Abstract
Purpose
Treatment of focal full-thickness chondral or osteochondral defects of the talus remains a challenge. The aim of this study was to evaluate the postoperative success and the long-term efficacy of matrix associated autologous chondrocyte implantation in these defects.
Methods
Matrix associated autologous chondrocyte implantation (MACI) was applied in 22 consecutive patients (mean age 23.9 years) with full-thickness chondral or osteochondral lesions of the talus. The average defect-size was 1.94 cm² (range 1–6). In case of osteochondritis dissecans (n = 13) an autologous bone graft was performed simultaneously. Follow-ups were routinely scheduled up to 63.5 (±7.4) months, consisting of clinical evaluation and magnetic resonance imaging.
Results
The AOFAS score improved significantly from 70.1 to 87.9/92.6/93.5/95.0/95.5 and 95.3 points at three, six, 12, 24, 36 and 63.5 months, respectively. On a visual analogue scale, pain intensity decreased from 5.7 (±2.6) to 0.9 (±0.8) while subjective function increased from 5.3 (±2.3) to 8.9 (±0.9) at final follow-up (each p < 0.001). The Tegner score rose significantly from 2.4 (±1.2) to 4.7 (±0.6). The MOCART score improved from 62.6 (±19.4) at three months to 83.8 (±9.4) at final follow-up. No significant differences were found between lesions caused by osteochondritis dissecans or trauma and between first- or second-line treatments. For all scores, the most benefit was seen within the first 12 months with stable results afterwards. No major complications were noted.
Conclusions
Matrix associated autologous chondrocyte implantation is capable of significant and stable long-term improvement of pain and functional impairment caused by focal full-thickness chondral and osteochondral talus lesions.
Introduction
In focal deep chondral or osteochondral lesions (OCL) of the talus, conservative treatment is restricted to stable defects in younger patients [1]. In any other condition, operative cartilage repair is indicated to restore physiological joint function and avoid secondary degeneration [2]. Common operative treatment options consist of bone marrow stimulating procedures (microfracturing, drilling, bone grafting) and osteochondral cylinder transfer (OCT) [1]. Most bone marrow stimulation procedures can be performed arthroscopically but often result in fibrocartilage and subsequent deterioration [3]. Therefore, these techniques are recommended for smaller talus defects not exceeding 1.5 cm² [4]. OCT can be delicate because of the incongruency of the donor- and recipient-site. Moreover, persistent gapping and donor-site morbidity have to be kept in mind [5]. Autologous chondrocyte implantation (ACI) is a cell-based cartilage repair technology using ex vivo cultivated chondrocytes. It has been established for the treatment of full cartilage defects of the knee joint in clinical routine with proven long-term efficacy [6]. For some years matrix associated ACI (MACI) has emerged abandoning the periosteal flap and thereby providing easier and time-saving handling [7]. This study reports on the results of matrix associated ACI for the treatment of deep chondral or osteochondral defects of the talus after five years.
Material and methods
Chondral or osteochondral lesions of the talus were diagnosed preoperatively by radiographs and magnetic resonance imaging (MRI). At index arthroscopy, the lesions were staged according to the ICRS classification [8]. Inclusion criteria were symptomatic full-thickness chondral or OCL including osteochondritis dissecans (OD) of the talus grade III to IV. All patients had a history of failed conservative or previous operative treatment. Exclusion criteria were: an unresolved ankle joint instability, a history of ankle fracture, septic ankle arthritis or rheumatic disease and neuromuscular disorders.
Clinical evaluation was based on the AOFAS score [9] rating the outcome as excellent (100–90 points), good (89–80 points), fair (79–70 points) or poor (<70 points). Additionally, the Tegner score [10] and a 10-point visual analogue scale (VAS) for the patient’s self-assessment of pain and function were conducted at three, six, 12, 24, 36 and 60 months. MRI was scheduled three, six, 12, 24 and 60 months postoperatively with coronal and sagittal planes (T1- and T2- sequences, 1.5 T, Magnetom Symphony, Siemens, Germany) and interpreted by the Magnetic Resonance Observation of Cartilage Repair Tissue scoring system (MOCART) [11].
