Abstract
Purpose
A prospective study was undertaken to evaluate the diagnostic and prognostic significance of serum levels of vascular endothelial growth factor (VEGF) in patients with primary localised osteosarcoma.
Methods
Serum VEGF levels were measured by an enzyme-linked immunosorbent assay (ELISA) in blood samples collected prechemotherapy, postchemotherapy, and postsurgery in 40 patients with histologically proven primary osteosarcoma. Comparison was made between serum VEGF level of healthy controls (n = 10) and prechemotherapy patient sera to evaluate its diagnostic potential. Serum VEGF levels of patients with and without metastasis were compared. Immunohistochemical staining was done to establish the correlation between serum and tissue VEGF expression. The Kaplan–Meier curve was used for survival analysis
Results
No significant relationship was observed between serum VEGF levels and age, gender, tumour size, local recurrence or histopathological subtypes of osteosarcoma. We observed significantly raised mean serum VEGF in patient sera compared with healthy controls (p = 0.001). Significant fall in mean serum VEGF level was observed following chemotherapy (p = 0.001). Patients who developed metastases had significantly higher serum VEGF levels compared with the nonmetastatic group (P = 0.001). Serum VEGF levels correlated well with VEGF expression in tissues.
Conclusion
Serum VEGF levels might prove to be of diagnostic, predictive and prognostic value in patients with primary osteosarcoma, although further studies with larger sample size and longer follow-up is needed to support the hypothesis.
Introduction
Osteosarcoma is the most common malignant bone tumour in adolescents and young adults. Despite recent advances in multimodality treatments consisting of aggressive neoadjuvant chemotherapy and wide tumour resection, mortality and morbidity rates from high-grade osteosarcoma remains high. Approximately 32–49 % of patients whose life expectancy after being diagnosed with high-grade osteosarcoma is usually less than 60 months [1]. New blood-vessel formation (angiogenesis) is a fundamental event in the process of tumour growth and metastatic dissemination [2]. Vascular endothelial growth factor (VEGF), a homodimeric protein, is the most potent stimulator of angiogenesis [3]. It is a major angiogenic factor that induces endothelial cell proliferation and increases permeability of the vascular endothelium [4]. Tissue expression of VEGF correlates well with tumour progression and has been shown to be of prognostic significance in many malignancies [5–9]. Serum VEGF level may be a surrogate marker of tumour VEGF expression in patients with osteosarcoma, as—similar to tissue expression—it has prognostic significance [10–16], with significant association between a high VEGF expression in the primary tumour and poor prognosis [17, 18]. Due to the central role of VEGF in tumour angiogenesis, the VEGF–VEGF receptor (VEGFR) pathway has become a major focus of research and antiangiogenic drug development in oncology [3]. Although a close relationship between a high serum VEGF levels and poor prognosis has been suggested [19, 20], the lack of prospective evaluation has made its value uncertain for predicting prognosis in patients with osteosarcoma. Measuring serum VEGF levels using enzyme-linked immunosorobent assay (ELISA) appears to be a more promising quantification procedure than immunohistochemical staining for tissue VEGF [4]. The aim of this study was to evaluate the potential role of serum VEGF assay as a diagnostic, predictive, and prognostic marker in terms of pulmonary metastasis and survival in patients with osteosarcoma.
Material and methods
This prospective study was conducted in the Department of Orthopaedics of a tertiary care referral centre from November 2007 to November 2009 after approval by the institutional ethics committee. Patients with histopathologically proven [core biopsy from the most active part of the lesion as seen on magnetic resonance imaging (MRI) images and histopathological analysis] diagnosis of osteosarcoma without prior chemotherapy or radiotherapy were invited to take part in the study. All participants had high-grade, localised extremity osteosarcoma (Enneking stage II), and all provided written informed consent. Staging work-up included noncontrast computed tomography (NCCT) of the chest to rule out pulmonary metastasis, MRI and X-ray for local staging and bone scan to rule out distant skeletal metastasis. Patients with secondary malignancies, prior chemoradiotherapy or surgery for the malignancy, or with pulmonary or nonpulmonary distant metastasis at presentation were excluded from the study. All patients were managed according to the standardised treatment protocol consisting of neoadjuvant chemotherapy (three cycles of doxorubicin and cisplatin were administered with a minimum of a 21-day interval between consecutive cycles), followed by appropriate surgical management and postoperative adjuvant chemotherapy (three cycles of doxorubicin and cisplatin). Prechemotherapy, postchemotherapy, and postsurgery blood samples (5 ml) were collected for serum VEGF analysis. VEGF half-life in circulation is only three minutes; postchemotherapy and postsurgery samples were collected one day after the last cycle of chemotherapy and surgery, respectively, giving adequate time for changes to manifest. Blood samples from ten healthy age- and sex-matched volunteers were collected to act as control. If there was a delay in measuring VEGF levels, then the serum was separated and stored at −20° to avoid loss of bioactive serum VEGF. Human VEGF ELISA kit (Bender Medsystem, Austria) was used to assess serum VEGF levels. To analyse the correlation between serum and tissue VEGF levels, the tumour tissue obtained during biopsy was subjected to immunohistochemical staining (avidin–biotin complex method) for VEGF. The volume of primary tumour was assessed on longitudinal and transverse MRI performed before biopsy and calculated with formula: π/6 × height × width × depth [21].
