Abstract
Purpose
Osteotomies of the proximal femur and stable fixation of displaced femoral neck fractures are demanding operations. An LCP Paediatric Hip Plate™ was developed to make these operations safer and less demanding. The article focuses on the surgical technique and critically analyses the device.
Methods
Between 2006 and 2008, 30 hips in 22 patients underwent surgery. Patients’ demographics, perioperative details, postoperative outcome and complications were retrospectively collected and analysed.
Results
Patients’ diagnoses included persistent congenital hip dysplasia (n = 4), neuropathic hip dysplasia (n = 9), idiopathic ante/retroversion (n = 8), femoral neck fracture (n = 3), Perthes’ disease (n = 2), deformity after slipped capital femoral epiphysis (SCFE), congenital femoral neck pseudarthrosis, deformity after pelvic tumour resection and malunion following proximal femoral fracture (one each). In 21 of 22 patients, the postoperative radiographs showed corrections as planned. Two cases had to be revised for screw loosening. Intraoperative handling using the plate was excellent in all cases.
Conclusions
In our case series of 30 hip operations, the LCP Paediatric Hip Plate™ was shown to be safe and applicable in the clinical setting with excellent results and a low complication rate. We consider that the LCP Paediatric Hip Plate™ is a valuable device for correction of pathological conditions of the proximal femur and for fixation of displaced femoral neck fractures in children. Larger studies should be carried out to better quantify the risk of clinically relevant complications.
Introduction
For congenital pathological conditions of the proximal femur as well as acquired pathological conditions of this region, there are several implants available for correction via an inter- or subtrochanteric osteotomy with or without rotation. Such implants include angular blade plates [1, 19], dynamic compression plates (DCP) [7], locking compression plates (LCP) [8], external fixators (monolateral, ring fixator) [11] and the Richards intermediate hip screw [6]. Femoral neck fractures [20] can be stabilised by plates, Kirschner wires or screws [18]. Inter- or subtrochanteric osteotomies are associated with a variety of problems such as prominent hardware, infections, haematoma, chisel malposition, neck fractures, blade loosening, loss of correction or avascular necrosis of the femoral head, especially using angular blade plates [1, 6].
The LCP Paediatric Hip Plate™ was developed to make inter- and subtrochanteric osteotomies and the treatment of femoral neck fractures safer and less demanding.
In this article, we focus on the surgical technique of this plate system for different indications and perform a critical analysis of the device.
Patients and methods
Description of the LCP Paediatric Hip Plate™
The LCP Paediatric Hip Plate™ family has a universal design for both sides of the femur and is available in sizes of 2.7 mm for babies up to a maximum body weight of 15 kg, 3.5 mm for children from three to four years up to seven to eight years (body weight ± 35 kg) and 5.0 mm for older children up to adolescence. Different plate designs are available for varus correction (with a screw angle of 100° or 110°), for valgus correction (140°) and for fracture treatment and rotational osteotomies (120°, 130°). All plates have three neck screws, which are mandatorily locking screws. While these screws are parallel in the valgus and fracture plates, in the varus plate the distal femoral neck screw is divergent and goes towards the calcar. In the shaft area, all plates have three combi-holes. Angular stability is provided by locking screws [15]. Hip spica cast immobilisation is generally not necessary. In addition, due to the low bone-plate contact while using locking screws, the risk of disturbances to periosteal circulation is minimised.
At the time of this study, the fracture plate still had four shaft holes and a 120° neck/shaft angle, and the valgus plate had a shaft neck angle of 150°. The 2.7-mm varus plate was also not yet available.
Surgical technique
All corrections are planned preoperatively as usual either by the fixed neck/shaft angle technique or calculated neck/shaft angle technique [14]. Apart from bilateral rotational osteotomies where a supine or prone patient positioning is mandatory to obtain a symmetrical result, a lateral position is used.