Statistics of descriptive data were calculated by standard formulas (arithmetic mean, standard deviation, range, frequency). Comparison between paired data and independent groups was performed by using the Student’s t test or rank-sum test for not normally distributed values. Factor analysis of patient’s age, BMI, defect size, time-point of symptom onset was calculated using the Pearson product–moment correlation coefficient (r). A P value less than 0.05 was regarded as statistically significant. Statistical analysis was performed using SigmaStat (Systat Software, version 3.1, San Jose, CA, USA) software package.
Study population
Between 2003 and 2007, a total of 22 consecutive patients (17 male, five female, age range 15–43, Ø 23.9) years, mean BMI 24.0 (±5.6) kg/m²) with full-thickness chondral or osteochondral lesions (ICRS III-IV°) of the talus (17 medial, five lateral, 15 right, seven left) contributed to this study. Thirteen patients suffered from OD, and nine patients reported a major trauma (sprain). Fifteen patients had no previous surgical interventions on the ankle joint. The other seven patients had an average of 1.3 (1–2) preceding operations consisting of fragment removal and drilling (3×), retrograde drilling (3×), retrograde drilling and bone grafting (1×), fragment removal (1×) or drilling (1×). Symptom onset ranged from 1 to 36 (Ø13.2) months. The defect-size ranged from 1 to 6 cm² with a mean of 1.94 (±1.0) cm². In two cases of large OD the cartilage specimen was taken from the medial trochlear rim of the ipsilateral knee. All 13 patients with OD and one patient with post-traumatic lesion received an autologous cancellous bone grafting. In three patients a medial malleolar osteotomy was employed, one of them already had a malleolar osteotomy. One anterior triangular distal tibial wedge osteotomy was used for defect exposure. One reconstruction of the lateral collateral ankle ligaments was performed. Irrespective of malleolar osteotomy, an incision length from 4 to 5.5 cm was sufficient for the approach.
Operative technique
Primarily, all patients underwent index ankle arthroscopy in supine straight leg position using anteromedial and anterolateral standard portals. The talus lesion was staged according to the ICRS classification [8]. Unstable fragments were removed. Routinely, a full-thickness specimen was harvested from the anterior rim of the talus cartilage by an angulated curette (Aesculap AG, Tuttlingen, Germany).
Chondrocytes were subsequently cultivated and processed (Genzyme Biosurgery, Neu-Isenburg, Germany) and afterwards dispersed onto a porcine collagen type I/III scaffold (MACI®).