Statistical analyses were performed using SPSS 17 software. The Mann–Whitney U test was used to compare serum concentrations of patients with those of the control group. Correlation was analysed using Pearson correlation test. Matched-paired samples (pretherapeutic and post-therapeutic) were analysed with the Wilcoxon matched-pairs signed-rank test. Kaplan–Meier curves for overall (OS) and disease-free (DFS) survival were analysed using the log-rank test.
Results
Of 40 patients with histopathological diagnosis of osteosarcoma, 27 were males and 13 were females; mean age of the patient was 15.98 years. Ten healthy volunteers (mean age 21.45 years) were selected as controls. Mean follow-up period from diagnosis was 15 (range 8–24) month. Mean tumour volume was 2,272.55 cm3, with a standard deviation (SD) 3,439.88 cm3. Nineteen patients developed pulmonary metastasis during the study period. Mean and median serum VEGF level in patients with osteosarcoma was 4,854.48 pg/ml and 4,545.00 pg/ml, respectively, (SD 2,678.56 pg/ml).
Association between VEGF and different clinicopathological variables
No significant relationship was observed between serum VEGF and age (p = 0.86), gender (p = 0.145), or tumour volume (p = 0.336). Serum VEGF levels in patients with osteosarcoma were significantly higher than in healthy controls (p < 0.001).
Association between serum VEGF and different histopathological subtypes of osteosarcoma
On the basis of histopathological examination of core biopsy specimens from 40 patients, the following osteosarcoma subtypes were diagnosed;
High-grade conventional (n = 24, mean serum VEGF level; 5,306.47 pg/ml)
Chondroblastic (n = 12, mean serum VEGF level; 2,405.57 pg/ml)
Telangiectatic (n = 2, mean serum VEGF level; 4,615.19 pg/ml)
Parosteal (n = 2, mean serum VEGF level;3,314.70 pg/ml)
We did not observe a correlation between these histopathological subtypes and serum VEGF level (p = 0.386).
Correlation between tissue expression and serum VEGF levels in osteosarcoma patients
Results of immunohistochemical staining of biopsy tissue for VEGF levels were interpreted in terms of percentage of cells with VEGF expression and differential staining pattern [degree of staining with haematoxylin and eosin (H&E)], and the relationship was tested by one-way analysis of variance (ANOVA). Statistically significant correlation was found between high serum VEGF levels and a high percentage of cells showing VEGF expression (p = 0.001) and degree of staining (p = 0.002) (Tables 1 and 2) (Figs. 1a, b, 2a, b, and 3a, b).
Table 1.
Correlation between tissue and serum in terms of percentage of cells staining positive for vascular endothelial growth factor (VEGF)
| Cells showing VEGF expression | No. | Mean (pg/ml) | Standard deviation (pg/ml) | Standard error (pg/ml) | 95 % confidence Interval for mean (pg/ml) | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| <50 % | 10 | 2,253.85 | 694.895 | 219.745 | 1,756.75 | 2,750.95 |
| >50 % | 10 | 7,005.99 | 3,240.477 | 1,024.729 | 4,687.89 | 9,324.09 |
| Total | 20 | 4,629.92 | 3,338.501 | 746.511 | 3,067.45 | 6,192.39 |
Table 2.
Correlation between tissue and serum expression of vascular endothelial growth factory (VEGF) in terms of differential staining
| Staining | No. | Mean (pg/ml) | Standard deviation (g/ml) | Standard error (pg/ml) | 95 % confidence interval for mean (pg/ml) | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| 1+ | 7 | 2,235.22 | 850.264 | 321.370 | 1,448.86 | 3,021.59 |
| 2+ | 5 | 3,420.09 | 1,567.860 | 701.168 | 1,473.33 | 5,366.84 |
| 3+ | 8 | 7,481.42 | 3,486.434 | 1232.641 | 4,566.69 | 1,0396.16 |
| Total | 20 | 4,629.92 | 3,338.501 | 746.511 | 3,067.45 | 6,192.39 |
Fig. 1.
