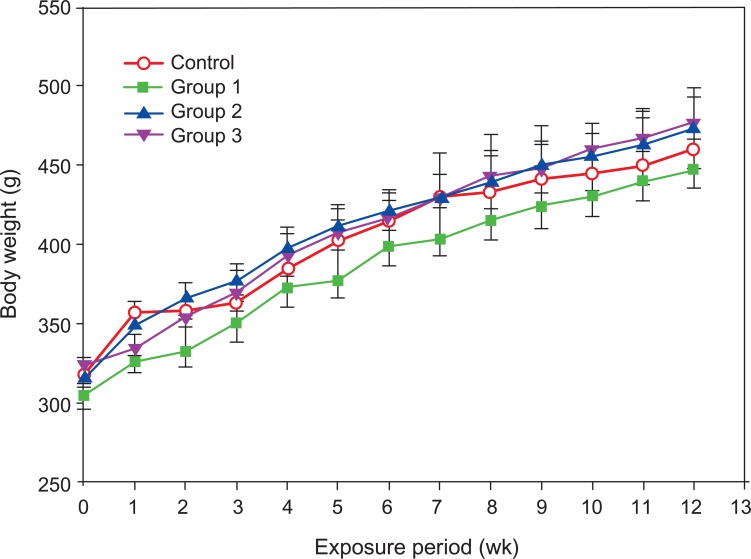
II. Body Weight
There were no statistical differences in body weight gain between the control group and the three agglomerated carbon black exposed groups during the 13-week exposure period (Figure 2).
Figure 2.
Body weights of male Sprague-Dawley rats exposed to carbon black for 13 weeks. Error bars indicate the standard error of the mean. Statistical significance was determined by ANOVA followed by Duncan's post hoc test (p<0.05). There were no statistically significant differences between the control group and the three carbon black exposure groups.
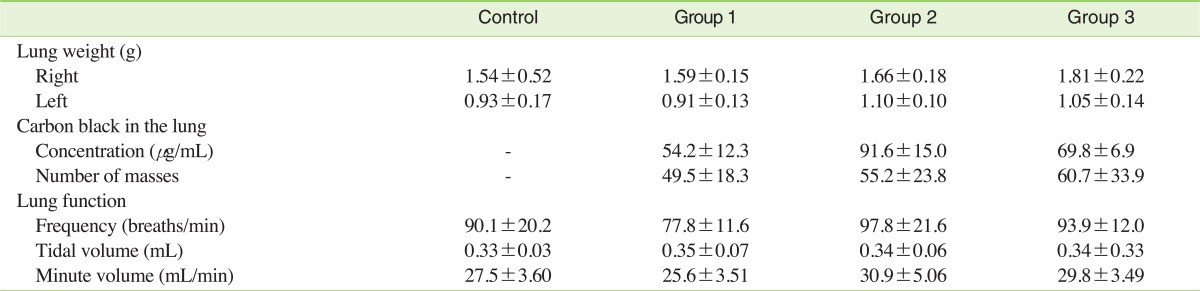
III. Pulmonary Function and Lung Burden of Carbon Black
Lung weights of group 2 and group 3 were a little higher than the control group and group 1, and distinct carbon black masses were found in the lung tissues of group 1, group 2 and group 3. Although the number of carbon black masses in lung tissue was highest in group 3, the carbon black concentration in the lung was highest in group 2. There were no statistically significant differences in pulmonary function between the control group and the three agglomerated carbon black exposed groups (Table 2). No histopathological symptoms were found to be associated with carbon black exposure (Figure 3A).
Table 2.
Lung burden of carbon black in male Sprague-Dawley rats exposed to carbon black for 13 weeks
Values are presented as mean±SD.
Statistical significance was determined (standard deviation) by ANOVA followed by Duncan's post hoc test (p<0.05).
There were no statistically significant differences between the control group and the three carbon black exposure groups.
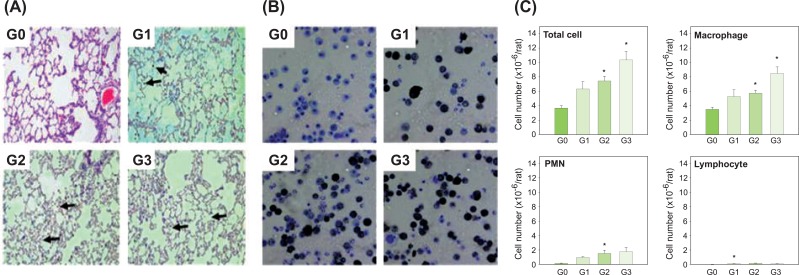
Figure 3.