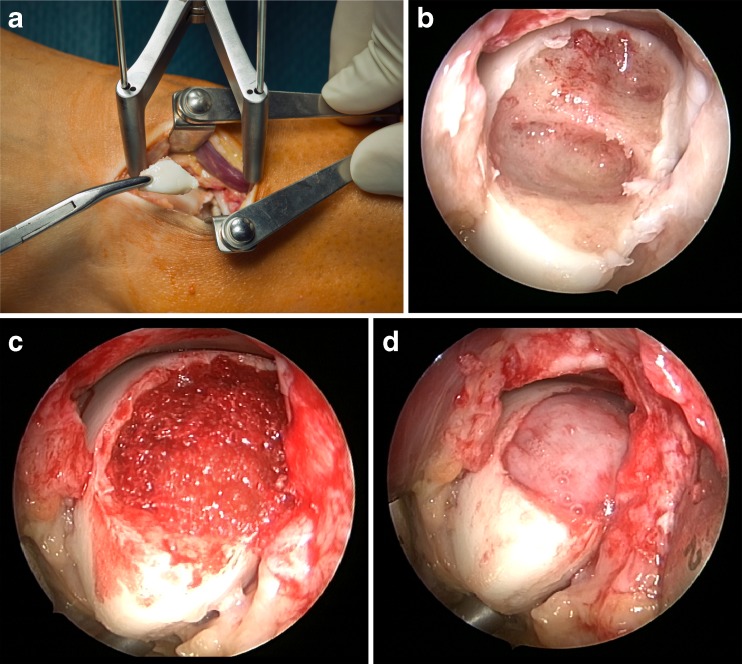
The MACI implantation was carried out through a standard anteromedial or anterolateral ankle approach. If necessary, an additional anterior triangular tibial wedge osteotomy was used. For a defect localisation posterior to the talar dome, a malleolar osteotomy was primarily performed. Using a joint distractor (Integra, Plainsboro, NJ, USA), the defect was meticulously debrided and a stable vertical rim of healthy cartilage was created. For subchondral lesions, a viable bone stock was prepared and cancellous bone grafting from the distal tibia was performed routinely using the same skin incision. A template of the defect was handcrafted from aluminium foil and transferred to the scaffold. The scaffold was then fixed by fibrin glue (Tissucol® Duo S Immuno, Baxter, Germany), (Fig. 1). Stability was critically checked by cycling the ankle joint to maximal plantar flexion and dorsal extension. In such cases, a malleolar osteotomy was refixed by two 4.5-mm malleolar screws under fluoroscopy. After wound closure the ankle joint was casted temporarily for one week in neutral position. Full range of motion was allowed immediately afterwards. No ankle bracing was provided. Postoperative rehabilitation consisted of six weeks of partial weight-bearing with 20 kg for six weeks, passive mobilisation, muscle strengthening and propioception training. Impact sports activities were not permitted for six months.
Fig. 1.
MACI implantation into a typical lateral OCL of the left talus (defect-size 3.6 cm²). The defect was exposed and the unstable fragment removed (a). A vital subchondral bone stock was prepared (b). The defect was filled with autologous cancellous bone graft from the distal anterior tibia (c). The cell loaded scaffold (MACI) was applied and sealed with fibrin glue (d)
Results
The mean follow-up was 63.5 (±7.4) months. From 21/22 patients a final follow-up at five years was available. One patient could not be located at five years, however, contributed to 36 months follow-up.
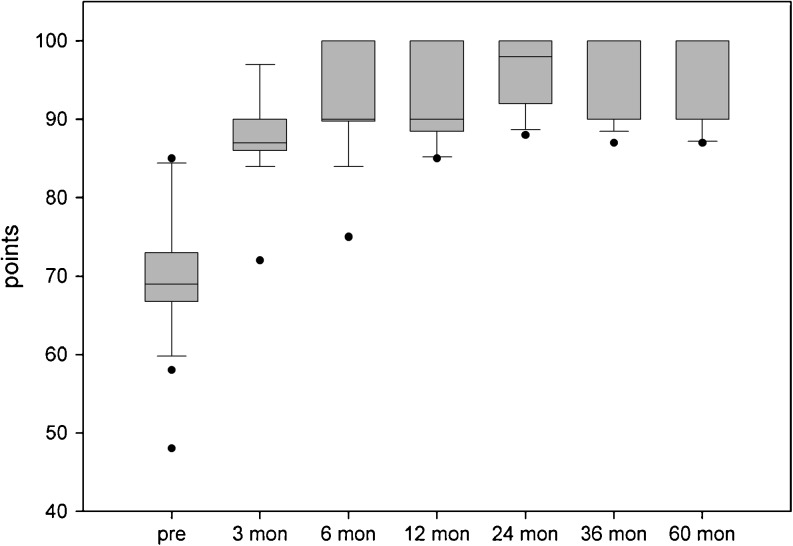
The overall AOFAS score significantly increased from 70.1 (±8.3) points preoperatively to 95.3 (±5.6) points at five years (p < 0.001, Fig. 2). No limitation in walking distance was reported. A minor limitation of dorsal extension (<10°) was found in three patients. A supporting insole was provided in 13 patients postoperatively. Ankle bandage support was used by two patients for sports. Four patients reported a temporary hypoaesthesia with regard to the superficial nerve area. No major complications occurred. In case of harvesting the cartilage specimen from the ipsilateral knee (n = 2), no donor-site morbidity was seen.
