Abstract
Chronic obstructive pulmonary disease affects millions worldwide. It is America’s third leading cause of death, and results in significant morbidity and cost. Although many therapies exist and are being developed to alleviate symptoms and decrease morbidity and mortality in chronic obstructive pulmonary disease, most have only been studied in placebo-controlled efficacy studies in highly selected populations. Comparative effectiveness and translational research in chronic obstructive pulmonary disease will require the development of infrastructures to support collaboration between researchers and the stakeholders who generate, disseminate and use new knowledge. Methodologies need to evolve to both prioritize research questions and to conduct collaborative comparative effectiveness research studies. Given the impracticality of testing every clinical intervention in comparative pragmatic trials for comparative effectiveness research in chronic obstructive pulmonary disease, we advocate expanding methodology that includes the use of observational databases with serially performed effectiveness analyses and quasi-experimental designs that include following healthcare changes longitudinally over time to assess benefit, harm, subgroups and cost.
Keywords: chronic obstructive pulmonary disease, comparative effectiveness research, data warehouse, emphysema, health services research, outcome research, quasi-experimental design, registry, translational research
Chronic obstructive pulmonary disease (COPD) affects 12–24 million people and is a leading cause of morbidity and mortality in the USA [1,101,102]. COPD recently became America’s third leading cause of death and results in as many as 800,000 hospital admissions annually in the USA [102–104]. Approximately one in four patients hospitalized for COPD are readmitted within 30 days of discharge [2–4,103,104]. Healthcare costs related to COPD are estimated to be approximately US$32–40 billion each year [5,103,104].
Many therapies exist to decrease symptoms related to COPD, reduce hospitalizations or exacerbations, and potentially slow disease progression [6–8,105,106]. However, the vast majority of therapies demonstrated to be beneficial in COPD have been studied in placebo-controlled efficacy studies in highly selected populations enriched with patients most likely to benefit, and in research settings in which patients receive intensive support to optimize self-management and to avoid or minimize medication-related adverse events. Very few clinical trials have prospectively compared therapeutic alternatives [9,10]. Much of the comparative research evidence in COPD has been based on observational studies [11,12]. Furthermore, studies in real world populations of COPD that have looked at adoption of therapies that do have efficacy evidence have found underuse and wide variations in care delivery across the USA [2,4,13,14]. These considerations highlight the need to identify the most effective therapies in real world patients with COPD and to design and test efforts to translate this evidence into healthcare for the millions of patients with COPD. Despite the potentially large impact on improving the demonstrated deficiencies in the translation of efficacy research-supported COPD therapies, quality measurement and improvement science for the treatment and outcomes of patients with COPD has also lagged [15].
In short, there is a need for translational and comparative effectiveness research (CER) in COPD. We developed a consortium of investigators and stakeholders (COPD Outcomes-based Network for Clinical Effectiveness and Research Translation [CONCERT]) and conducted two research development conferences (Setting effectiveness and translational research priorities to improve COPD care, 21–22 May 2009 in San Diego, CA, USA, and Setting effectiveness and translational research priorities to improve COPD care, 20–21 May 2010 in New Orleans, LA, USA). In this review, we discuss considerations for CER in COPD translational research, comment on CER methodology and exemplify some current CER in COPD.
CER delineated for COPD
US governmental and private sector initiatives have attempted to refocus medical research on exploring comparisons of clinical strategies in a way that has culminated in the field of CER. Amongst federal mandates and recent high-profile public discourses, definitions, priorities and approaches for organizing and conducting CER have emerged. The Institute of Medicine (IOM), an independent, nonprofit organization that works outside of the US federal government to provide unbiased and authoritative advice to decision-makers and the public about health issues as an arm of the National Academy of Sciences, offers a broad description for CER that includes “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policymakers to make informed decisions that will improve healthcare at both the individual and population levels”[16]. The IOM definition spans a spectrum from traditional efficacy research through to health services delivery research to highlight the translation of evidence into practice with an overt goal of improving the individual and population health. Thus, the IOM has set the stage for health policy and implementation discourses that include further refinements of the definition.