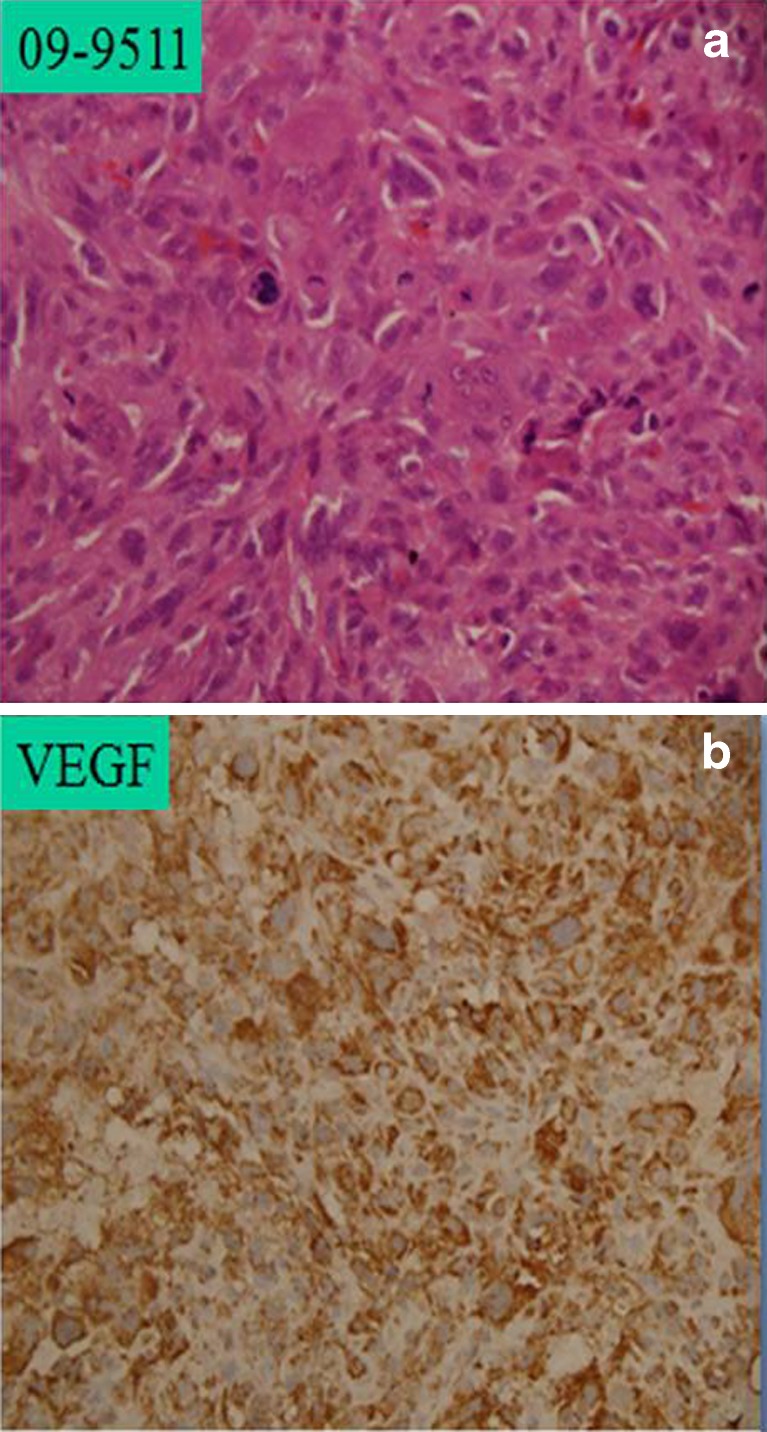
a Prechemotherapy high-grade osteosarcoma with 3+ haematoxylin and eosin (H&E) staining. b Prechemotherapy with strong immunopositivity for vascular endothelial growth factor (VEGF), approximately >95 % cells showing VEGF expression
Fig. 2.