Fig. 2.
AOFAS score results
On a visual analogue scale (0–10 max.), the mean pain intensity reduced from 5.7 (±2.6) to 0.9 (±0.8) while the subjective functional status markedly increased from 5.3 (±2.3) to 8.9 (±0.9) at final follow-up. Both differences were statistically significant (each p < 0.001).
The preoperative Tegner activity level 2.4 (±1.2) differed obviously from the level reported before the onset of symptoms (4.9 ± 0.6, p < 0.001). It increased markedly to 2.8 (±1.5) at three months, 4.3 ± 1.3 at six months, 4.5 ± 0.6 at 12 months, 4.5 ± 0.7 at 24 months, 4.6 ± 0.7 at 36 months and 4.7 ± 0.6 at five years. The difference between the preclinical level and the level at five years was not significant (p = 0.53). Four patients did not reach their exact previous activity level (4 vs. 5 [3×], 5 vs. 6 [1×]) while the vast majority did. Moreover, one patient exceeded his preoperative level (6 vs. 5).
The overall MOCART score improved steadily from three to 24 months and was stable afterwards (Fig. 3). At 12 months, a complete defect filling was seen in each patient. No graft hypertrophy was observed. No patient with bone grafting or a history of bone marrow stimulation (drilling) revealed a regular subchondral bone signal (Fig. 4). There was no correlation between the mean overall MOCART score and the AOFAS score at final follow-up (p = 0.261).
Fig. 3.
MOCART score results
Fig. 4.
Representative preoperative coronal and sagittal MRI (T2-weighted) of an OD on the medial talus ICRS grade III (2.4 cm², AOFAS score 72 points) (a, b). At 63 months, the defect is completely filled by smooth repair tissue. Persistent irregularities of the subchondral bone, small cyst after cancellous bone grafting (MOCART score 70 points, AOFAS score 90 points) (c, d)
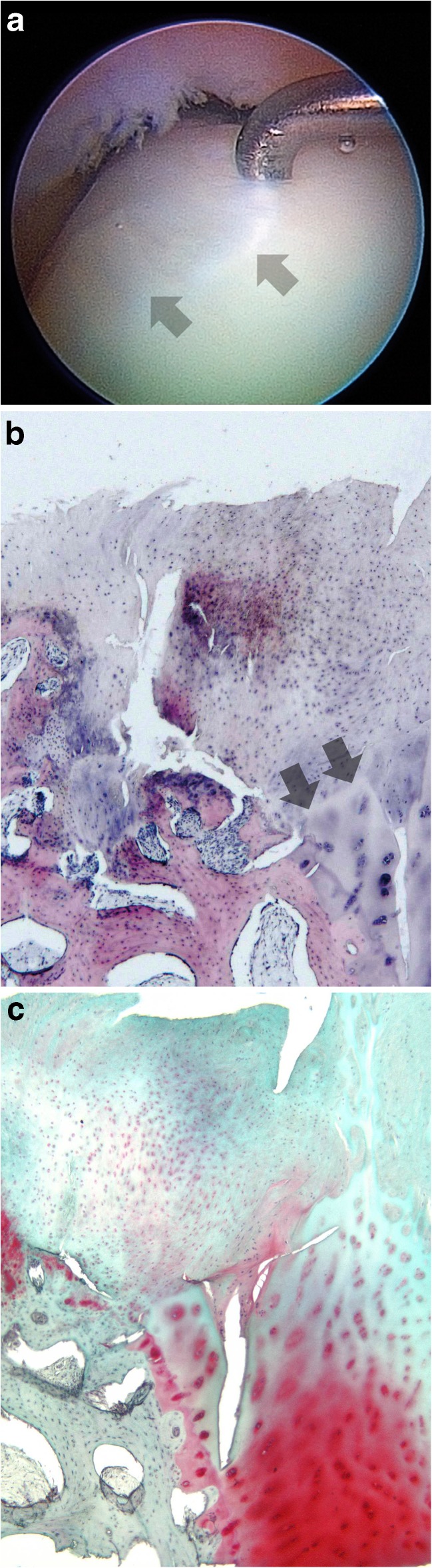
In two of those three patients with medial malleolar osteotomy a full-thickness biopsy was obtained during hardware removal (Table 1). The appearance of the transplant concerning surface structure, defect filling and integration was categorised using Brittberg’s scoring [8]. Based on criteria of cellularity, matrix composition and architectural structure both repair tissues showed hyaline-like aspects (Fig. 5).