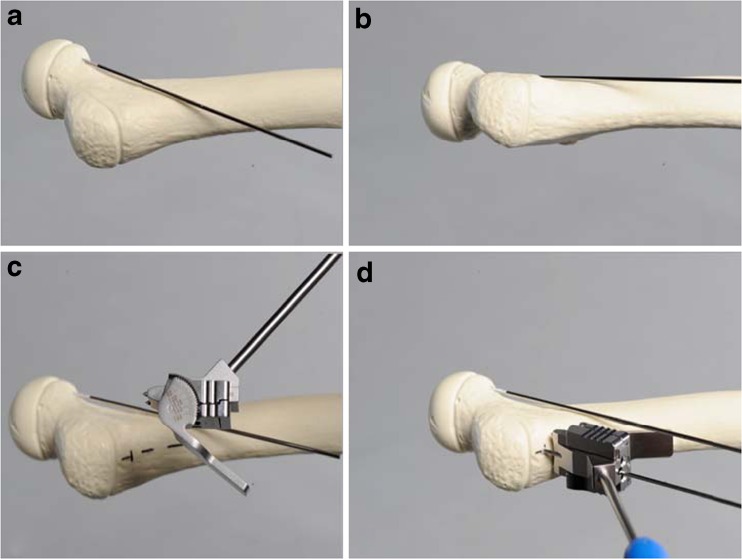
A standard lateral approach to the proximal femur is performed. A guide wire is placed on the anterior surface of the femoral neck to mark the anteversion of the femoral neck. Under an image intensifier, the parallel alignment of the so-called anteversion Kirschner wire to the anterior aspect of the femoral neck is checked (Fig. 1a, b). The preoperatively calculated angle for the positioning wire is set on a specific positioner for the aiming block (Fig. 1c).
Fig. 1.
a, b Correct placement of the so-called anteversion Kirschner wire. c How to set the preoperative calculated positioning wire angle on the positioner for the aiming block. d Correct placement of the aiming block and the positioning Kirschner wire on the lateral aspect of the proximal femur
The optimal entry point for the positioning Kirschner wire, located five, six or eight millimetres, depending on the plate size, distal to the trochanteric epiphysis in the anteroposterior (AP) view, is used. The positioning wire is inserted parallel to the anteversion Kirschner wire in the centre of the femoral neck in the axial view (Fig. 1d). Correct localisation of the positioning Kirschner wire is crucial for a successful operation. The positioning Kirschner wire is not removed until the plate is fixed on the femur with at least two locking screws to prevent plate rotation.
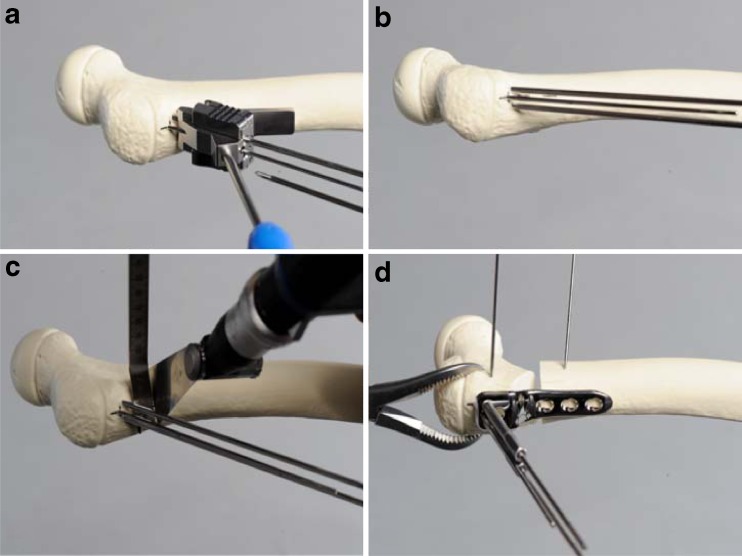
The Kirschner guide wires for the proximal screws are inserted with the help of the aiming block for screws (Fig. 2a, b). Optimal position of the guide wires is verified with the image intensifier in AP and axial views to ensure that the femoral neck epiphysis is not penetrated. For the 3.5- and 5.0-mm plates, the guide wires have the same diameter of 2.8 mm. It is mandatory that the corresponding (3.5/5.0) aiming block for the aiming Kirschner wires is used. The tip should end five millimetres distal to the growth plate. If a neutral position of the plate is required, the plate should align with the femoral shaft axis. For additional flexion or extension, the plate can be rotated in the sagittal plane.
Fig. 2.
a, b Insertion of the Kirschner guide wires with the help of the aiming block in the femoral shaft. c Optimal positioning of the osteotomy. d Fixation of the proximal fragment to the plate (to a 3.5 varus plate in this bone model). The two Kirschner wires in the ventral aspect of the femur in the proximal and distal fragments are inserted in case a derotation is planned together with the varus correction
For the 3.5-mm plate, the osteotomy is performed ten millimetres distal to the Kirschner guide wires for the calcar screw and in a 5.0-mm plate 13–16 mm (Fig. 2c, d). The osteotomy is done perpendicular to the femoral shaft. If rotation is planned, two Kirschner wires are inserted proximal and distal to the planned osteotomy to control rotation. For safe and better control of the proximal fragment, the use of a reduction forceps is strongly recommended. Manipulation of the neck wires should be avoided. Fixation of the proximal fragment must be done using three locking screws.