The Department of Health and Human Services (DHHS), the federal department of the executive branch of the US government responsible for providing a wide range of health benefits and services to the American public through programs such as the CDC, the Centers for Medicare and Medicaid Services (CMS) and the NIH, is the major funder of medical research in the USA and has been charged under the recent Patient Protection and Affordable Care Act of 2010 to establish methodological standards for CER and policies for research funding. The Federal Coordinating Council for Comparative Effectiveness defines CER as research that “provides information on the relative strengths and weakness of various medical interventions. Such research will give clinicians and patients valid information to make decisions that will improve the performance of the USA healthcare system” [17,18]. And specifically this cross-institute council indicated that CER is the “conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in ‘real world’ settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence-based information to patients, clinicians, and other decision-makers, responding to their expressed needs, about which interventions are most effective for which patients under specific circumstances.”
Also established in the 2010 Patient Protection and Affordable Care legislation was the Patient-Centered Outcomes Research Institute (PCORI), which has been charged with moving forward from these definitions to establish a functional approach and practical strategies to develop and organize the infrastructure for prioritizing and conducting CER. The research questions most appropriate for CER in COPD need to be defined and prioritized. Given the emphasis in the DHHS definition of CER on end users (patients, clinicians and other decision-makers), prioritization of CER questions requires input from the diverse perspectives of stakeholders who generate, disseminate and will hopefully use new knowledge.
Another US federal branch of the DHHS, the Agency for Healthcare Research and Quality (AHRQ), focuses on the health delivery end of the research spectrum and proposed steps for conducting CER with an expressed goal of furthering the development of the research infrastructure as follows [19,107]:
Identify new and emerging clinical interventions
Review and synthesize current medical research
Identify gaps between existing medical research and the needs of clinical practice
Promote and generate new scientific evidence and analytic tools
Train and develop clinical researchers
Translate and disseminate research findings to diverse stakeholders
Reach out to stakeholders via a citizens’ forum
Each of the institutes within the NIH, AHRQ and CMS has chosen to focus its funding upon different aspects of CER infrastructure and research. The NIH as the major funder of basic science and clinical trials is targeting trial infrastructure such as expanding clinical trial networks and developing novel requests for applications and other investigator-initiated grants; the AHRQ continues its focus on review and synthesis of existing evidence while advancing novel approaches for CER methodology; and the CMS is focusing on infrastructure and database development through initiatives such as the Multi-Payer Research Database [17,20]. With multiple branches of the US government emphasizing the adoption of meaningful use of electronic medical records, it is anticipated that databases available for CER will expand in the future.
Characterizations of CER recognize that for this evolving research paradigm to succeed in improving health across the USA, it must assess a wide range of health-related outcomes across diverse patient populations and subgroups. This strategy is in contradistinction to traditional efficacy research that narrowly defines its research population to avoid contamination by confounds to the major clinical effect under study. The result of a narrowed population is its compromise to external validity or what has been termed generalizability; for example, can a physician apply the results of an efficacy study to a particular patient or patient population. Extensive exclusion and inclusion criteria of efficacy studies or randomized controlled trials (RCTs) may severely limit generalizability or application of evidence. Thus, CER strives to conduct research in a more usable way across broad populations of ‘real world’ patients with the goal that health can be improved. Moreover, CER suggests that interventions for comparison are equally broad, spanning such therapies as medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies. In addition, the field of CER and its champions, such as the IOM and PCORI, are engaged in expanding data sources and methods of assessment to achieve efficient, impactful studies with active dissemination of results that can and will be useful in healthcare [18,20–22]. Implicit across the definitions of CER is the need for novel methodology to include broad stakeholder input to prioritize research topics and engage in subsequent practical translation. The imperative that CER “responds to the expressed needs of patients, clinicians, and other decision-makers” necessitates ways to bring researchers, providers, administrators, patients and payers together to develop research agendas and apply the generated evidence [16,17,20,107]. The new networks and institutes, such as the PCORI, have begun to establish methods groups to identify optimal ways to synthesize and conduct CER.