Histopathological findings in the lungs and leukocytes in BAL fluid of male Sprague-Dawley rats exposed to carbon black for 13 weeks. (A) Histopathological findings. (B) Cytospun Leukocytes in BAL fluid. (C) Numbers of leukocytes in the BAL fluid. Arrows show masses of carbon black in the lungs (20x magnification). Error bars indicate the standard error of the mean. Statistically significant significances were determined by ANOVA followed by Duncan's post hoc test (p<0.05). G0, control; G1, carbon black dispersed by vortex; G2, carbon black dispersed by vortex+sonication; G3, carbon black dispersed and stabilized with albumin. BAL, bronchoalveolar lavage; PMN, polymorphonuclear leukocytes. *Statistically significant compared to control.
IV. BAL Cell Differentiation
Most macrophages in the BAL fluid from rats in group 1, group 2 and group 3 contained carbon black masses, but there was no indication that macrophages had been damaged by carbon black (Figure 3B). The numbers of total cells, macrophages and PMNs were increased by carbon black inhalation. However, statistically significant differences were found only between the control group and the two sonicated groups (group 2 and group 3) in terms of total cells and macrophages, and between the control group and group 2 for PMNs (Figure 3C).
V. LDH, Albumin, and Cytokines in BAL
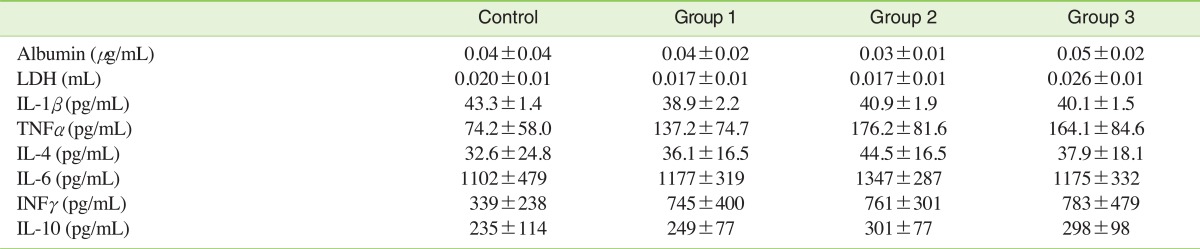
LDH was slightly increased in group 3 compared with control, but no statistical significance was found. Albumin and inflammatory cytokine levels did not change following exposure to carbon black aerosols (Table 3).
Table 3.
Albumin, LDH and inflammatory cytokine levels in the BAL fluid of male Sprague-Dawley rats exposed to carbon black for 13 weeks
Values are presented as mean±SD.
LDH, lactate dehydrogenase; BAL, bronchoalveolar lavage; IL, interleukin; TNF, Tumor necrosis factor; INF, Interferon.
Statistical significance was determined (standard deviation) by ANOVA followed by Duncan's post hoc test (p<0.05).
There were no statistically significant differences between the control group and the three carbon black exposure groups.
DISCUSSION
Carbon black has been massively used as a filler in the rubber tire industry since the early 19th century. Although Henderson et al. have claimed that the current TLV is too high for the workplace, the current TLV of 3.5 mg/m3 based on toxicity data reported by Nau et al. [12] in 1962 has not yet been changed.
Carbon black has been suspected as a cause of respiratory disease, cardiovascular disorders and cancer. However, there is little consistency in the results from epidemiological studies. Robertson and Ingalls [13] reported that carbon black did not increase the incidence of cancer, respiratory disease or cardiovascular disorders in workers at a carbon black manufacturing facility. Crosbie [14] and Küpper et al. [15] reported that carbon black did not cause serious respiratory effects. Sorahan et al. [16] also could not confirm a relationship between carbon black and increased standard mortality ratio in a cohort study from 1951 and 1996, whereas Gardiner et al. [17-19] reported that carbon black causes a decrease in respiratory function and induces chronic respiratory inflammation.
Recently, interest in the toxicity of carbon black, especially for nano-sized carbon black, has increased because many studies have reported that nano-sized particles are more toxic than large particles such as fine particles [1,2]. Furthermore, unknown deaths in Korea have been reported in a tire manufacturing facility. Some experts insisted that nano-sized carbon black aggravated cardiovascular symptoms and it might be the cause of unknown deaths of tire manufacturing facility. Therefore, both for hazard identification of nano-sized carbon black and to verify the possibility that nano-sized carbon black may have contributed to the deaths at the tire manufacturing facility, a re-evaluation of the toxicity of nano-sized carbon black is needed.