Correction (varus/valgus/rotation) is applied and the distal fragment is fixed by two to three locking or cortical screws. A cortical compression screw in the distal hole can be used for interfragmentary compression of the plate to the bone. The screw should be changed to a locking screw before the end of the operation. To avoid screw loosening, the locking screw heads need to be fixed correctly.
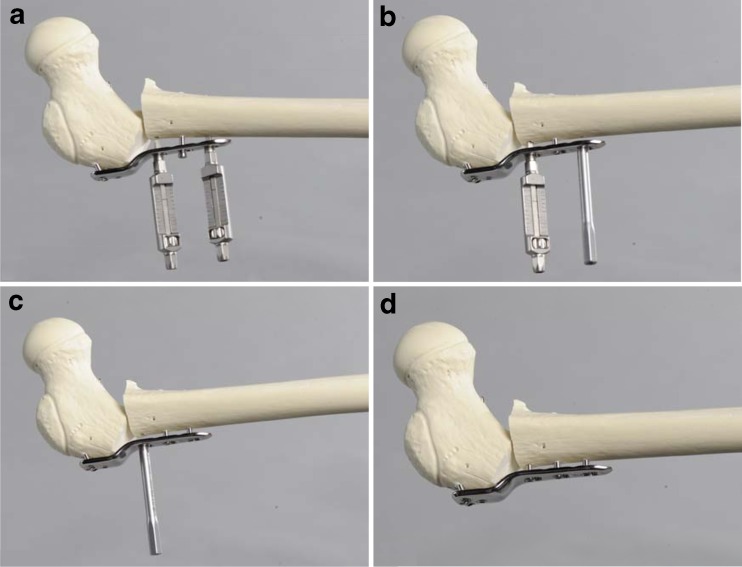
For additional intraoperative medialisation, a special instrument was developed in which two guides are necessary. The planned medialisation is set on the medialisation guides. The guides are then fixed in the locking portions of the proximal and distal shaft combi-holes. Once the plate is fixed to the shaft by a locking screw, the medialisation guides can be removed and the combi-holes must be filled with locking screws. The plate must always be fixed to the shaft with locking screws, if medialisation was performed (Fig. 3).
Fig. 3.
Additional medialisation by using the medialisation device. a Fixation of both devices on the plate. b, c After medialisation, stepwise refixation of the plate by refixation of the locking screws. d Final result
Patient inclusion and data collection
The LCP Paediatric Hip Plate™ has been used since 2006 in our clinic. As our clinic is a market preferences evaluation clinic (MPE clinic) and European Conformity (CE) marking was launched in November 2005, approval was given to use the plate before the 2007 US Food and Drug Administration (FDA) approval. In a retrospective case series, 30 proximal femur operations in 22 patients were evaluated and used for analysis of the surgical technique. Indications for which the plate was used included: hip dysplasia, Perthes’ disease, idiopathic ante/retroversion of the femoral neck, femoral neck fractures and complications after slipped capital femoral epiphysis (SCFE).
Statistical analyses
Data were collected directly in the clinic using an MS Access database 2003 (Microsoft Corporation, Redmond, WA, USA) and transferred into Intercooled Stata version 11 (StataCorp LP, College Station, TX, USA) for analyses. Study parameters were analysed and tabulated using standard descriptive statistics.
Results
Twenty-two children (30 hips) with pathological conditions of the hip and proximal femur underwent surgery at our clinic in the analysis period. An overview of each patient’s diagnosis and demographics is presented in Table 1. Figures 4 and 5 illustrate two clinical cases in which the LCP Pediatric Hip Plate™ was used. All but two patients with unilateral operations were operated upon in a lateral position. Of the eight patients with bilateral operations, four had rotational osteotomies and were operated upon in a supine position. Operation time ranged from 30 to 120 minutes per side, including additional operations such as a triple osteotomy (n = 3) and an open reduction of a hip (n = 1). Preoperative femoral deformities, intraoperative corrections and outcome for each patient are shown in Table 2. The postoperative radiographs corresponded with the preoperative planned corrections in 21 of 22 patients. In one case, the corrected femoral neck had less valgus than planned due to insufficient preoperative planning and suboptimal placement of the Kirschner guide wires for the neck screws.