General methodological approaches for consideration in CER for COPD
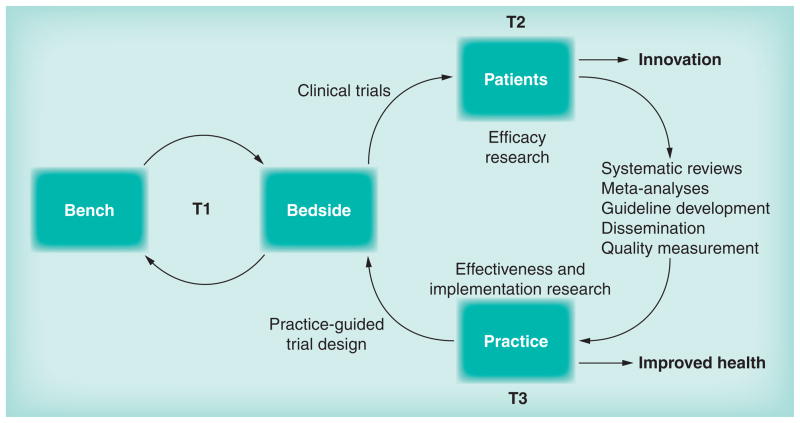
CER is concerned with the conduct and synthesis of systematic research comparing different interventions and strategies to prevent, diagnose, treat and monitor health conditions. Its purpose is to inform patients, providers and decision-makers about which interventions are most effective for patients under specific circumstances [17]. CER may employ observational or clinical trial methodologies to compare strategies for care available in typical healthcare settings, provided by typical healthcare providers, addressing a comprehensive array of health-related outcomes. In general, outcomes are favored to be patient centered and the research should be conducted in ‘real world’ diverse and representative patient populations. Translational research includes three stages: T1 is the classic bench-to-bedside (or rather human) translation; T2 constitutes efficacy research and clinical trials; and T3 is translational research into health delivery, effectiveness and implementation that explores general medical practice [23]. Thus, CER methods have the opportunity to evolve toward the translation of research findings that emerge from traditional efficacy research, in which comparisons between alternatives (including no treatment or placebo) are conducted in controlled clinical trials within narrowly selected patient populations (i.e., those who are presupposed to benefit) and in resource-intensive research settings in which interventions are administered by investigators and their research staff. Comparative efficacy studies include research to evaluate the effects of interventions, thus measuring outcomes related to underlying disease mechanisms or clinical changes to the disease from interventions. In short, comparative efficacy studies are critical to answering questions such as ‘Can this intervention work?’ and ‘How does this intervention work?’ under ideal and carefully controlled research conditions, whereas comparative effectiveness studies may address translational questions such as ‘Which interventions when translated into practice result in care improvements and increase the likelihood of health benefits?’ Ultimately, it is the efficacy research that leads to innovations and effectiveness research that leads to practice change and improved health (Figure 1).
Figure 1. Translational research model.
Efficacy (T2) leads to innovation while effectiveness and implementation (T3) lead to health improvement.
A straightforward approach to CER would be to apply traditional efficacy methods such as the strong research design of the RCT. However, not every important CER question can be answered by large RCTs. Even pragmatic strategies that attempt to minimize investigator control of interventions, reduce exclusions and maximize generalizability have limitations due to resource availability, sufficient patients or sample size for enrollment, and lengthy time needed for outcome evaluation and follow-up. As trial synthesis and meta-analysis currently rely on evidence from randomized clinical trials, one can anticipate tension in how to apply these methods to CER with sufficient representativeness and generalizability that will require expanded intermediary study designs. New approaches to observational designs should thus be part of the methodological development to realize the promise of CER and translational science. Synergy of these approaches, such as large database development, with health information technology mandates may create unique opportunities with a national evolution to electronic medical records and interoperability. For COPD, novel opportunities for translational and effectiveness methodologies may evolve to effectively and efficiently evaluate CER questions and guide subsequent care innovation.