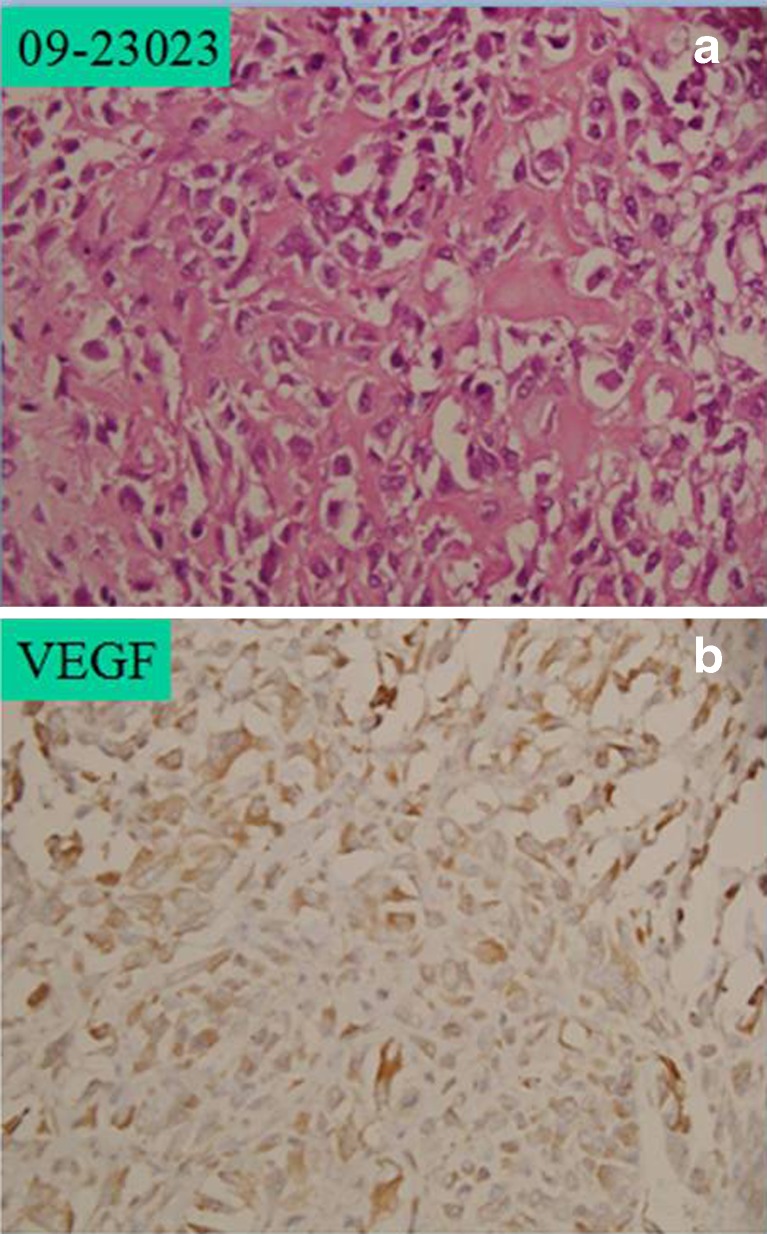
a Prechemotherapy high-grade osteosarcoma with 2+ haematoxylin and eosin (H&E) staining. b Prechemotherapy with intermediate immunopositivity for vascular endothelial growth factor (VEGF), approximately >50 % cells showing VEGF expression
Fig. 3.
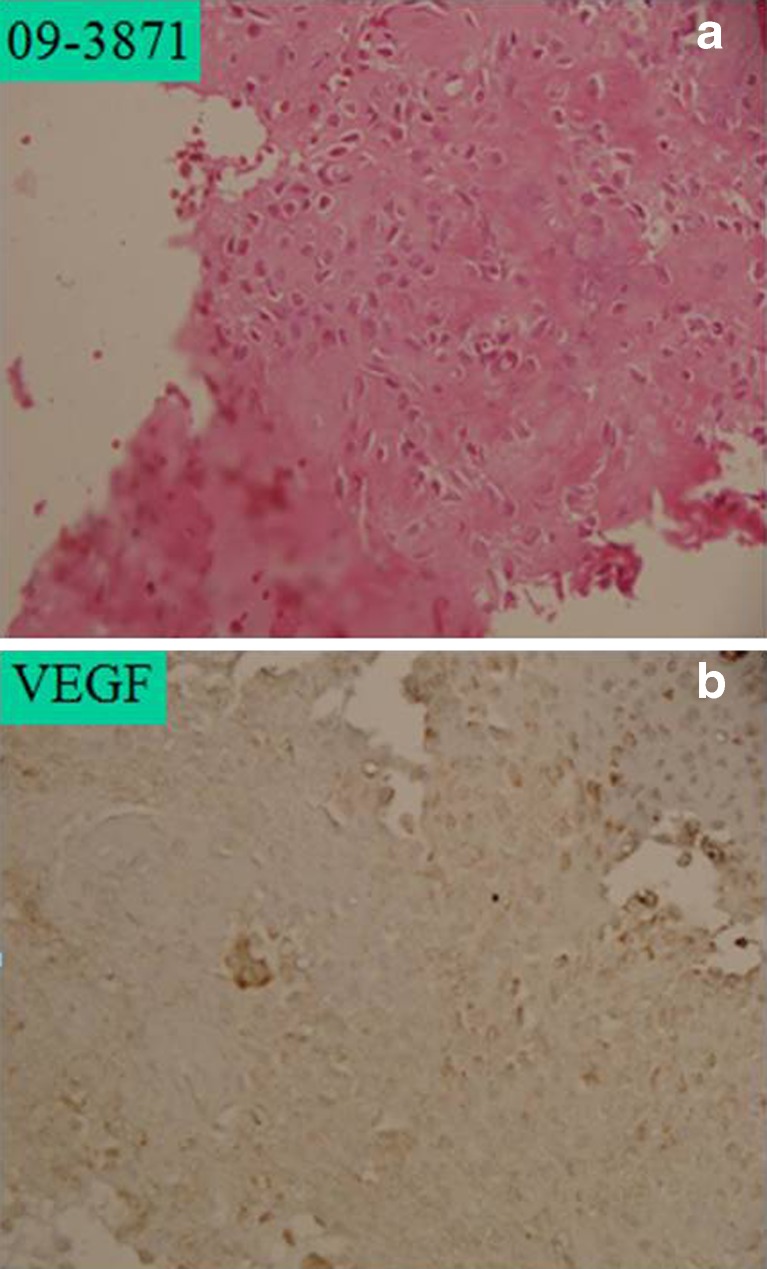
a Prechemotherapy high-grade osteosarcoma with 1+ hematoxylin and eosin (H&E) staining. b Prechemotherapy with low immunopositivity for vascular endothelial growth factor (VEGF), approximately 10 % cells showing VEGF expression
Serum VEGF level following therapeutic intervention
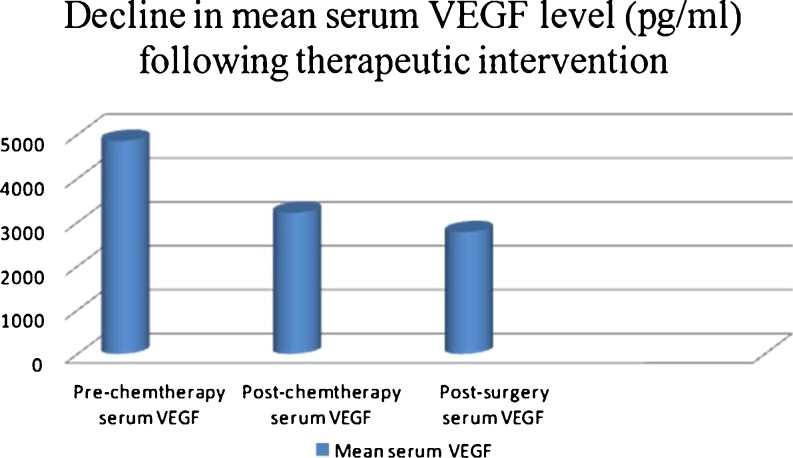
Mean serum VEGF levels measured following three cycles of chemotherapy demonstrated a statistically significant decline compared with prechemotherapy levels (p = 0.001). No statistical correlation was found on comparison of postchemotherapy and postsurgery serum VEGF levels (mean postsurgery level 2,774.08 pg/ml, p = 0.480); however, the overall trend of decline in serum VEGF levels prechemotherapy, postchemotherapy, and postsurgery (p = 0.005) was statistically significant (Fig. 4).
Fig. 4.
Decline in serum vascular endothelial growth factor (VEGF) level following chemotherapy (p = 0.001) and surgery (p = 0.480) in patients with osteosarcoma
Association of serum VEGF level, pulmonary metastasis, and local recurrence
Patients who developed pulmonary metastasis had higher baseline (prechemotherapy) mean serum VEGF compared with those who did not during a maximum follow-up of 24 months (p = 0.001). This suggests the prognostic role of VEGF in osteosarcoma patients. There was no correlation observed between serum VEGF level and local recurrence (Table 3).
Table 3.
Relationship between mean serum vascular endothelial growth factor (VEGF) level and pulmonary metastasis
| Pulmonary metastasis | Number of patients | Mean serum VEGF level | P value |
|---|---|---|---|
| Yes | 19 | 6278.78 pg/ml | 0.001 |
| No | 21 | 3565.77 pg/ml |
Overall survival in osteosarcoma patients in relation to various clinico pathological variables (Table 4)
Table 4.