Table 1.
Diagnosis and demographics of all 22 patients treated operatively with the new LCP Paediatric Hip Plate
| ID | Age at OP (years) | Gender | Weight (kg) | Diagnosis left hip/femoral neck | Diagnosis right hip/femoral neck | Mobility |
|---|---|---|---|---|---|---|
| 1 | 2.0 | Girl | 14 | Persistent hip dysplasia, congenital | Walker | |
| 2 | 11.0 | Girl | 50 | Situation after SCFE/SUFE | Walker | |
| 3 | 8.0 | Girl | 26 | Idiopathic ante/retroversion | Idiopathic ante/retroversion | Walker |
| 4 | 12.5 | Girl | 30 | Neuropathic hip dysplasia (CP, MMC), situation after operation 6 years ago | Non-walker | |
| 5 | 7.0 | Girl | 20 | Neuropathic hip dysplasia (CP, MMC) | Non-walker | |
| 6 | 1.0 | Boy | 8 | Neuropathic hip dysplasia (CP, MMC) | Non-walker | |
| 7 | 16.0 | Boy | 69 | Idiopathic ante/retroversion | Idiopathic ante/retroversion | Walker |
| 8 | 11.5 | Girl | 43 | Persistent hip dysplasia, congenital | Persistent hip dysplasia, congenital | Walker |
| 9 | 10.5 | Boy | 26 | Perthes’ disease | Walker | |
| 10 | 16.5 | Girl | 43 | Fracture: type I/II/III | Walker | |
| 11 | 6.0 | Boy | 24 | Congenital femoral neck pseudarthrosis | Walker | |
| 12 | 13.0 | Girl | 75 | Idiopathic ante/retroversion | Idiopathic ante/retroversion | Walker |
| 13 | 10.0 | Boy | 27 | Perthes’ disease | Walker | |
| 14 | 11.0 | Girl | 35 | Fracture: type I/II/III | Walker | |
| 15 | 9.5 | Girl | 28 | Idiopathic ante/retroversion | Idiopathic ante/retroversion | Walker |
| 16 | 3.0 | Girl | 14 | Neuropathic hip dysplasia (CP, MMC) | Neuropathic hip dysplasia (CP, MMC) | Non-walker |
| 17 | 10.0 | Boy | 34 | Neuropathic hip dysplasia (CP, MMC) | Neuropathic hip dysplasia (CP, MMC) | Walker |
| 18 | 14.0 | Boy | 62 | Situation after pelvic tumour resection and radiation | Walker | |
| 19 | 16.0 | Girl | 45 | Malunion after proximal femoral fracture, polytrauma | Walker | |
| 20 | 9.5 | Girl | 24 | Persistent hip dysplasia, congenital | Walker | |
| 21 | 14.0 | Boy | 80 | Fracture: type I/II/III | Walker | |
| 22 | 4.0 | Girl | 25 | Neuropathic hip dysplasia (CP, MMC) | Neuropathic hip dysplasia (CP, MMC) | Walker |
SUFE slipped upper femoral epiphysisCP cerebral palsy, MMC myelomeningocele
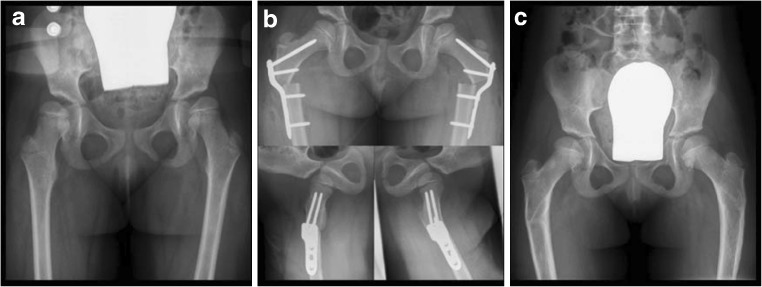
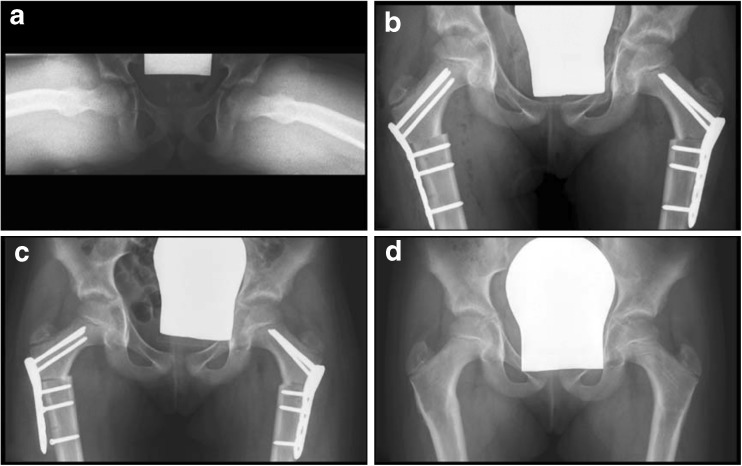
Fig. 4.