One example of this kind of research that can ultimately also help guide implementation and care improvement can come from conducting CER within linked registries that have been organized to monitor and guide quality of care [24,25]. By conducting translational and CER research across linked registries for patients and providers in real world conditions, researchers and policymakers can explore CER across a wide representative sample of US hospitals and monitor effects of healthcare policy and care improvement efforts serially over time. Methods that employ sophisticated modeling techniques to control for confounding and selection bias have evolved and the large samples reduce opportunities for error [26–32]. In addition, subgroups such as those in racial and ethnic minorities, the socioeconomically disadvantaged and those with multimorbid conditions can be assessed for differences in responses, risks and benefits.
In some circumstances, confounding and selection bias cannot be definitively excluded in observational designs and thus large randomized clinical trials will be needed to assess the comparative effectiveness of some treatment alternatives. However, even in these trials, observational studies can guide the development and design of pragmatic RCTs by providing essential preliminary data on relevant comparators, outcomes and estimations of sample size. Even when a definitive RCT answers a particular CER question, databases and registries will be beneficial for studying implementation and practice change. Such resources will additionally enable ongoing surveillance and quality monitoring across patient-focused outcomes that can further guide practice changes. Large, representative, multisite registries, preferably enhanced in the future with clinical information derived from increased use of electronic medical records, may provide the necessary data monitoring of the effects of practice change, including assessing for harm in real world settings. With sustained funding, registry-conducted CER could document care delivery and provide quality measurement that can assist ongoing care enhancement and compliment experimental design by informing future clinical trials.
Mixed methods and quasi-experimental designs should also be considered as part of the comprehensive approach to CER in COPD [26–32]. Research in real world settings may not always have the luxury of random assignment or it may be difficult to devise a control group because of ethical objections to withholding an intervention or components of the intervention from one of the study groups. In addition, the researcher may not have the level of control of the delivery of the intervention in the real world setting that can be found in an experimental setting. Quasi-experimental designs can be a strong alternative to randomized clinical trials when research is conducted in real world settings. The trade-off involved with conducting research in the sometimes difficult real world setting is increased external validity and generalizability to other real world clinical settings.
Quasi-experimental designs, such as the regression discontinuity design, have been shown, when analyzed correctly, to lead to unbiased estimates of the difference between groups [26–28,31]. In this design, assignment to groups is based on some continuous variable; individuals who exceed some threshold are assigned to the experimental group and those that do not are assigned to the control group. This type of design mimics what happens in the clinical setting, where, for example, patients are prescribed blood pressure medicine only if their blood pressure exceeds safe limits. Interrupted time series and nonequivalent control group designs are other useful approaches in community-based research, in which outcomes are followed over time [30]. While not as strong as the regression discontinuity or interrupted time series designs, the nonequivalent control group design can be strengthened through the use of certain design elements and statistical analyses [27,31,32]. In this design, naturally occurring groups of participants are compared. The groups could be, for example, patients treated at different clinics or treated by different healthcare teams.
Given the impracticality of testing every clinical intervention in comparative pragmatic trials, we advocate expanding methodology that includes the use of linked registries, serially performed rigorous observational effectiveness analyses and quasi-experimental designs for CER in COPD. To further ensure that the benefits promised by CER (whether by observational, RCT or quasi-experimental design) are indeed realized, we would further recommend follow-up studies to evaluate time trends in quality metrics, health outcomes to monitor for possible harms and subgroup variations, and costs analyses to be considered as components of implementation.
