Abstract
Studies with 15N indicate that appreciable generation of NH4+ from endogenous sources accompanies the uptake and assimilation of exogenous NH4+ by roots. To identify the source of NH4+ generation, maize (Zea mays L.) seedlings were grown on 14NH4+ and then exposed for 3 d to highly labeled 15NH4+. More of the entering 15NH4+ was incorporated into the protein-N fraction of roots in darkness (approximately 25%) than in the light (approximately 14%). Although the 14NH4+ content of roots declined rapidly to less than 1 μmol per plant, efflux of 14NH4+ continued throughout the 3-d period at an average daily rate of 14 μmol per plant. As a consequence, cumulative 14NH4+ efflux during the 3-d period accounted for 25% of the total 14N initially present in the root. Although soluble organic 14N in roots declined during the 3-d period, insoluble 14N remained relatively constant. In shoots both soluble organic 14N and 14NH4+ declined, but a comparable increase in insoluble 14N was noted. Thus, total 14N in shoots remained constant, reflecting little or no net redistribution of 14N between shoots and roots. Collectively, these observations reveal that catabolism of soluble organic N, not protein N, is the primary source of endogenous NH4+ generation in maize roots.
Short-term studies with 13NH4+ have provided estimates of NH4+ influx, efflux, and cytoplasmic concentration in spruce (Kronzuker et al., 1995a, 1995b). As the NH4+ concentration in the external solution increased from 10 to 1500 μm, cytosolic NH4+ levels increased from 2 to 33 mm and efflux increased from 10% to 35% of influx. Similar rates were reported for rice: as external NH4+ levels increased from 2 to 1000 μm, cytosolic NH4+ levels increased from 3 to 38 mm and efflux rose from 11% to 29% of influx (Wang et al., 1993). The half-lives of cytosolic NH4+ were 8 and 15 min for rice and spruce, respectively.
During short-term exposure of cereal seedlings to 15NH4+ solution, efflux of endogenous 14NH4+ exceeded the total 14NH4+ initially present in the root tissues (Morgan and Jackson, 1988a, 1988b). Moreover, after a 5-h pretreatment in 15NH4+ solution, the subsequent efflux of 15NH4+ to the ambient 14NH4+ solution was greater than the initial NH4+ content of the root (Morgan and Jackson, 1989). Thus, appreciable generation of NH4+ from endogenous organic N sources accompanies concurrent uptake and assimilation of NH4+ by roots.
The efflux of NH4+ provides only a minimal estimate of endogenous NH4+ generation because part of the NH4+ is likely reassimilated. In support of this possibility, when NH4+ assimilation via Gln synthetase was blocked with Met sulfoxamine, NH4+ generation in maize roots was estimated to be 50% faster than concurrent NH4+ uptake (Jackson, et al., 1993). The potential for substantial generation, recycling, and efflux of endogenous NH4+ in roots is thus indicated.
A crucial question raised by these observations is whether protein turnover is the source of endogenous NH4+ generation, or if recycling of intermediates of the NH4+ assimilation pathway, such as Gln, is the source. To address this question, maize (Zea mays) seedlings that had been grown on 14NH4+ were exposed to highly labeled 15NH4+ for 3 d. It was hypothesized that if protein turnover is the source of NH4+ and if part of this NH4+ is subject to efflux and translocation to the shoot, a decline in endogenous 14N protein in the root should occur as new protein is synthesized from the entering 15NH4+.
The fact that 15NH4+ was applied during six diurnal periods also permitted us to (a) directly measure of the diurnal pattern of NH4+ fluxes into and out of roots, (b) compare 15NH4+ uptake and assimilation by roots during successive light and dark periods, and (c) determine the relationship of the latter processes to carbohydrate levels in shoots and roots.