A 4-year-old girl with cerebral palsy. a Bilateral subluxation due to valgus deformity of the femoral shaft. b A bilateral intertrochanteric varus osteotomy together with a derotation was performed. c The postoperative course was uneventful, and the plates could be removed bilaterally 5 months after the initial operation
Fig. 5.
a A 9.5-year-old girl with bilateral idiopathic retroversion of 10°. b A bilateral derotation osteotomy was done and fixed by a 3.5-mm 120° LCP Paediatric Hip Plate. c Two weeks after the operation, the girl complained of right-sided pain in the proximal femur. The X-ray showed loosening of the femoral shaft screws from the DC holes. d A revision was performed to change and refix the plate. The further course was uneventful with plate removal 10 months after the initial operation
Table 2.
Preoperative deformities, intraoperative corrections, outcomes and complications of the 22 patients operated with the LCP Paediatric Hip Plate
| ID | Site | Varus/valgus | Ante/retrocurvation | Operation | Postoperative results | Complications |
|---|---|---|---|---|---|---|
| 1 | L | Valgus >160° | Retroversion >20° | Varus 30°, rotation 30° | As planned | |
| 2 | L | Valgus >130–140° | Anatomical | Varus 25°, rotation 10°, extension 15° | As planned | |
| 3 | L | Valgus >130–140° | Anteversion >50–60° | Rotation 30° | As planned | Broken neck screw was detected at implant removal without clinical relevance |
| R | Valgus >130–140° | Anteversion >50–60° | Rotation 30° | As planned | ||
| 4 | L | Valgus >160° | Anteversion >50–60° | Varus 30°, rotation >30°, extension 25° | As planned | |
| 5 | R | Valgus >160° | Anteversion >50–60° | Varus 30°, rotation >30°, extension 15° | As planned | |
| 6 | R | Valgus >160° | Anteversion >60° | Varus 30°, rotation >30°, extension 20° | As planned | |
| 7 | L | Anatomical | Anteversion >50–60° | Rotation 30° | As planned | |
| R | Anatomical | Anteversion >50–60° | Rotation 30° | As planned | ||
| 8 | L | Valgus >150–160° | Anatomical | Varus 30° | As planned | |
| R | Valgus >150–160° | Anatomical | Varus 30° | As planned | ||
| 9 | R | Anatomical | Anatomical | Varus 25° | As planned | |
| 10 | R | Anatomical | Anatomical | Valgus 10° | Less valgus than planned | |
| 11 | R | Varus <100° | Anteversion 10–20° | Valgus >30°a, rotation 15°, extension 10° | As planned | |
| 12 | L | Varus 100–110° | Retroversion >20° | Valgus 15°a, rotation 25° | As planned | Screw loosening, delayed healing, refixation of plate 15 months after initial operation in the same surgery as plate removal on contralateral side |
| R | Varus 100–110° | Retroversion >20° | Valgus 15°a, rotation 25° | As planned | ||
| 13 | L | Anatomical | Anatomical | Varus 20°, rotation 10°, extension 10° | As planned | |
| 14 | R | Anatomical | Anatomical | Anatomical | As planned | |
| 15 | L | Anatomical | Retroversion >10–20° | Rotation 25° | As planned | |
| R | Anatomical | Retroversion >10–20° | Rotation 25° | As planned | Screw loosening from DC hole, reoperation with refixation | |
| 16 | Valgus >150–160° | Anteversion >40–50° | Valgus >30a, rotation 30°, extension 20° | As planned | ||
| R | Valgus >150–160° | Anteversion >40–50° | Valgus >30a, rotation 30°, extension 20° | As planned | ||
| 17 | L | Valgus >160° | Anteversion >40–50° | Varus 30°, rotation 25° | As planned | |
| R | Valgus >160° | Anteversion >40–50° | Varus 30°, rotation 25° | As planned | Delayed healing at 2 months (partial weight-bearing, no surgical intervention) | |
| 18 | R | Varus >100° | Retroversion >10–20° | Valgus >30°a, rotation 10° | As planned | |
| 19 | R | Anatomical | Anteversion >30–40° | Rotation 30° | As planned | |
| 20 | L | Valgus >160° | Anteversion >40–50° | Varus >30°, rotation 30°, extension 20° | As planned | |
| 21 | R | Anatomical | Anatomical | Valgus 10°a | As planned | |
| 22 | L | Valgus >160° | Anteversion >20–30° | Varus >30°, rotation 20° | As planned | Screw loosening detected at implant removal without clinical relevance |
| R | Valgus >160° | Anteversion >20–30° | Varus >30°, rotation 20° | As planned | Screw loosening detected at implant removal without clinical relevance |
aAnalysis of the postoperative X-rays revealed that correction with the valgus plate caused an unwanted medialisation and, therefore, a suboptimal mechanical axis, although the planned correction was achieved and the children had no complaints
Using the valgisation plate, an unwanted medialisation was observed. The 120° fracture plate also seemed to be less anatomical than expected.