Now, it is generally accepted that increased surface area is one of the main causes of the increased toxicity of nano materials. For carbon black, many studies in cell culture systems [20-22], in instillation systems [23-29] and in inhalation systems [30-32] have verified that if the particle size decreases, the toxicity increases because the surface area has increased. However, the effect of aggregation on the toxicity of carbon black has not been reported. In the risk assessment of chemicals in the workplace, the effect of agglomeration on toxicity is also important because nano materials, especially well-agglomerated nano materials, might agglomerate before workers are exposed in the workplace. Therefore, this study was performed to minimize the knowledge gap regarding nano-sized carbon black, toxicity and agglomeration.
To make the different states of agglomerates, Printex 90 aerosols were generated in two steps. Namely, Printex 90 was dispersed in distilled water as the first step and the dispersed Printex 90 was aerosolized through an orifice in the nose-only inhalation chamber as the second step. For the first step, three different methods were adapted to make different agglomerates in the distilled water: 1) vortex only for group 1; 2) vortex+sonication for group 2 and 3) vortex+sonication+dispersion in a stabilizer for group 3. The polydispersity indices of group 1, group 2 and group 3 were 0.85, 0.33 and 0.70, respectively; this means that only the particles in group 2 were well-dispersed. Although the average diameters of carbon black in group 1, group 2 and group 3 were 7.14 µm, 0.22 µm and 1.88 µm, respectively, in water suspension, the diameters of the aerosols in the inhalation chamber were 1.52 µm, 1.30 µm and 0.97 µm for group 1, group 2 and group 3, respectively. It seems that the agglomeration states in water solution did not affect on the agglomeration state of aerosols in the inhalation chamber; this was because large agglomerates in the water solution were eliminated or smashed while passing through the orifice and the small carbon black aggregates re-agglomerated in the inhalation chamber. TEM analysis supported the results on aerodynamic particle sizes measured by the cascade impactor.
The average concentrations were 8.84 mg/m3, 8.60 mg/m3 and 8.97 mg/m3 for group 1, group 2 and group 3, respectively over the 13-week exposure period. These concentrations were slightly higher than the level of 7 mg/m3 that Driscoll et al. [5] and Elder et al. [31] found to be associated with a mild to moderate respiratory effect. In this study, there were no changes in body weight, cytokine levels in the BAL fluid or blood biochemical and hematological parameters. However, mild changes were found in the total and differential cell counts in the BAL fluid; these results correspond to previous results preformed [5,31].
There was little difference in the toxic effect between the three different aggregated carbon black exposed groups, even though the deposition of carbon black in the lungs of rats in group 2 and group 3 was higher than in group 1 in this study. Sometimes the relationship between agglomeration and the surface area of particles can be explained by the fractal dimension (Df). Namely, Df can often be used to characterize agglomerate morphology through the following equation; NP = A(Rg/Ro)Df, where Np is the number of primary particles in the aggregate, A is a dimensionless prefactor, Ro is the primary particle radius and Rg is the characteristic radius of the aggregate. Katrinak et al. [33] classified particles as fractal-like aggregates with 1.35 < Df < 1.89 and possibly non-fractal particles with Df > 2 (the surface area decreases if the fractal dimension is > 2). In our study, the surface area was not changed by agglomeration or sonication as determined using the Brunauer, Emmett, Teller method (data not shown), whereas carbon black aerosols seemed to be a little better dispersed in group 2 and group 3 (more fractal-like particles) than in group 1 (non-fractal-like particles) based on TEM morphology (Figure 1A). This is similar to the results of Kobayashi et al. [34], who reported that agglomeration causes minimal effects of the toxicity of titanium dioxide because agglomeration does not have a considerable affect on the surface area of titanium dioxide.
In conclusion, mild respiratory toxicity occurred in rats exposed to nano-sized carbon black for 13 weeks at a concentration of approximately 9 mg/m3 through nose-only inhalation; there were few differences in toxicity between the different agglomeration states.
ACKNOWLEDGEMENTS
The authors thank Dr. Jenny Roberts and Dr. Vincent Castranova at NIOSH in Morgantown, WV, USA for their helpful discussions and for providing carbon black, Printex 90.
Footnotes
The authors have no conflict of interest to declare on this study.