Exemplary CER work: treatment of acute exacerbation of COPD
A recent example of CER in COPD comes from the work by Lindenauer and colleagues published in 2010 [11]. Using a large administrative data set from linked cohorts across the premier health-care system, the investigators compared non-intensive care unit-hospitalized patients receiving intravenous or oral steroids for acute COPD exacerbations. The use of steroids is one of the few guideline-recommended care interventions with efficacy research support [6–8,105,106]. The guidelines advocate the use of systemic cortico-steroids for hospitalized COPD exacerbations and generally suggest oral prednisone equivalents of 30–40 mg daily if tolerated, or if it is unable to be used orally, then to use equivalent intravenous doses. However, the best efficacy data for steroids come from the seminal paper by Neiwoehner and colleagues in 1999 who conducted a three-arm RCT where all patients initially received 125-mg intravenous methylprednisolone every 6 h for 72 h followed by different oral prednisone daily tapers [33]. The guideline recommendations are influenced by the increased side effects associated with higher corticosteroid dosages.
One of the key findings of the Lindenauer et al. study was the documentation of care delivery that differs greatly from guidelines, perhaps being significantly influenced by the seminal efficacy study [11,33]. Lindenauer and colleagues identified intravenous doses that ranged from 120 to 800 mg/day prednisone equivalents in the vast majority of patients with those that did receive oral dose receiving 20–80 mg/day prednisone equivalents.
The investigators went on to develop a composite patient-centered outcome based on treatment failure, the need for mechanical ventilation after hospital day 2, readmission within 30 days and inpatient mortality. The study’s major finding was that there was no worse outcome with low-dose oral steroids compared with high-dose intravenous delivery. The investigators found a trend to better outcomes with low-dose oral steroids with an unadjusted treatment failure odd ratio of 0.91 (95% CI: 0.83–1.00). They further demonstrated rigorous CER observational methods to adjust for sources of bias, including propensity score and covariate adjustment, matched sample unbalanced covariate adjustment, and dose effect modeling assuming a 10% increase in the proportion of oral steroid use in hospitals.
The accompanying editorial from two CONCERT investigators noted that there are two “options for moving forward. One approach would be to conduct a large-scale, pragmatic, noninferiority clinical trial [requiring] 30,000 [to 120,000] patients” [25]. This calculation came from the effect sizes in the Lindenauer paper demonstrating the utility of observational studies for planning of subsequent RCTs. Alternatively, clinicians and healthcare systems could “advocate for translating these research findings into clinical practice now and for developing implementation and dissemination campaigns to facilitate uptake, including the development and testing of quality metrics.” Well noted is that “caution should be exercised when advocating a change in clinical practice based on observational research” alone. But here indeed lies another opportunity for CER within linked registries, “enabling ongoing surveillance of care quality and patient outcomes [and] can be used to track changes in practice, patient adherence, as well as benefits and harms in real world settings.”
Thus, observational data repositories that are refreshed over time with sustained funding could further assess any potential harm from practice changes as well as explore benefit in subgroups, including those typically under-represented in efficacy research (e.g., those with multiple comorbid conditions or race/ethnic minorities). Data sources could further be used to document the quality of care delivery and provide useful quality measurement to track and assist efforts to enhance care. Such monitoring over time could provide further CER evidence that improvements in health and outcomes occur with research translation and implementation [25].
Consortium for CER in COPD: CONCERT
In response to the emerging CER landscape and prior identification of the need for better research translation, implementation and outcome analyses in COPD, we have established a consortium for COPD research with an explicit focus on CER. CONCERT is an interdisciplinary and multi-institutional team of investigators dedicated to improving the outcomes of patients with COPD through the development of effective methods to translate biomedical knowledge into routine practice [108]. With research funding support from AHRQ and the National Heart, Lung, and Blood Institute (NHLBI), CONCERT has begun working in the CER arena in COPD, including successfully engaging a national group of stakeholder organizations to identify and prioritize a translational research agenda in acute and chronic COPD care.