In only five of the 22 children was a hip spica applied due to the underlying disease or family situation. In four children with bilateral rotational operations, weight-bearing with crutches was allowed immediately after operation. Overall, partial or full weight-bearing was allowed in 14 of the 22 patients. Two patients were permitted free movement without weight-bearing, one child was allowed only passive motion and five children were immobilised in a hip spica cast due to impaired compliance.
No intraoperative complication was reported. In 20 of the 22 children, the first follow-up radiograph after six to eight weeks showed consolidation of the osteotomy. In a child who underwent bilateral rotational osteotomies, radiographs showed delayed healing with screw loosening on one side eight months postoperatively. Although the child achieved pain-free mobility, it was decided to revise that side while the plate on the contralateral side was removed under the same anaesthesia. Another child had incomplete healing after six weeks resulting in a two month extra period of partial weight-bearing without additional surgical intervention. One child with bilateral operations complained of right-sided hip pain after two weeks. A radiograph revealed loosening of all three femoral shaft screws from the DC holes (Fig. 5). No local problems such as a pseudobursa or seroma were recorded.
In 29 of 30 hips, the plate was removed after a mean duration of nine months (range 6–34 months). All plate removals were uneventful except for one screw breakage.
Discussion
The objective of this study was to critically analyse the application of the LCP Paediatric Hip Plate™ in an early phase and observe the learning curve of this operation. The focus was therefore set on this specific implant and its handling. Khouri and colleagues as well as Rutz and Brunner presented their first results with the LCP Paediatric Hip Plate™ in children with neurological disorders and severe osteoporosis [10, 16]. Both groups reported good results in proximal femoral osteotomies in their patients with a low complication rate. In our clinic, the LCP Paediatric Hip Plate™ is used in a broad range of indications including many diseases of the hip and proximal femur requiring varus/valgus or rotational osteotomies. In femoral neck fractures which require open reduction, we also use the implant successfully. In general, the applicability and handling of this hip plate was excellent. The planned correction could be achieved as expected in all but one osteotomy. For this case, the suboptimal outcome was caused by insufficient preoperative planning and intraoperative misplacement of the Kirschner guide wires for femoral neck screws and was not plate related.
Osteotomies for correction of congenital dysplastic hip sequelae and acquired pathological conditions of the hip and proximal femur have been previously described [12]. To stabilise these osteotomies, various techniques have been published [5, 8, 11, 13, 19]. However, none of the reported techniques appeared to be superior and complication rates up to 9 % have been described [1, 6]. In our series, the rate of clinically relevant complications was 6.6 % (two reoperations for screw loosening), which is in accordance with previously published data [10, 16].
In the light of these results and our experience, a critical analysis of the LCP Paediatric Hip Plate™ and the implantation technique has been made: the concept of the plate allows exact positioning. Any inadequate placement of guide wires can be corrected without causing damage to the femoral neck. This is one of the most valuable features of the plate, especially in comparison to blade plate designs. Once the Kirschner guide wires are placed correctly, the plate devices do not allow for incorrect plate placement.
The 100° and 110° screw angles of the varus LCP Paediatric Hip Plate™ proved to be adequate. Because of the unique offset for each plate size, an additional medialisation can be easily and precisely performed using the specially developed medialisation device. This is in contrast to the traditional blade plate system, in which the plate has to be removed for additional medialisation once it has been applied.