This article is available from: http://e-eht.org/
References
- 1.Ferin J, Oberdöster G, Penney DP, Soderholm SC, Piper HC. Increased pulmonary toxicity of ultrafine particles? I. Particle clearance, translocation, morphology. J Aerosol Sci. 1990;21(3):381–384. [Google Scholar]
- 2.Oberdöster G, Ferin J, Finkelstein G, Wade P, Corson N. Increased toxicity of ultrafine particles? II. Lung lavage studies. J Aerosol Sci. 1990;21(3):384–387. [Google Scholar]
- 3.Sager TM, Porter DW, Robinson VA, Lindsley WG, Schwegler-Berry DE, Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1(2):118–129. [Google Scholar]
- 4.Bihari P, Vippola M, Schultes S, Praetner M, Khandoga AG, Reichel CA, et al. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part Fibre Toxicol. 2008;5:14. doi: 10.1186/1743-8977-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driscoll KE, Carter JM, Howard BW, Hassenbein DG, Pepelko W, Baggs RB, et al. Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicol Appl Pharmacol. 1996;136(2):372–380. doi: 10.1006/taap.1996.0045. [DOI] [PubMed] [Google Scholar]
- 6.Henderson RF, Barr EB, Cheng YS, Griffith WC, Hahn FF. The effect of exposure pattern on the accumulation of particles and the response of the lung to inhaled particles. Fundam Appl Toxicol. 1992;19(3):367–374. doi: 10.1016/0272-0590(92)90175-h. [DOI] [PubMed] [Google Scholar]
- 7.Kim SC, Chen DR, Qi C, Gelein RM, Finkelstein JN, Elder A, et al. A nanoparticle dispersion method for in vitro and in vivo nanotoxicity study. Nanotoxicology. 2010;4(1):42–51. doi: 10.3109/17435390903374019. [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Kang MG, Cho HW, Han JH, Chung YH, Rim KT, et al. Effect of nano-sized carbon black particles on lung and circulatory system by inhalation exposure in rats. Saf Health Work. 2011;2(3):282–289. doi: 10.5491/SHAW.2011.2.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Occupational Safety and Health (NIOSH) Occupational exposure to titanium dioxide. [cited 2012 Jan 10]. Available from: http://www.cdc.gov/niosh/docs/2011-160/pdfs/2011-160.pdf.
- 10.New Energy and Industrial Technology Development Organization (NEDO) Nano material risk assessment report titanium dioxide (TiO2) Kanagawa: NEDO; 2011. pp. v1–v29. (Japanese) [Google Scholar]
- 11.Rudd CJ, Strom KA. A spectrophotometric method for the quantitation of diesel exhaust particles in guinea pig lung. J Appl Toxicol. 1981;1(2):83–87. doi: 10.1002/jat.2550010207. [DOI] [PubMed] [Google Scholar]
- 12.Nau CA, Neal J, Stembridge VA, Cooley RN. Physiological effects of carbon black. IV. Inhalation. Arch Environ Health. 1962;4:415–431. doi: 10.1080/00039896.1962.10663179. [DOI] [PubMed] [Google Scholar]
- 13.Robertson JM, Ingalls TH. A case-control study of circulatory, malignant, and respiratory morbidity in carbon black workers in the United States. Am Ind Hyg Assoc J. 1989;50(10):510–515. doi: 10.1080/15298668991375083. [DOI] [PubMed] [Google Scholar]
- 14.Crosbie WA. The respiratory health of carbon black workers. Arch Environ Health. 1986;41(6):346–353. doi: 10.1080/00039896.1986.9935777. [DOI] [PubMed] [Google Scholar]
- 15.Küpper HU, Breitstadt R, Ulmer WT. Effects on the lung function of exposure to carbon black dusts. Results of a study carried out on 677 members of staff of the DEGUSSA factory in Kalscheuren/Germany. Int Arch Occup Environ Health. 1996;68(6):478–483. doi: 10.1007/BF00377873. [DOI] [PubMed] [Google Scholar]
- 16.Sorahan T, Hamilton L, van Tongeren M, Gardiner K, Harrington JM. A cohort mortality study of U.K. carbon black workers, 1951-1996. Am J Ind Med. 2001;39(2):158–170. doi: 10.1002/1097-0274(200102)39:2<158::aid-ajim1003>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner K, Trethowan NW, Harrington JM, Rossiter CE, Calvert IA. Respiratory health effects of carbon black: a survey of European carbon black workers. Br J Ind Med. 1993;50(12):1082–1096. doi: 10.1136/oem.50.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardiner K. The methodological problems of multinational epidemiological studies with particular reference to carbon black studies. Occup Med (Lond) 1995;45(5):247–255. doi: 10.1093/occmed/45.5.247. [DOI] [PubMed] [Google Scholar]