MATERIALS AND METHODS
Plant Culture
Maize (Zea mays L. cv Pioneer 3320) caryopses were germinated at 30°C in contact with 0.1 mm CaSO4. After 30 h, uniform seedlings were selected and their seminal roots excised. Cultures of eight seedlings each were transplanted into 160 L of basal nutrient solution, pH 6.0, containing 0.125 mm (NH4)2SO4, 1.25 mm K2SO4, 0.25 mm Ca(H2PO4)2, 1 mm CaSO4, 46 μm B, 9 μm Mn, 0.8 μm Zn, 0.3 μm Cu, 0.1 μm Mo, and 54 μm Fe as ferric diethylenetriamine pentaacetate. The solution was aerated with compressed air that had been washed with H2SO4 and water to remove ambient NH4+. A combination of sodium vapor and metal halide lamps provided 1140 μE m−2 s−1 illumination at canopy height during a 14-h photoperiod (7 am to 9 pm). The average air temperature during the experiment was 23.4°C ± 1.6°C. Both pH and [NH4+] of the nutrient solution were checked daily and maintained at pH 6.0 and 0.25 mm, respectively, by continuous injection of Ca(OH)2 and (NH4)2SO4.
Experimental Procedure
At the beginning of the dark period on the 7th d after imbibition, four cultures were harvested and the rest were transferred to fresh basal nutrient solution containing 0.22 mm NH4+ labeled with 96.1 atom % 15N. An initial sample of 15NH4+ nutrient solution was taken for subsequent analysis. Four additional cultures were harvested and a solution sample was taken at the beginning (7 am) and end (9 pm) of each light period on the 8th, 9th, and 10th d. The pH and [NH4+] of the nutrient solution were checked daily and maintained as described above by continuous injection of Ca(OH)2 and (15NH4)2SO4 containing 99.8 atom % 15N.
At harvest, the roots were dipped five times in 0.1 mm CaSO4, excised, blotted lightly, and weighed. After weighing the shoots, which included the remaining seed pieces, all tissue samples were freeze dried, weighed, ground, and mixed thoroughly.
N and 15N Analysis
Shoots, roots, and nutrient solutions were analyzed for NH4+ and its 15N enrichment (Jackson et al., 1993). In addition, shoots and roots were analyzed for soluble and insoluble N and their respective 15N enrichments. These fractions were separated by extraction with acidified (pH 3.0) 80% (v/v) ethanol. Organic N in the extract and residue was converted to NH4+ by Kjeldahl digestion (McKenzie and Wallace, 1954), and the NH4+ was quantified by spectrophotometric analysis (Smith, 1980). NH4+ was recovered by diffusion, converted to dinitrogen gas by a freeze-layer procedure (Volk and Jackson, 1979), and analyzed for 15N enrichment by MS.
Total N and 15N analyses were used to calculate the tissue contents of six isotopic N species: 14NH4+, 15NH4+, soluble 14N, soluble 15N, insoluble 14N, and insoluble 15N. Because 14NH4+ and 15NH4+ were subtracted from soluble 14N and soluble 15N, these fractions represent soluble organic N constituents. Protein is the primary constituent of the insoluble-N fraction.
Carbohydrate Analysis
To obtain comparable tissue samples for nonstructural carbohydrate analysis, the experiment was repeated and the seed pieces were discarded at harvest. Insoluble and soluble carbohydrates of shoots and soluble carbohydrates of roots were assayed by enzymatic and spectrophotometric procedures after separation by extraction with 80% (v/v) ethanol (Jones et al., 1977).
RESULTS