Considering the operative technique, the femoral head physis should generally not be perforated by femoral neck screws. However, for special indications such as proximal femoral neck fractures, screws may need to be placed in the femoral head. Although the risk of premature closure increases with penetration of the physis, it is much less than the risk of nonunion, pin breakage and avascular necrosis [3]; fracture stability is therefore more important than preservation of the proximal femoral physis [4]. The size of the plate and screws is largely dependent on the patient’s age and weight: for children with a body weight of less than 15 kg, the recommended screw diameter is 2.7 mm; in children up to 35 kg or more, the screw diameters are 3.5 and 5.0 mm, respectively. If penetration of the physis is absolutely required or cannot be avoided, the positioning Kirschner wires for the femoral neck screws must be correctly placed before the screws are inserted to avoid multiple perforation of the physis by the screws. Removal of the implant should be done rather early in such cases as soon as complete healing and remodelling is achieved. In addition, screw perforation of the head must be strictly avoided; under an image intensifier, it is mandatory to control the subchondral placement of screws in all directions.
We prefer a lateral position of the patient as it provides: (1) better access and view of the proximal femur, (2) better and unrestricted manipulation of the guiding block and (3) less operating assistance is required. This approach also allows for a more sterile environment, less radiation for the safety of both patient and surgeon and better pictures due to the closer position of the fluoroscope to the patient.
No hip spica cast is needed, even in bilateral operations; however, it might be indicated depending on the patient’s condition, underlying disease or compliance.
Loss of correction or plate breakage was not observed in our patient cohort. One child showed loosening of all three femoral shaft screws from the DC holes, which resulted in a revision operation. Retrospective analysis of the postoperative radiographs revealed a technical failure with incorrect placement of the femoral shaft screws.
In 28 of the 30 hips, good consolidation was radiologically confirmed within the expected healing time. In two children, radiological signs of delayed healing were observed. One required revision. No patient developed massive callus formation, which was probably achieved by primary bone healing due to angular stability of the implant.
Because of the plate’s anatomical shape, no severe local irritations such as seroma or pseudobursa were observed. This is of special interest in children with cerebral palsy who often have a bony habitus. Finally, plate removal was uneventful except for one screw breakage, which had no further consequences. Therefore in our case study, removal of the LCP Paediatric Hip Plate™ seemed to be superior to published results with other implants [2, 9, 17].
This study had an influence on further plate design adaptations that are now definitively available: (1) the shaft-neck angle in the fracture plate was changed from 120 to a more anatomical 130°; (2) the shaft screws in the fracture plate were reduced from four to three holes to facilitate plate insertion and fixation with less muscle stripping; (3) the valgisation plate design was altered to avoid pathological medialisation and involved changing the shaft-neck angle from 150 to 140°; and (4) lastly, the development of a 2.7-mm sized plate was stimulated.
This study has limitations; in particular its retrospective design, its lack of a control group including existing osteotomy devices and its small sample size are relevant. Nevertheless, this initial experience encourages us to believe that the LCP Paediatric Hip Plate™ will prove to be a valuable device for a broad range of indications. A larger series should be implemented to better quantify the risk of clinically relevant complications. Comparative studies reporting superiority of the device compared with other, already widely used devices are pending.