Comparison of overall survival data for various groups of patients with osteosarcoma
| Variable | No. | Mean survival (months) | 95 % confidence interval (months) | Survival at 1 year | P value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Gender | ||||||
| Male | 27 | 15.57 | 13.92 | 17.21 | 83.5 % | 0.667 |
| Female | 13 | 15.87 | 13.63 | 18.11 | 73.8 % | |
| Age | ||||||
| <20 years | 31 | 15.45 | 13.89 | 17.00 | 81.6 % | 0.327 |
| >20 years | 9 | 16.88 | 14.48 | 17.08 | 88.9 % | |
| Pulmonary metastasis | ||||||
| Yes | 19 | 14.63 | 12.58 | 16.69 | 72.4 % | 0.088 |
| No | 21 | 16.98 | 15.62 | 18.35 | 94.7 % | |
P value (log rank test) for comparison of survival data between groups. Significance p < 0.05 (univariate analysis of prognostic factors for overall survival)
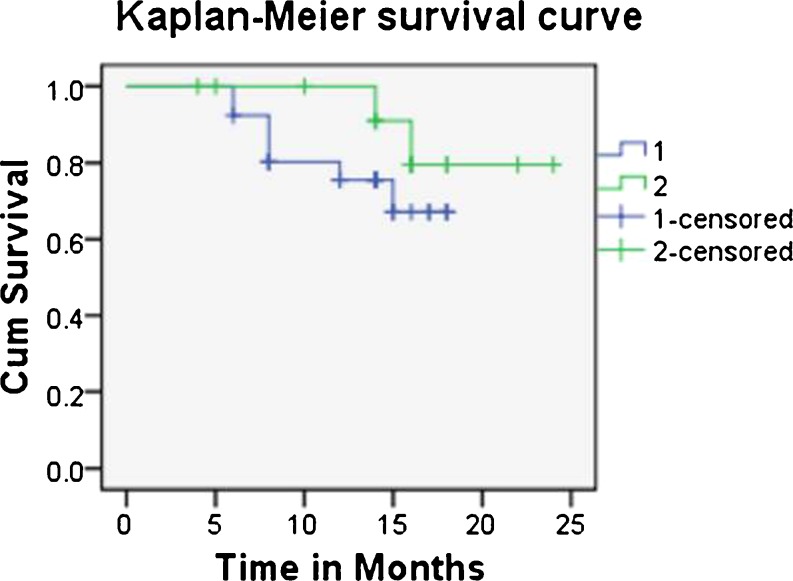
There was no significant difference in terms of OS between genders. Mean survival in male patients was 13.92 (95 % CI 13.92–17.21) months and in female patients 15.87 (95 % CI: 13.63–18.11) months from diagnosis. Patients over 20 years had slightly better survival at year (88.9 %) compared with patients under 20 years (81.6 %). Nineteen of 40 patients developed pulmonary metastasis; their prognosis was poor (mean survival 14.63 months, CI 12.58–16.69), with a one year survival rate of 72.4 % compared with patients without pulmonary metastasis (one year survival 94.9 %, mean survival 16.88 months). Patients with mean serum VEGF level over 3,500 pg/ml had poor mean OS of 15.34 months compared with 22.18 months in patients with mean serum VEGF under 3,500 pg/ml (p = 0.228) (Table 5, Fig. 5)
Table 5.
Comparison of overall survival (OS) with mean serum vascular endothelial growth factor (VEGF) level as the variable in patients with osteosarcoma
| Variable (mean serum VEGF level) | Number of patients | Mean survival (month) | 95 % CI (lower limit in months) | 95 % CI (upper limit in months) | Cumulative survival at 2 years | P value |
|---|---|---|---|---|---|---|
| >3,500 pg/ml | 26 | 15.34 | 13.62 | 17.05 | 67.2 % | 0.282 |
| <3,500 pg/ml | 14 | 22.18 | 19.92 | 23.44 | 79.5 % |
CIconfidence interval, P (log rank test) value for the comparison of survival data between groups. Significance p < 0.05
Fig. 5.
Kaplan–Meier survival estimate as a function of prechemotherapy serum vascular endothelial growth factor (VEGF) level in the osteosarcoma group with 95 % confidence intervals (CI). Ticks represent censoring of patients at that time point (1: >3,500 pg/ml, 2: <3,500 pg/ml; months)
Discussion
The role of VEGF as a regulator of angiogenesis in both normal tissue and neoplastic growth is well documented. VEGF and VEGFR bring about regulation of vascular and lymphatic endothelium and play a key role in tumour progression by promoting angiogenesis and lymph angiogenesis [22–24]. VEGF expression in neoplastic tissue quantified by immunohistochemical analysis has been shown to correlate with prognosis. Similarly, genetic detection of VEGF expression by tumour tissue correlates with prognosis. However, both these methods required advanced laboratory support for detection and estimation and have not gained significant clinical utility. VEGF can also be detected and quantified in serum using ELISA. Serum VEGF levels correlate well with tissue VEGF expression. Measuring VEGF level is advantageous as a simple and rapid procedure. Significant association between serum VEGF level and microvessel density in osteosarcoma patients have been reported [4]. In our analysis statistically significant correlation was observed between serum and tissue expression levels of VEGF before any therapeutic intervention in terms of percentage of cells showing VEGF expression (P = 0.001) and microscopic differential staining pattern in the biopsy specimen (P = 0.002). Serum VEGF level was significantly higher in patients compared with healthy controls (p = 0.001). Several other studies also reported high serum VEGF levels in patients with bone sarcoma compared with healthy controls [4, 19, 21, 25]. No significant correlation was documented between serum VEGF expression and age, sex, and tumour volume, which is consistent with finding of other studies [4, 19, 21, 25]. Interestingly, a study on VEGF levels in ten patients shows higher VEGF expression in female compared with male (p < 0.05) patients, but the small sample size may have contributed to that significant correlation [17]. The large tumour size in that study group may be attributed to relative lack of awareness about the disease in the Indian population and significant delay in reaching the referral centres. Some studies [17–19] correlated histological types of osteosarcoma with VEGF expression and found no significant association; we found a similar observation in our study (p = 0.386). Only four patients developed local recurrence over the entire follow-up period.