Changes in Endogenous 14N Fractions
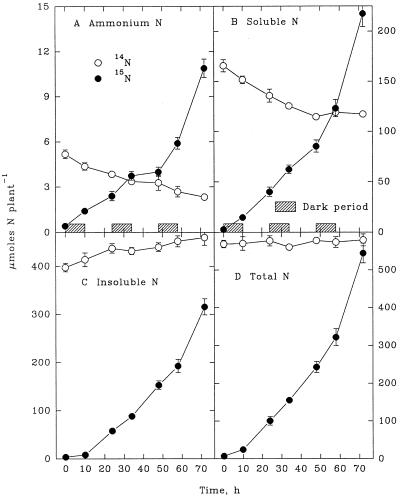
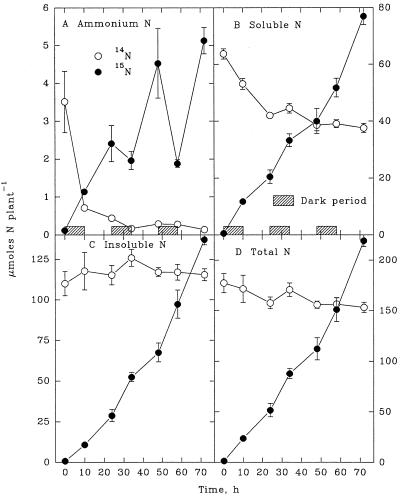
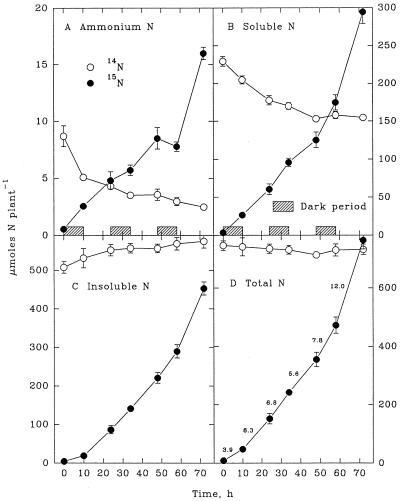
An appreciable increase in insoluble 14N in shoots (Fig. 1C) was balanced by an equivalent decline in the 14NH4+ and soluble-14N fractions (Fig. 1, A and B). Thus, the total endogenous 14N in shoots remained constant during the 3-d exposure to 15NH4+ (Fig. 1D). By contrast, the insoluble 14N of roots remained relatively constant (Fig. 2C), even though 14NH4+ and soluble 14N decreased appreciably (Fig. 2, A and B). As a consequence, total 14N in the root declined during the 3-d exposure to 15NH4+ (Fig. 2D). On a whole-plant basis, the changes in 14N (Fig. 3) reflected those in the shoot, which contained more than 75% of the total 14N in the plant.
Figure 1.
Shoot contents of NH4+ (A), soluble N (B), insoluble N (C), and total N (D) derived from endogenous (14N) sources and from exogenously supplied NH4+ (15N) during a 3-d continuous exposure of maize seedlings to highly labeled 15NH4+. Each value is the mean of four replicates ± se.
Figure 2.
Root contents of NH4+ (A), soluble N (B), insoluble N (C), and total N (D) derived from endogenous (14N) sources and from exogenously supplied NH4+ (15N) during a 3-d continuous exposure of maize seedlings to highly labeled 15NH4+. Each value is the mean of four replicates ± se.
Figure 3.
Whole-plant contents of NH4+ (A), soluble N (B), insoluble N (C), and total N (D) derived from endogenous (14N) sources and from exogenously supplied NH4+ (15N) during a 3-d continuous exposure of maize seedlings to highly labeled 15NH4+. Each value is the mean of four replicates ± se. The diurnal rates of 15NH4+ uptake (in micromoles per gram fresh weight per hour) are noted in D.
Estimation of NH4+ Fluxes into and out of Roots
During each diurnal period a complete balance sheet of the changes in 14NH4+ and 15NH4+ in the uptake solution was compiled. The procedure is illustrated in Table I using data from the initial 10-h dark period. Even though an appreciable quantity of 99.8 atom % 15N was injected to maintain the [NH4+], the 15N enrichment of the nutrient solution declined from 96.1 to 93.5 atom % 15N, reflecting a release of 14NH4+ from the root. The net rate of release, 4.3 μmol plant−1 h−1, is less than the actual rate because of concurrent uptake of 14NH4+ from the nutrient solution. The latter can be estimated from the fact that 14NH4+ and 15NH4+ are taken up in proportion to their average molar concentrations in the nutrient solution during any given period. The calculation (Table I, line G) reveals that 14NH4+ was taken up at a rate of 2.6 μmol plant−1 h−1. This rate, when added to the net rate of 14NH4+ release, provides an estimate of “true” 14NH4+ release (Table I, line J). Similar calculations were made throughout the experiment to quantify 14NH4+ release from the root in successive diurnal periods (Table II). Both the uptake and release of NH4+ were greater in light than in darkness. NH4+ release remained appreciable even in the final light period, when it accounted for 4.8% of the total 14N initially present in roots harvested on the 7th d. As a consequence, cumulative 14NH4+ release during the 3-d period was equivalent to 25% of the total 14N initially present in the root.
Table I.