Published research by CONCERT investigators has demonstrated that the care and outcomes of patients with COPD vary substantially across the USA, and that many patients are not benefiting from guideline-recommended therapies supported by efficacy research [4,13]. One potential contributor to such practice variation is the lack of data about the effectiveness of treatment strategies in ‘real world’ clinical practice. We also surmise that gaps in health services knowledge about how best to implement evidence-based care in diverse clinical settings contribute to poor translation. Such gaps in effectiveness knowledge may lead to limited healthcare decision-making amongst patients and clinicians. These factors and other aspects, such as healthcare costs associated with the novel adoption of new and often more expensive treatments, have been implicated as reasons for poor translation of clinical efficacy data into best clinical practices and have stimulated the NIH to develop a COPD Awareness Campaign [109]. On the basis of the emerging research paradigms, these findings have increased interest in comparisons of existing interventions, including pharmacologic agents, devices, and strategies of care to improve outcomes and the efficiency with which healthcare is delivered. Through a series of Consensus Conferences (‘Setting effectiveness and translational research priorities to improve COPD care’) held in 2009 and 2010, CONCERT explored various topics and research questions amongst its diverse transdisciplinary group.
CONCERT is a multi-institutional and interdisciplinary consortium of investigators at six US medical centers (Baystate Medical Center, Kaiser Permanente Northwest and The Center for Health Research, University of Chicago, University of Illinois at Chicago, University of North Carolina at Chapel Hill, and University of Washington) with expertise in comparative effectiveness, cost–effectiveness, epidemiology, pharmacoepidemiology, statistics, clinical trials, performance measurement, quality improvement, behavioral psychology and multi-center data coordination. Investigators within CONCERT also have clinical expertise in hospital medicine, pulmonary medicine, critical care medicine and behavioral science, and provide care to diverse patient populations with COPD in managed care and nonmanaged care settings. The network engaged private, governmental and patient advocacy groups through support from an R13 conference grant from the AHRQ (HS017894) to identify and discuss research topics and questions in COPD. The uniqueness of the stakeholder group includes its broad representation and extensive expertise of participants that spans insurers/health plans, professional organizations, quality improvement organizations, government and private research entities, and patient advocacy groups (Box 1). As part of the methods of developing prioritized topics for CER in COPD, CONCERT worked with its workshop participants who were solicited from its stakeholder groups and identified research questions for consideration. The solicitation had the expressed objective of identifying real world translational research questions and strategies to accelerate adoption and implementation of interventions to improve COPD care. General topics in COPD that were considered are summarized in Box 2–5.
Box 1. Stakeholder groups who participated in CONCERT Consensus Conferences.
|
COPD: Chronic obstructive pulmonary disease.
Box 2. Research topics in chronic obstructive pulmonary disease care coordination.
|
COPD: Chronic obstructive pulmonary disease.
Box 5. Research topics in transitions in chronic obstructive pulmonary disease care.
|
COPD: Chronic obstructive pulmonary disease.
Box 3. Research topics in chronic obstructive pulmonary disease care.
|
COPD: Chronic obstructive pulmonary disease.
Box 4. Research topics in acute chronic obstructive pulmonary disease care.
|
AE-COPD: Acute exacerbations of chronic obstructive pulmonary disease; COPD: Chronic obstructive pulmonary disease; NIV: Noninvasive ventilation.
In a series of two conferences coinciding with the American Thoracic Society meetings (‘Setting effectiveness and translational research priorities to improve COPD care’) the CONCERT consortium worked to develop a research agenda across four areas:
Chronic COPD care
COPD care coordination
Acute COPD care
Transitions in COPD care
Advancing the methodology for consensus building amongst stakeholders in COPD, CONCERT adapted modified Delphi techniques for ranking and the use of analytical hierarchy processes for developing prioritized agendas [34–37]. A separate report summarizing the results of the Consensus Conferences is in development.