Table 1.
Characteristics of biopsy samples
| Case number | Defect origin | Defect-size (cm²) | tbiopsy (months) | Brittberg scorea | Brittberg ratinga | AOFAS score (pre/final) |
|---|---|---|---|---|---|---|
| 1 | OD | 6 | 11 | 4/4/4 | normal | 68/100 |
| 2 | Trauma | 2.8 | 12 | 4/3/4 | nearly normal | 66/89 |
aBrittberg-score categories: filling, integration, surface (each 0 to 4 pts)
Fig. 5.
Case no. 1: The arthroscopic view shows the former defect filled completely by a smooth, well integrated but softer regenerative tissue (arrow heads) (a). A full core biopsy detects regenerative tissue tightly bonded with native hyaline cartilage on the right (arrow heads). Partly uneven surface. Numerous, viable cells with chondrocytic phenotype in the superficial zone. The junctional zone shows an increased bone remodelling after cancellous bone grafting. No tide mark is detectable (hematoxylin-eosin, magnification ×5) (b). High content of glycosaminoglycans (GAG) in native cartilage, lower GAG content in regenerative tissue (safranin-O, magnification ×5) (c)
The OD subgroup (n = 13) and the trauma subgroup (n = 9) consisted of patients of corresponding age (23.3 ± 8.3 vs. 26.4 ± 6.1 years, p = 0.12) and defect-size (2.1 ± 1.2 vs. 1.7 ± 0.5 cm², p = 0.46). Starting at the same AOFAS baseline score (70.4 ± 9.3 vs. 69.6 ± 5.8), trauma patients had a significant better score than OD patients only at three months (90.9 ± 4.6 vs. 85.7 ± 4.6, p = 0.03). At final follow-up, there was no statistical score difference (94.7 ± 5.7 [OD] vs. 96.1 ± 5.0 [trauma]).
Patients representing the second-line treatment group (n = 7) had similar demographic and baseline data compared to the first-line treatment group (n = 15), (age 26.0 ± 9.4 vs. 23.1 ± 6.6 years [p = 0.43], defect-size 1.8 ± 0.6 vs. 2.0 ± 1.1 cm² [p = 0.59]). Their AOFAS scores were slightly lower (92.4 ± 4.8 vs. 96.4 ± 5.3 at 60 months) but with significance only at six months postoperatively (85.4 ± 5.5 vs. 95.4 ± 4.6, p = 0.01).
Age, gender, BMI, defect localisation (medial/lateral), defect-size and duration of symptoms did not influence the score results (data not shown).
Discussion
In comparison to the knee, few studies of periosteal-based ACI in the ankle joint report success rates varying from 81.2 % to 100 % [12–16]. In the longest follow-up found in literature, Giannini et al. [17] accounted a series of ten patients (defect size 3.1 cm²) with a follow-up of ten years. The AOFAS score was 37.9 points at baseline, 89.4 points at 12 months, 93.9 at 36 months and 92.7 points at final follow-up. Five biopsies at hardware removal were described as organised like hyaline cartilage.