Calculation of NH4+ fluxes into and out of maize roots
| Measurement | Volume | [NH4+] | Atom % 15N | I 14NH4+ | II 15NH4+ | Rate |
|---|---|---|---|---|---|---|
| L | mm | % | μmol 160 L−1 | μmol plant−1 10 h−1 | ||
| A. Initial (9 pm) | 160 | 0.223 | 96.10 | 1392 | 34,288 | |
| B. Injection | 0.09756 | 113 | 99.80 | 22 | 11,002 | |
| C. Final (7 am) | 160 | 0.231 | 93.55 | 2384 | 34,576 | |
| D. Change (C − A − B) | +970 | −10,714 | ||||
| E. Mean concentration (A + C)/2 | 1888 | 34,432 | ||||
| F. Net 15NH4+ uptake (DII/227 plants) | 47.2 | |||||
| G. 14NH4+ uptakea(F[EI/EII]) | 2.6 | |||||
| H. Total NH4+ uptake (F + G) | 49.8 | |||||
| I. Net 14NH4+ release (DI/227 plants) | 4.3 | |||||
| J. “True” 14NH4+ releaseb(I + G) | 6.9 | |||||
Rates of 15NH4+ uptake, 14NH4+ uptake, and 14NH4+ release by 227 maize seedlings were calculated from [15NH4+] and [14NH4+] in the nutrient solution at the beginning (9 pm) and end (7 am) of the first 10-h dark period, during which 97.56 mL of 113 mm 15NH4+ (99.8 atom % 15N) was injected to maintain the total [NH4+] close to its initial level (0.223 mm).
14NH4+ uptake can be estimated from net 15NH4+ uptake because the two isotopic species are taken up in proportion to their mean molar concentrations in the nutrient solution during the uptake period.
Net 14NH4+ release = true 14NH4+ release − 14NH4+ uptake. Therefore, true 14NH4+ release = net 14NH4+ release + 14NH4+ uptake.
Table II.
Diurnal rates of 15NH4+ uptake, 14NH4+ uptake, and 14NH4+ release by maize roots
| Day | Diurnal Period | Length | Total (15NH4+ + 14NH4+) Uptake | True 14NH4+ Release | True Root 14N Releasea |
|---|---|---|---|---|---|
| h | μmol plant−1 | period−1 | % | ||
| 7–8 | Dark | 10 | 49.8 | 6.9 | 4.0 |
| 8 | Light | 14 | 103.1 | 11.6 | 6.7 |
| 8–9 | Dark | 10 | 63.9 | 0.2 | 0.1 |
| 9 | Light | 14 | 177.3 | 11.1 | 6.4 |
| 9–10 | Dark | 10 | 90.6 | 5.2 | 3.0 |
| 10 | Light | 14 | 193.3 | 8.3 | 4.8 |
| Total | 678.0 | 43.3 | 25.0 |
Roots of maize seedlings were exposed continuously to highly labeled 15NH4+ for 3 d. NH4+ flux rates were calculated from periodic analysis of the uptake solution, as illustrated in Table I.
14NH4+ release of the total 14N present in roots harvested on the 7th d (174 μmol 14N root−1).
It is important to note that the data in Table II cannot be compared directly with the data in Figure 2. Table II shows the true release of 14NH4+ from roots, which consists of measured net release of 14NH4+ plus the concurrent, calculated uptake of 14NH4+. By contrast, Figure 2 portrays the net change in root 14N, which reflects only net release of 14NH4+ from the root, provided that no interchange of 14N between roots and shoots occurs.
Uptake and Assimilation of Applied 15NH4+
The rate of 15NH4+ uptake by maize seedlings was similar during light and dark periods (Fig. 3D). A small but measurable amount of the entering 15NH4+ accumulated as NH4+ in the shoot, and the amount increased appreciably as the plants developed (Fig. 1A). By contrast, 15NH4+ accumulation in the root exhibited a diurnal pattern (Fig. 2A). Except for the initial dark period, root 15NH4+ increased during illumination and declined in darkness.
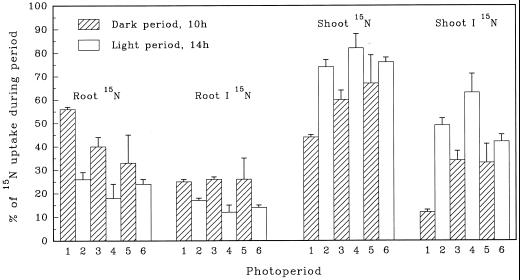
Most of the entering 15NH4+ was assimilated into the soluble-15N and insoluble-15N fractions (Fig. 3, B and C). Although the patterns of accumulation in shoots (Fig. 1, B and C) and roots (Fig. 2, B and C) were similar to those in the whole plant, diurnal differences in assimilation and translocation were evident (Fig. 4). During each dark period a greater percentage of the entering 15NH4+ was retained in the root and incorporated into the insoluble-15N fraction. Conversely, during each light period a greater percentage of the entering 15NH4+, or metabolites thereof, was translocated to the shoot and incorporated into the shoot insoluble-15N fraction.