In addition, with funding from the NHLBI (RC2 HL101618), CONCERT has also designed and developed a comprehensive data repository for patients with evidence of obstructive lung disease across its six sites called the COPD Data Hub. The intent of this Data Hub is to support novel CER studies in diverse COPD patient populations that span managed care and non-managed care settings linked to the research agenda developed during the CONCERT Consensus Conferences. In this respect, CONCERT is attempting to respond to the DHHS definition for CER, which emphasizes the need for stakeholder input in the conduct of CER studies. The COPD Data Hub links administrative and electronic sources of clinical data across the six CONCERT clinical centers and if successfully maintained with sustaining funding will provide longitudinal data on care practices and health outcomes across diverse, real world, healthcare settings. The COPD Data Hub was designed to be flexible and incorporate data from varied healthcare settings that include university-affiliated medical centers, a community medical center, a health maintenance organization and a government-operated veteran medical center. Preliminary data from 2006 to 2008 (pending 2009–2010 data collection) includes 146,240 unique patients of whom 58.3% were female at nonveteran sites; 68.4% were Caucasians and 14.3% African–American, and the average patient age was 61.8 years (standard deviation: 13.5) [38,39]. These findings suggest that the COPD Data Hub will include a substantially greater diversity of patients (e.g., higher proportion of women and greater diversity by race) than has been typically possible in recently published large-scale clinical trials of efficacy [9,10]. The COPD Data Hub infrastructure will facilitate the development of appropriately designed and ‘shovel-ready’ observational CER studies. Furthermore, CONCERT has enriched the COPD Data Hub by collecting additional detailed clinical and questionnaire information on a sample of 1200 patients selected from the COPD Data Hub. With added data of baseline spirometry, 6-min walk distance and quality of life metrics, the Network can further validate and leverage the usefulness of the larger COPD Data Hub by linking it to disease severity, functional status and various patient-centered outcomes. In addition, recruited individuals can serve to accelerate enrollment into subsequent CER prospective studies. Using the Data Hub, CONCERT has recently conducted a preliminary analysis of recommended care components that might be used to comprise a quality of care bundle for hospitalized acute exacerbations of COPD. Targeting priorities from our Consensus Conferences, we explored interventions to improve care delivery. Drawing from clinical practice guidelines, we identified components of recommended care such as the use of bronchodilators, supple mental oxygen, noninvasive positive pressure ventilation and corticosteroids. We then used a cross-sectional design across a random sample of acute COPD hospitalizations in the year 2008 from our Data Hub. Using existing data in the COPD Data Hub supplemented with chart abstraction, we explored how often we could find evidence for care delivery for the recommended components of care for hospitalized COPD acute exacerbation [39]. From 242 unique patients across our six sites, we identified variation and low delivery of all components of a possible care bundle (Figure 2). These findings supported the development of a multisite research application to improve the care of hospitalized COPD acute exacerbation.
Figure 2. Processes of care and summary measures at each CONCERT site.
CONCERT: Chronic obstructive pulmonary disease Outcomes-based Network for Clinical Effectiveness and Research Translation.
Conclusion
In general, CER in COPD will require the development of infrastructures to support collaboration between researchers and the stakeholders who generate, disseminate and use new knowledge. Methodologies need to evolve to both prioritize research questions with stakeholder input and to conduct collaborative CER studies of high potential value. Stakeholder representation should routinely include patients, clinicians, professional organizations, payers, policymakers, regulators, funding agencies and researchers. Given the impracticality of testing every clinical intervention in comparative pragmatic trials for CER in COPD, we advocate expanding methodologies to include the use of observational databases with serially performed effectiveness analyses and quasi-experimental designs. To further ensure that the benefits promised by CER are indeed realized, we would further recommend follow-up studies in linked registries to evaluate trends over time in quality metrics and health outcomes. Such monitoring will further allow monitoring for possible harms, analyses within subgroups or in those with comorbidities, and explorations of cost as a component of implementation research.