- 19.Gardiner K, van Tongeren M, Harrington M. Respiratory health effects from exposure to carbon black: results of the phase 2 and 3 cross sectional studies in the European carbon black manufacturing industry. Occup Environ Med. 2001;58(8):496–503. doi: 10.1136/oem.58.8.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koike E, Kobayashi T. Chemical and biological oxidative effects of carbon black nanoparticles. Chemosphere. 2006;65(6):946–951. doi: 10.1016/j.chemosphere.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Totlandsdal AI, Refsnes M, Låg M. Mechanisms involved in ultrafine carbon black-induced release of IL-6 from primary rat epithelial lung cells. Toxicol In Vitro. 2010;24(1):10–20. doi: 10.1016/j.tiv.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Mroz RM, Schins RP, Li H, Drost EM, Macnee W, Donaldson K. Nanoparticle carbon black driven DNA damage induces growth arrest and AP-1 and NFkappaB DNA binding in lung epithelial A549 cell line. J Physiol Pharmacol. 2007;58 Suppl 5(Pt 2):461–470. [PubMed] [Google Scholar]
- 23.Tin-Tin-Win-Shwe, Yamamoto S, Ahmed S, Kakeyama M, Kobayashi T, Fujimaki H. Brain cytokine and chemokine mRNA expression in mice induced by intranasal instillation with ultrafine carbon black. Toxicol Lett. 2006;163(2):153–160. doi: 10.1016/j.toxlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61(5):442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DM, Stone V, Findlay P, MacNee W, Donaldson K. Increased inflammation and intracellular calcium caused by ultrafine carbon black is independent of transition metals or other soluble components. Occup Environ Med. 2000;57(10):685–691. doi: 10.1136/oem.57.10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen NR, Møller P, Jensen KA, Vogel U, Ladefoged O, Loft S, et al. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part Fibre Toxicol. 2009;6:2. doi: 10.1186/1743-8977-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CC, Chen SH, Ho SH, Yang CY, Wang HD, Tsai ML. Proteomic analysis of proteins from bronchoalveolar lavage fluid reveals the action mechanism of ultrafine carbon black-induced lung injury in mice. Proteomics. 2007;7(23):4388–4397. doi: 10.1002/pmic.200700164. [DOI] [PubMed] [Google Scholar]
- 28.Vesterdal LK, Folkmann JK, Jacobsen NR, Sheykhzade M, Wallin H, Loft S, et al. Pulmonary exposure to carbon black nanoparticles and vascular effects. Part Fibre Toxicol. 2010;7:33. doi: 10.1186/1743-8977-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sager TM, Castranova V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part Fibre Toxicol. 2009;6:15. doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter JM, Corson N, Driscoll KE, Elder A, Finkelstein JN, Harkema JN, et al. A comparative dose-related response of several key pro- and antiinflammatory mediators in the lungs of rats, mice, and hamsters after subchronic inhalation of carbon black. J Occup Environ Med. 2006;48(12):1265–1278. doi: 10.1097/01.jom.0000230489.06025.14. [DOI] [PubMed] [Google Scholar]
- 31.Elder A, Gelein R, Finkelstein JN, Driscoll KE, Harkema J, Oberdörster G. Effects of subchronically inhaled carbon black in three species. I. Retention kinetics, lung inflammation, and histopathology. Toxicol Sci. 2005;88(2):614–629. doi: 10.1093/toxsci/kfi327. [DOI] [PubMed] [Google Scholar]
- 32.Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, et al. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol. 2004;195(1):35–44. doi: 10.1016/j.taap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Katrinak KA, Rez P, Perkes PR, Buseck PR. Fractal geometry of carbonaceous aggregates from an urban aerosol. Environ Sci Technol. 1993;27(3):539–547. [Google Scholar]
- 34.Kobayashi N, Naya M, Endoh S, Maru J, Yamamoto K, Nakanishi J. Comparative pulmonary toxicity study of nano-TiO(2) particles of different sizes and agglomerations in rats: different short- and long-term post-instillation results. Toxicology. 2009;264(1-2):110–118. doi: 10.1016/j.tox.2009.08.002. [DOI] [PubMed] [Google Scholar]