Figure 4.
Diurnal increments of total 15N and insoluble 15N (I 15N) in shoots and roots of maize seedlings as percentages of 15N uptake during each of six successive photoperiods. Each value is the mean of four replications. se values are indicated by vertical bars.
Diurnal Changes in Dry Matter and Carbohydrate
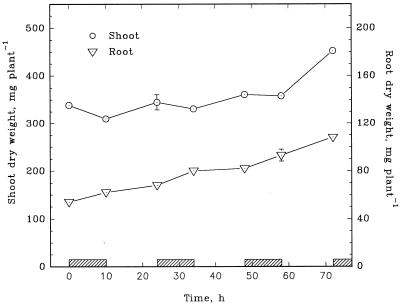
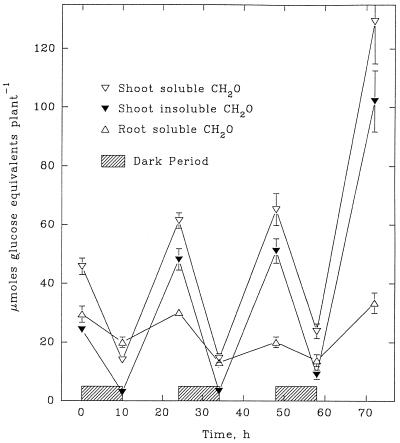
The dry weight of maize shoots generally increased during light periods and declined during dark periods (Fig. 5). By contrast, root weight increased at least as rapidly in darkness as in light. Although the carbohydrate content of both shoots and roots exhibited a typical diurnal pattern (Fig. 6), the pattern in roots was attenuated considerably.
Figure 5.
Dry weights of maize shoots and roots harvested at the beginning and end of each photoperiod during a 3-d exposure to highly labeled 15NH4+. Each value is the mean of four replications. se bars are shown when they are larger than the symbols.
Figure 6.
Soluble and insoluble carbohydrate contents of shoots and soluble carbohydrate contents of roots during a 3-d continuous exposure of maize seedlings to 14NH4+. Each value is the mean of four replicates ± se.
DISCUSSION
Source of 14NH4+ Generation in Roots
Morgan and Jackson (1988a, 1988b, 1989) demonstrated that significant generation of NH4+ occurs in roots during uptake and assimilation of exogenously supplied NH4+. They suggested that organic N degradation, NH4+ assimilation, and NH4+ influx and efflux can be modified by environmental and nutritional conditions that alter the pool size of NH4+ in roots.
Throughout the 3-d exposure of maize seedlings to 15NH4+, during which appreciable synthesis of insoluble 15N occurred in roots, the endogenous insoluble 14N of roots remained relatively constant. However, 14NH4+ and soluble 14N in roots declined significantly. There was evidence of 14N-protein synthesis in the shoot, apparently at the expense of the pool of soluble 14N in the shoot. The ultimate source of 14N for protein synthesis in the shoot remains tentative, however, because of the likelihood of soluble-14N interchange between the root and shoot as a consequence of amino acid cycling (Lambers et al., 1982; Cooper and Clarkson, 1989). In spite of this possibility, no measurable net transport of endogenous 14N from roots to shoots occurred during the 3-d exposure to 15NH4+. Collectively, these data reveal that soluble organic N, rather than protein, is the primary source of endogenous NH4+ generation in maize roots.