However, conventional ACI alters the surrounding intact cartilage by suturing the periosteal flap and carries the risk of periosteal hypertrophy or delamination [11]. Malleolar osteotomy is obligatory for the approach. By substituting a biodegradable scaffold, these disadvantages can be avoided. Matrix associated autologous chondrocyte implantation using a porcine collagen I/III scaffold (here: MACI®) is a representative for scaffold ACI and was demonstrated to be successful in the knee joint [7].
In our study, the preoperative functional disability was characterised as 12 “poor” and seven “fair” gradings in the AOFAS score, while only three patients were categorised as “good”. At five years, four “good” and 17 “excellent” classifications were found. Best AOFAS score increases were seen within 12 months with stable results afterwards. On questioning, nearly all patients were satisfied (4/22) or very satisfied (17/22) with their result. The majority of patients returned to their previous activity level. One patient was dissatisfied due to a minor lack of dorsal ankle extension after medial malleolar osteotomy. Nevertheless, all other patients would repeat this procedure regardless.
MRI has become more important for morphological evaluation of the repair tissue after cartilage repair procedures in the ankle [18, 19]. In our study, the MOCART score increased over time indicating radiological maturation towards a normal hyaline signal of the repair tissue. However, incomplete bone remodelling after previous damage to the subchondral bone and lamina was observed subsequently but did not affect the clinical outcome. In conformity with the study of Aurich [19], we could not detect correlation between the MOCART score and AOFAS score.
There are few substantial reports about scaffold ACI in the ankle joint. Giannini et al. [20] presented 16 patients treated for osteochondral lesions featuring a significant AOFAS score increase from 54.2 points to 89 points at 12 months. Giza et al. [21] contributed ten patients with full-thickness lesion and stated an improvement of the AOFAS score from 61.2 to 74.7 points at one year and 73.3 points at two years thus reflecting a success rate of 50 %. Schneider et al. [22] applied scaffold ACI in osteochondral defects in 20 patients. Despite significant AOFAS improvement from 60 to 87 points at 21.1 months, two failures needing further procedures were reported. Lee et al. [23] found age and size as influencing factors of cartilage repair as observed by second-look arthroscopy in 38 patients 12 months after ACI using a fibrin gel and mandatory osteotomy.
Two studies of arthroscopic scaffold ACI can be found in literature. Aurich et al. [19] reported an increase of the AOFAS from 58.6 to 80.4 points at a mean follow-up of 24.5 months representing 64 % excellent and good and 36 % fair and poor results. They treated 18 patients with a mean defect size of 1.5 cm². The final MOCART score of 62.4 points did not correlate with clinical findings. In another study by Giannini et al. [24], the mean preoperative AOFAS score of 38 patients (mean defect-size 1.6 cm²) was 57.2 points and increased to 86.8 points at 12 months and to 89.5 points at 36 months. There were 25 excellent (55.0 %), 13 good (27.5 %), six fair (12.5 %), and two poor (5 %) ratings at final follow-up. Both studies treated selected populations consisting of primary interventions without bone grafting [19] or post-traumatic lesions excluding OD [24].
As in any other study of MACI on the talus, our study is a prospective case-series only [25]. With respect to the literature, our study contributes a reasonable sample size and dedicated long-term follow-up emphasising the longevity of MACI in the talus. The quota of good or excellent results in literature for matrix-associated techniques on the talus lesions vary from 50 % to 100 % reflecting nonhomogeneous populations and pathologies addressed, different study designs, follow-ups, scaffolds and different surgical techniques. The results of our study are consistent with these findings reflecting a high and stable postoperative outcome in the AOFAS score and patient-derived evaluation. In contrast to other shorter-termed studies, we could not detect age, size, previous intervention or duration of symptoms as influencing factors for the clinical outcome [11, 17, 19, 23]. ACI is a proven sophisticated cell-based technique for cartilage repair. Nevertheless, the disadvantages of a cost-intensive two-step procedure have to be considered. For further evidence, long-term comparative studies comparing different repair strategies for OCL in the talus must be set up.