We tried to establish a correlation between prechemotherapy serum VEGF level and response to neoadjuvant chemotherapy and found that decline in serum VEGF level following neoadjuvant chemotherapy was statistically significant (p = 0.001). Similar findings are reported by several authors [4, 21, 25, 26]. We found no statistically significant decline in serum VEGF concentration following surgery in osteosarcoma patients (p = 0.480) but the overall trend of decline in serum VEGF level prechemotherapy—postchemotherapy—postsurgery was statistically significant (p = 0.005). Therefore, VEGF status might be of potential value for predicting the effectiveness of conventional chemotherapy in patients with osteosarcoma, although this statement cannot be generalised because a standard chemotherapy protocol was used for all patients in the study, and other chemotherapy regimens can show different response and prognosis. Questions remain as to whether an insufficient decrease in VEGF during the course of neoadjuvant chemotherapy could be indicative for the change in chemotherapy prior to surgery. Further studies are required to answer this question by comparing the effect of different standard chemotherapy regimens on serum VEGF level. Several studies show that VEGF can be up-regulated in tumour tissues of various histologies; therefore, it seems likely that tumour tissue is an important source of circulating VEGF in tumour patients. This explains the decline in serum VEGF following chemotherapy and surgical excision of the tumour. Although serum VEGF level was higher in the relapse group compared with the no-relapse group, association was insignificant (0.249). This is in contrast with findings of other studies [4, 27] and is probably due to low sample size and shorter follow-up period. The role of VEGF in chemosensitivity and chemoresistance in vivo is conflicting [28]: Cases with pulmonary metastasis showed higher serum VEGF levels compared with those without pulmonary metastasis (p = 0.001). Similar findings are reported by other authors [21, 28]. No correlation was observed between VEGF expression and OS. We chose a cutoff value of 3,500 pg/ml to compare survival, as mean serum VEGF level in patients with pulmonary metastasis, which is an independent factor for survival, was over 3,500 pg/ml. In contrast, some authors [18, 21, 27, 28] report a high incidence of pulmonary metastasis in patients with high VEGF and subsequently low survival rate and overall poor prognosis.
To conclude, we observe no correlation between serum VEGF, age, sex, tumour volume, or different histopathological subtypes of osteosarcoma. However, we did observe a significant decline in serum VEGF levels following three cycles of chemotherapy with doxorubicin and cisplatin, suggesting its predictive role, but this statement cannot be generalised, as different standard chemotherapy regimens are being used worldwide, and further study with other regimens is required to support this hypothesis. The other conclusion that can be made from our study is that patients with localised osteosarcomas when treated with three cycles of preoperative chemotherapy and adequate resection showed a higher risk of developing pulmonary metastases if serum VEGF levels were elevated. This reflects only a higher tumour volume of micrometastasis at the time of diagnosis. A trend for a poor response to the given chemotherapy regimen cannot be stated with certainty, but one observation that can be made is high serum levels of VEGF, which might be an indication for a more aggressive type of chemotherapy than the one used. Studies with a larger sample size and longer follow-up will be required to support the diagnostic and prognostic role of VEGF in osteosarcoma patients.
The findings of a correlation between high serum levels and poor prognosis may have a therapeutic implication. Active angiogenesis plays an important role in tumour progression in osteosarcoma patients. The use of antiangiogenic therapy in combination with systemic cytotoxic drugs may suppress tumour angiogenesis and lead to better lesion control.