Release of 14NH4+ from Roots
During the first 24 h of exposure to 15NH4+ the release of endogenously derived 14NH4+ from the root into the nutrient solution was equivalent to 10.7% of the initial 14N content of the root (Table II). A slower but measurable release of 14NH4+ continued throughout the subsequent 2-d period. This occurred even though the content of 14NH4+ in the root was less than 1 μmol after 24 h of exposure to exogenous 15NH4+ (Fig. 2A), indicating a continual generation of 14NH4+ from endogenous 14N pools. Thus, it is clear that the source of 14NH4+, presumably the soluble-14N pool of the root, was not replaced completely by synthesis of soluble 15N from entering 15NH4+. In support of this premise are the observations that the root soluble-14N pool declined only 22 μmol root−1 (34%) during the first 24 h, and remained relatively constant at 40 μmol root−1 thereafter (Fig. 2B). This occurred even though the root soluble-15N pool increased from near 0 to more than 200 μmol root−1 during the 3-d period. One interpretation of these observations is that the endogenous soluble-14N pool, the putative source of 14NH4+ generation in roots, occupies a different cellular or intracellular “compartment” than that of recently synthesized soluble 15N. For example, synthesis of soluble 15N might occur primarily in the root tip (0–2 cm), where NH4+ uptake is maximal (Cruz et al., 1995), whereas NH4+ generation might occur in the more mature regions of the root.
Diurnal Use of Exogenous 15NH4+
Although effective absorption and assimilation of 15NH4+ occurred in both dark and light periods, diurnal differences in utilization were observed (Fig. 4). More of the entering 15N was retained by the root in the dark (33%–56%) than in the light (18%–26%). A similar retention of NH4+ was reported for perennial ryegrass by Ourry et al. (1996). We also observed that more of the entering 15NH4+ was incorporated into root insoluble 15N in the dark (approximately 25%) than in the light (approximately 14%). Conversely, the synthesis of shoot insoluble 15N was enhanced by light.
In contrast to the data reported here, Ourry et al. (1996) found that the rate of NH4+ uptake by perennial ryegrass declined during darkness. However, the plants were exposed to a lower light intensity (500 μmol m−2 s−1) and a lower [NH4+] (20 μm) than were used in our study. Restricted rates of NH4+ uptake in the dark were also reported for both fescue and timothy grass when grown at 20 μm NH4+ (Macduff et al., 1997). Finally, the rate of NH4+ uptake in the dark by barley grown under light-limited conditions (350 μmol m−2 s−1) was only 50% of the rate in the light (Rigano et al., 1996), perhaps reflecting a limitation of NH4+ uptake by carbohydrate supply.
Several lines of evidence suggest that both the uptake and assimilation of NH4+ are regulated by carbohydrate supplied from the shoot. First, Massimino et al. (1981) reported that NH4+ uptake by maize declined within 2 h after lowering the light intensity to restrict photosynthesis. Second, after bean plants had been ringed, NH4+ uptake and root soluble carbohydrate content declined concurrently (Michael et al., 1970). When exogenous Suc was supplied to the roots of ringed plants, however, the rate of NH4+ uptake exceeded that of intact plants. Third, Lewis et al. (1987) found that a much higher proportion of 14C derived from photosynthetic CO2 fixation was allocated to the root when N was supplied as NH4+ rather than as NO3−.
Based on these observations we hypothesized that diurnal differences in retention and incorporation of NH4+ into macromolecules were related to changes in the diurnal supply of carbohydrate to the root. To examine this possibility, the changes in carbohydrate contents of the shoot and roots in a duplicate experiment were compared with 15NH4+-assimilation rates in the original experiment (Table III). It was assumed that (a) the carbohydrate changes in the replicate experiment were comparable to those in the original study, (b) assimilation of the entering 15NH4+ occurred exclusively in the root, (c) the initial product of NH4+ assimilation in the root was Gln, and (d) the assimilation of 1 mol of 15NH4+ into Gln requires 0.505 mol of Glc equivalents to supply the necessary reductant, ATP, and C skeletons (Schubert, 1980). Using these assumptions, the C required for net 15NH4+ assimilation during each diurnal period was estimated.
Table III.