Conclusion
MACI is capable of significant and stable long-term improvement of pain and functional impairment caused by focal full-thickness chondral and osteochondral talus lesions. The minimally invasive technique described proved successful both for chondral and osteochondral defects and for first- and second-line treatments.
Acknowledgments
Conflict of interest
One of the authors has declared a potential conflict of interest: Genzyme Biosurgery provided reimbursement for educational activities (S.A.). The authors did not receive any financial support for the development and preparation of this article.
References
- 1.Zengerink M, Struijs PA, Tol JL, Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 3.Buckwalter JA, Lohmander S. Operative treatment of osteoarthrosis. Current practice and future development. J Bone Joint Surg Am. 1994;76:1405–1418. doi: 10.2106/00004623-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004;25:168–175. doi: 10.1177/107110070402500311. [DOI] [PubMed] [Google Scholar]
- 5.Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84:237–244. doi: 10.1302/0301-620X.84B2.11735. [DOI] [PubMed] [Google Scholar]
- 6.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 9.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 10.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop. 1985;198:43–49. [PubMed] [Google Scholar]
- 11.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291–303. doi: 10.1016/S1083-7515(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 13.Koulalis D, Schultz W, Heyden M (2002) Autologous chondrocyte transplantation for osteochondritis dissecans of the talus. Clin Orthop Relat Res 395:186–192 [DOI] [PubMed]
- 14.Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179–183. doi: 10.1302/0301-620X.87B2.15376. [DOI] [PubMed] [Google Scholar]
- 15.Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88:303–308. doi: 10.2106/JBJS.E.00033. [DOI] [PubMed] [Google Scholar]
- 16.Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med. 2009;37:274–284. doi: 10.1177/0363546508325670. [DOI] [PubMed] [Google Scholar]
- 17.Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(Suppl 1):112S–118S. doi: 10.1177/0363546509349928. [DOI] [PubMed] [Google Scholar]
- 18.Choi YS, Potter HG, Chun TJ. MR imaging of cartilage repair in the knee and ankle. Radiographics. 2008;28:1043–1059. doi: 10.1148/rg.284075111. [DOI] [PubMed] [Google Scholar]
- 19.Aurich M, Bedi HS, Smith PJ, Rolauffs B, Muckley T, Clayton J, Blackney M. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 20.Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, Grigolo B, Berti L. Surgical treatment of osteochondral lesions of the talus in young active patients. J Bone Joint Surg Am. 2005;87(Suppl 2):28–41. doi: 10.2106/JBJS.E.00516. [DOI] [PubMed] [Google Scholar]
- 21.Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, Walton J. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 2010;31:747–753. doi: 10.3113/FAI.2010.0747. [DOI] [PubMed] [Google Scholar]
- 22.Schneider TE, Karaikudi S. Matrix-Induced Autologous Chondrocyte Implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810–814. doi: 10.3113/FAI.2009.0810. [DOI] [PubMed] [Google Scholar]
- 23.Lee KT, Lee YK, Young KW, Park SY, Kim JS (2012) Factors influencing result of autologous chondrocyte implantation in osteochondral lesion of the talus using second look arthroscopy. Scand J Med Sci Sports 22(4):510–515 [DOI] [PubMed]
- 24.Giannini S, Buda R, Vannini F, Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873–880. doi: 10.1177/0363546507312644. [DOI] [PubMed] [Google Scholar]
- 25.Niemeyer P, Salzmann G, Schmal H, Mayr H, Sudkamp NP (2011) Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. Oct 30. [Epub ahead of print] [DOI] [PubMed]