Conclusion
With the new LCP Paediatric Hip Plate™, a device was developed by which corrective osteotomies of the proximal femur and femoral neck fractures can be surgically treated in a safe way. Based on the biomechanical properties of the locking screws, weight-bearing is possible. Due to the different screw angles and plate designs together with the angular stability achieved by the locking screws, osteotomies of the proximal femur can be performed with a low complication rate after careful planning.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Beauchesne R, Miller F, Moseley C. Proximal femoral osteotomy using the AO fixed-angle blade plate. J Pediatr Orthop. 1992;12:735–740. [PubMed] [Google Scholar]
- 2.Becker CE, Keeler KA, Kruse RW, Shah SA. Complications of blade plate removal. J Pediatr Orthop. 1999;19:188–193. doi: 10.1097/00004694-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Blasier RD, Hughes LO. Fractures and traumatic dislocation of the hip in children. In: Beaty JH, Kasser JR, editors. Rockwood and Wilkins’ fractures in children. 5. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 913–939. [Google Scholar]
- 4.Boardman MJ, Herman MJ, Buck B, Pizzutillo PD. Hip fractures in children. J Am Acad Orthop Surg. 2009;17:162–173. doi: 10.5435/00124635-200903000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gordon JE, Pappademos PC, Schoenecker PL, Dobbs MB, Luhmann SJ. Diaphyseal derotational osteotomy with intramedullary fixation for correction of excessive femoral anteversion in children. J Pediatr Orthop. 2005;25:548–553. doi: 10.1097/01.bpo.0000158783.37602.cb. [DOI] [PubMed] [Google Scholar]
- 6.Hau R, Dickens DR, Nattrass GR, O’Sullivan M, Torode IP, Graham HK. Which implant for proximal femoral osteotomy in children? A comparison of the AO (ASIF) 90 degree fixed-angle blade plate and the Richards intermediate hip screw. J Pediatr Orthop. 2000;20:336–343. doi: 10.1097/00004694-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hogan KA, Blake M, Gross RH. Subtrochanteric valgus osteotomy for chronically dislocated, painful spastic hips. J Bone Joint Surg Am. 2006;88:2624–2631. doi: 10.2106/JBJS.E.00918. [DOI] [PubMed] [Google Scholar]
- 8.Huber H, Haefeli M, Dierauer S, Ramseier LE. Treatment of reduced femoral antetorsion by subtrochanteric rotational osteotomy. Acta Orthop Belg. 2009;75:490–496. [PubMed] [Google Scholar]
- 9.Kahle WK. The case against routine metal removal. J Pediatr Orthop. 1994;14:229–237. doi: 10.1097/01241398-199403000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Khouri N, Khalife R, Desailly E, Thevenin-Lemoine C, Damsin JP. Proximal femoral osteotomy in neurologic pediatric hips using the locking compression plate. J Pediatr Orthop. 2010;30:825–831. doi: 10.1097/BPO.0b013e31820156f2. [DOI] [PubMed] [Google Scholar]
- 11.Moens P, Lammens J, Molenaers G, Fabry G. Femoral derotation for increased hip anteversion. A new surgical technique with a modified Ilizarov frame. J Bone Joint Surg Br. 1995;77:107–109. [PubMed] [Google Scholar]
- 12.Müller ME. Die hüftnahen Femurosteotomien unter Berücksichtigung der Form, Funktion und Beanspruchung des Hüftgelenkes. Stuttgart: Thieme; 1971. [Google Scholar]
- 13.Müller ME, Allgöwer M, Schneider R, Willenegger H, editors. AO manual of internal fixation. Techniques recommended by the AO-ASIF Group. 3. Berlin: Springer; 1991. [Google Scholar]
- 14.Paley D. Hip joint considerations. In: Paley D, editor. Principles of deformity correction. Berlin: Springer; 2005. pp. 647–694. [Google Scholar]
- 15.Perren SM, Frigg R, Hehli M, Tepic S. Lag screw. In: Rüedi TP, Murphy WM, editors. AO principles of fracture management. 1. Stuttgart: AO Publishing-Thieme; 2000. pp. 157–168. [Google Scholar]
- 16.Rutz E, Brunner R. The pediatric LCP hip plate for fixation of proximal femoral osteotomy in cerebral palsy and severe osteoporosis. J Pediatr Orthop. 2010;30:726–731. doi: 10.1097/BPO.0b013e3181efb86b. [DOI] [PubMed] [Google Scholar]
- 17.Schmalzried TP, Grogan TJ, Neumeier PA, Dorey FJ. Metal removal in a pediatric population: benign procedure or necessary evil? J Pediatr Orthop. 1991;11:72–76. doi: 10.1097/01241398-199101000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Shrader MW, Jacofsky DJ, Stans AA, Shaughnessy WJ, Haidukewych GJ. Femoral neck fractures in pediatric patients: 30 years experience at a level 1 trauma center. Clin Orthop Relat Res. 2007;454:169–173. doi: 10.1097/01.blo.0000238794.82466.3d. [DOI] [PubMed] [Google Scholar]
- 19.Slongo TF. Intertrochanteric osteotomy of the proximal femur in childhood. Oper Orthop Traumatol. 2008;20:334–353. doi: 10.1007/s00064-008-1406-8. [DOI] [PubMed] [Google Scholar]
- 20.Slongo TF, Audigé L, AO Pediatric Classification Group Fracture and dislocation classification compendium for children: the AO pediatric comprehensive classification of long bone fractures (PCCF) J Orthop Trauma. 2007;21:S135–S160. doi: 10.1097/00005131-200711101-00020. [DOI] [PubMed] [Google Scholar]