Carbohydrate required for 15NH4+ assimilation by maize roots
| Day | Photoperiod | 15NH4+ Assimilation | Carbohydrate
|
|||
|---|---|---|---|---|---|---|
| Requirementa | Change in shoot | Change in root | Transport to rootb | |||
| μmol N plant−1 | μmol C plant−1 | |||||
| 7–8 | Dark | 39 ± 4 | 117 ± 13 | −319 ± 7 | −57 ± 10 | 60 ± 15 |
| 8 | Light | 102 ± 18 | 310 ± 53 | +556 ± 35 | +61 ± 7 | 371 ± 55 |
| 8–9 | Dark | 91 ± 11 | 275 ± 34 | −550 ± 12 | −103 ± 6 | 172 ± 37 |
| 9 | Light | 110 ± 24 | 333 ± 74 | +590 ± 67 | +42 ± 11 | 375 ± 64 |
| 9–10 | Dark | 120 ± 28 | 365 ± 84 | −501 ± 24 | −38 ± 13 | 327 ± 97 |
| 10 | Light | 288 ± 30 | 872 ± 91 | +1193 ± 147 | +118 ± 21 | 990 ± 108 |
The diurnal assimilation of 15NH4+ was measured during a 3-d exposure of maize seedlings to highly labeled 15NH4+. The minimal transport of C from shoot to root required to support 15NH4+ assimilation was calculated from the theoretical C requirement for 15NH4+ assimilation and the changes in tissue carbohydrate. Each value is the mean of four replicates ± se.
Calculation of the total C requirement for 15NH4+ assimilation is based on the assumption that assimilation of entering 15NH4+ occurs in the root, and that the initial product of NH4+ assimilation is Gln, which requires 0.505 mol of Glc equivalents mol−1 NH4+ to provide the necessary reductant, ATP, and C skeletons (Schubert, 1982).
Minimal C transport to the root required to support 15NH4+ assimilation is equal to the total C required for 15NH4+ assimilation plus the change in root C content.
As plants developed, the C required to support measured 15NH4+ assimilation increased from 117 μmol plant−1 in the first dark period to 872 μmol plant−1 during the final light period (Table III). Yet the carbohydrate content of the roots at the beginning of each dark period varied from 120 to 200 μmol plant−1 (i.e. 20–33 μmol Glc equivalents plant−1; Fig. 6). These data reveal that with the exception of the first dark period, there were insufficient carbohydrate reserves in the root to support assimilation of the 15NH4+ absorbed. However, there were adequate reserves in the shoot. Thus, appreciable transport of carbohydrate from the shoot to the root must have occurred during both dark and light periods.
Minimal estimates of the transport of C to the root required to support 15NH4+ assimilation can be calculated by adding the total C required for 15NH4+ assimilation to the accumulation of C by the root (Table III). Such estimates ranged from 60 μmol plant−1 during the first dark period to 990 μmol plant−1 during the final light period (Table III). It is of particular interest to note that during the three successive dark periods, 15NH4+ assimilation accounted for 19%, 31%, and 65%, respectively, of the decline in shoot carbohydrate. This reflects the facts that carbohydrate reserves in the root at the beginning of each dark period were relatively constant (12–19 μmol Glc equivalents plant−1), whereas the rate of 15NH4+ uptake and assimilation during the three successive dark periods increased from 3.9 to 7.8 μmol g−1 fresh weight root h−1 (Fig. 3D).
The increasing proportion of C reserves required to sustain the assimilation of entering NH4+ supports the concept that carbohydrate availability is involved in the regulation of NH4+ uptake and assimilation by the root. If so, the dark-enhanced assimilation of 15NH4+ into the insoluble-15N fraction of the root indicates that C availability is higher during dark periods relative to 15NH4+ uptake. This possibility is consistent with the diurnal pattern of 15NH4+ accumulation in roots (Fig. 2A). Although the 15NH4+ content of roots was low (<5 μmol), fluctuations likely reflect the changing diurnal equilibria between uptake and assimilation of 15NH4+. With the exception of the first dark period, the 15NH4+ content of roots declined in darkness and increased during the light. This suggests that in darkness the assimilation of 15NH4+ usually exceeded its uptake, indicating an adequate supply of carbohydrate. During illumination, however, the 15NH4+ content of roots increased, suggesting that the supply of carbohydrate was insufficient to assimilate all of the entering 15NH4+. Yet, measured carbohydrate levels in roots increased during illumination. Additional studies are in progress to examine this apparent anomaly.
ACKNOWLEDGMENT
We are grateful to P.V. Windsor for excellent technical assistance.
Footnotes
This work was supported by a grant from the Office of International Development of the U.S. Department of Agriculture and by the North Carolina Agricultural Research Service.