Acknowledgments
The authors have not received any fund or grant from any professional body or institution. We thank Dr. Shyam Prakash for helping in laboratory analysis of the serum samples. We also thank Mr. Guresh Kumar for statistical support.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Picci P, Mercuri M, Ferrari S, Alberghini M, Briccoli A, Ferrari C, et al. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol. 2010;21(6):1366–1373. doi: 10.1093/annonc/mdp502. [DOI] [PubMed] [Google Scholar]
- 2.Trueta J, Amato VP. The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg Br. 1960;42-B:571–587. doi: 10.1302/0301-620X.42B3.571. [DOI] [PubMed] [Google Scholar]
- 3.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 4.Kaya M, Wada T, Kawaguchi S, Nagoya S, Yamashita T, Abe Y, et al. Increased pre-therapeutic serum vascular endothelial growth factor in patients with early clinical relapse of osteosarcoma. Br J Cancer. 2002;86(6):864–869. doi: 10.1038/sj.bjc.6600201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132(5):541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 6.Linderholm B, Tavelin B, Grankvist K, Henriksson R. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J Clin Oncol. 1998;16(9):3121–3128. doi: 10.1200/JCO.1998.16.9.3121. [DOI] [PubMed] [Google Scholar]
- 7.Imoto H, Osaki T, Taga S, Ohgami A, Ichiyoshi Y, Yasumoto K. Vascular endothelial growth factor expression in non-small-cell lung cancer: prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115(5):1007–1014. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Tsujitani S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Expression of vascular endothelial growth factor correlates with hematogenous recurrence in gastric carcinoma. Surgery. 1999;125(2):195–201. doi: 10.1016/S0039-6060(99)70265-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith BD, Smith GL, Carter D, Sasaki CT, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol. 2000;18(10):2046–2052. doi: 10.1200/JCO.2000.18.10.2046. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19(4):1207–1225. doi: 10.1200/JCO.2001.19.4.1207. [DOI] [PubMed] [Google Scholar]
- 11.Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S. Elevation of serum level of vascular endothelial growth factor as a new predictor of recurrence and disease progression in patients with superficial urothelial cancer. Urology. 1999;53(2):302–307. doi: 10.1016/S0090-4295(98)00486-5. [DOI] [PubMed] [Google Scholar]
- 12.Werther K, Christensen IJ, Nielsen HJ. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer. 2002;86(3):417–423. doi: 10.1038/sj.bjc.6600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Circulating VEGF levels in the serum of gastric can… [Ann Surg. 2002]—PubMed—NCBI [Internet]. [cited 2012 Jan 2]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12131083
- 14.Teknos TN, Cox C, Yoo S, Chepeha DB, Wolf GT, Bradford CR, et al. Elevated serum vascular endothelial growth factor and decreased survival in advanced laryngeal carcinoma. Head Neck. 2002;24(11):1004–1011. doi: 10.1002/hed.10163. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BC, Ritchie JM, Broghammer CLW, Coffin J, Sorosky JI, Buller RE, et al. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin Cancer Res. 2002;8(10):3193–3197. [PubMed] [Google Scholar]
- 16.Poon RTP, Ho JWY, Tong CSW, Lau C, Ng IOL, Fan S-T. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 2004;91(10):1354–1360. doi: 10.1002/bjs.4594. [DOI] [PubMed] [Google Scholar]
- 17.Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6(2):572–577. [PubMed] [Google Scholar]
- 18.Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, et al. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer. 1999;35(7):1089–1093. doi: 10.1016/S0959-8049(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 19.Holzer G, Obermair A, Koschat M, Preyer O, Kotz R, Trieb K. Concentration of vascular endothelial growth factor (VEGF) in the serum of patients with malignant bone tumors. Med Pediatr Oncol. 2001;36(6):601–604. doi: 10.1002/mpo.1136. [DOI] [PubMed] [Google Scholar]
- 20.Rutkowski P, Kamińska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84(3):151–159. doi: 10.1002/jso.10305. [DOI] [PubMed] [Google Scholar]
- 21.Kaya M, Wada T, Nagoya S, Sasaki M, Matsumura T, Yamashita T. The level of vascular endothelial growth factor as a predictor of a poor prognosis in osteosarcoma. J Bone Joint Surg Br. 2009;91(6):784–788. doi: 10.1302/0301-620X.91B6.21853. [DOI] [PubMed] [Google Scholar]
- 22.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 23.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 24.Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7(2):199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 25.Pavlakovic H, Schütz V, Rössler J, Koscielniak E, Havers W, Schweigerer L. Quantification of angiogenesis stimulators in children with solid malignancies. Int J Cancer. 2001;92(5):756–760. doi: 10.1002/1097-0215(20010601)92:5<756::AID-IJC1253>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011;20(1):13–19. doi: 10.1016/j.suronc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Kreuter M, Paulussen M, Boeckeler J, Gerss J, Buerger H, Liebscher C, et al. Clinical significance of vascular endothelial growth factor-A expression in Ewing’s sarcoma. Eur J Cancer. 2006;42(12):1904–1911. doi: 10.1016/j.ejca.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 28.Mikulić D, Ilić I, Cepulić M, Orlić D, Giljević JS, Fattorini I, et al. Tumour angiogenesis. and outcome in osteosarcoma. Pediatr Hematol Oncol. 2004;21(7):611–619. doi: 10.1080/08880010490501015. [DOI] [PubMed] [Google Scholar]