LITERATURE CITED
- Cooper HD, Clarkson DT. Cycling of amino-nitrogen and other nutrients between shoots and roots in cereals: a possible mechanism integrating shoot and root in the regulation of nutrient uptake. J Exp Bot. 1989;40:753–762. [Google Scholar]
- Cruz C, Lips H, Martins-Loução Uptake regions of inorganic nitrogen in roots of carob seedlings. Physiol Plant. 1995;95:167–175. [Google Scholar]
- Jackson WA, Chaillou S, Morot-Gaudry J-F, Volk RJ. Endogenous ammonium generation in maize roots and its relationship to other ammonium fluxes. J Exp Bot. 1993;44:731–739. [Google Scholar]
- Jones MGK, Outlaw WH, Jr, Lowry OH. Enzymic assay 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzuker HJ, Siddiqi MY, Glass ADM. Analysis of 13NH4+ efflux in spruce roots. A test case for identification in compartmental analysis. Plant Physiol. 1995a;109:481–490. doi: 10.1104/pp.109.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzuker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of ammonium in spruce. Planta. 1995b;196:691–698. [Google Scholar]
- Lambers H, Simpson RJ, Beilharz VC, Dalling MJ. Translocation and utilization of carbon in wheat (Triticum aestivum) Physiol Plant. 1982;56:18–22. [Google Scholar]
- Lewis OAM, Fulton B, von Zelewski AAA. Differential distribution of carbon in response to nitrate, ammonium and nitrate + ammonium nutrition in wheat. In: Ullrich WR, Aparicio PJ, Syrett PJ, Castillo F, editors. Inorganic Nitrogen Metabolism. Berlin: Springer-Verlag; 1987. pp. 240–246. [Google Scholar]
- Macduff JH, Bakken AK, Dhanoa MS. An analysis of the physiological basis of commonality between diurnal patterns of NH4+, NO3− and K+ uptake by Phleum pratense and Festuca pratensis. J Exp Bot. 1997;48:1691–1701. [Google Scholar]
- Massimino D, André M, Richaud A, Massimino J, Vivoli J. The effect of a day at low irradiance of a maize crop. 1. Root respiration and uptake of N, P and K. Physiol Plant. 1981;51:150–155. [Google Scholar]
- McKenzie HA, Wallace HS. The Kjeldahl determination of nitrogen: a critical study of digestion conditions—temperature, catalyst, and oxidizing agent. Aust J Chem. 1954;7:55–70. [Google Scholar]
- Michael G, Martin P, Owassia I (1970) The uptake of ammonium nitrate in relation to carbohydrate supply of the roots. In EA Kirkby, ed, Nitrogen Nutrition of the Plant. Arthur Wigley & Sons, Leeds, UK, pp 22–29
- Morgan MA, Jackson WA. Suppression of ammonium uptake by nitrogen supply and its relief during nitrogen limitation. Physiol Plant. 1988a;73:38–45. [Google Scholar]
- Morgan MA, Jackson WA. Inward and outward movement of ammonium in root systems: transient responses during recovery from nitrogen deprivation in presence of ammonium. J Exp Bot. 1988b;39:179–191. [Google Scholar]
- Morgan MA, Jackson WA. Reciprocal ammonium transport into and out of plant roots: modifications by plant nitrogen status and elevated root ammonium concentration. J Exp Bot. 1989;40:207–211. [Google Scholar]
- Ourry A, Macduff JH, Prudhomme M-P, Boucaud J. Diurnal variation in the simultaneous uptake and “sink” allocation of NH4+ and NO3− by Lolium perenne in flowing solution culture. J Exp Bot. 1996;47:1853–1863. [Google Scholar]
- Rigano C, Rigano VM, Vona V, Carfagna S, Carillo P, Esposito S. NH4+ assimilation by roots of young barley plants, changes in pool of free glutamine and asparagine and respiratory oxygen consumption. Plant Physiol Biochem. 1996;34:683–690. [Google Scholar]
- Schubert KR (1980) The energy requirement for nitrogen fixation in nodulated legumes. In RJ Summerfield, AH Bunting, eds, Advances in Legume Science. Royal Botanic Gardens, Kew, UK, pp 85–96
- Smith VR. A phenol-hypochlorite manual determination of ammonium-nitrogen in Kjeldahl digests of plant tissue. Commun Soil Sci Plant Anal. 1980;11:709–722. [Google Scholar]
- Volk RJ, Jackson WA. Preparing nitrogen gas for nitrogen-15 analysis. Anal Chem. 1979;51:463–464. [Google Scholar]
- Wang YW, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. I. Fluxes and subcellular distribution of 13NH4+ Plant Physiol. 1993;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]