Future perspective
COPD is an increasingly prevalent and morbid disease worldwide with considerable consequences in human suffering, disability and economic cost. Over the next decade, substantial work will be needed to understand which therapies should be used in which patients and how efficiently they decrease symptoms, reduce hospitalizations or exacerbations, and potentially slow disease progression. For CER to impact individuals in real world populations with multimorbid illnesses, methodological techniques will need to evolve from the current reliance on placebo-controlled efficacy studies in highly selected populations to pragmatic and observational studies with rigorous study design. Registries and multisite cross-institutional databases are likely to play important roles in guiding the translation of efficacy research-supported COPD therapies and allowing and supporting quality measurement and improvement in the treatment of COPD. Serial real-time assessments in such registries will allow longitudinal follow-up and enable exploration into how healthcare delivery and implementation of changes based on CER actually effect individuals and subpopulations. This is an exciting time for research across diseases such as COPD and holds great promise for improving health if future funding adequately sustains the evolving infrastructures and political pressures do not undermine rational data gathering and transparent analysis and dissemination.
Executive summary.
Chronic obstructive pulmonary disease (COPD) affects millions worldwide and is the third leading cause of death in the USA, and results in significant morbidity and cost.
The Institute of Medicine defines comparative effectiveness research (CER) as “the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat and monitor a clinical condition or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers and policymakers to make informed decisions that will improve healthcare at both the individual and population levels.”
The USA 2010 Patient Protection and Affordable Care legislation established the Patient-Centered Outcomes Research Institute that has been charged with establishing a functional approach and practical strategies to develop and organize the infrastructure for prioritizing and conducting CER.
CER includes observational or clinical trial methodologies to compare strategies for care available in typical healthcare settings, provided by typical healthcare providers, addressing a comprehensive array of health-related outcomes.
New approaches to randomized trials, observational studies and evidence synthesis methodology will be needed to realize the promise of CER and translational science in COPD and across other diseases.
Given the impracticality of testing every clinical intervention in large randomized comparative trials, CER will benefit from expanding observational methodology that includes the use of linked registries with serially performed analyses and quasi-experimental designs for CER in COPD.
Acknowledgments
The authors gratefully acknowledge discourse at some meetings/presentations during the May 2011 American Thoracic Society International conference that helped inform portions of our manuscript, and the contributions to the CONCERT CER Consensus Conferences, including members of the Conference Organizing Committee (G Bauldoff, Ohio State University; KW Lin, Agency for Healthcare Research and Quality; R Smith, National Heart, Lung and Blood Institute; B Yawn, Olmsted Medical Center and CONCERT investigators as listed in author list), the CONCERT External Advisory Committee (SA Buist, Oregon Health and Sciences University; S Finn, Department of Veteran’s Affairs; E Lang, McGill University; D Mannino, University of Kentucky School of Medicine; P Pronovost, Johns Hopkins University; CS Rand, Johns Hopkins University; P Wyer, Columbia University) and the participants from the stakeholder organization that participated in one of the Consensus Conferences in 2009 and 2010 as listed in Box 1.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors financially acknowledge federal research funding for CONCERT projects in comparative effectiveness research in chronic obstructive pulmonary disease, including National Heart, Lung, and Blood Institute RC2 HL101618 and the Agency for Healthcare Research and Quality R13 HS017894, which helped inform this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance: United States, 1971–2000. MMWR Surveill Summ. 2002;51(6):1–16. [PubMed] [Google Scholar]
- 2.Medicare Payment Advisory Commission. Report to the Congress: Promoting Greater Efficiency in Medicare. MEDPAC; Washington, DC, USA: 2007. [Google Scholar]
- 3.Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228. doi: 10.3109/15412555.2010.481697. [DOI] [PubMed] [Google Scholar]
- 4.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 5.Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the US: a review and synthesis of the literature. COPD. 2006;3(4):211–218. doi: 10.1080/15412550601009396. [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 7.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 9.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 11.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 12.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 13.Mularski RA, Asch SM, Shrank WH, et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–1850. doi: 10.1378/chest.130.6.1844. [DOI] [PubMed] [Google Scholar]
- 14.Bratzler DW, Oehlert WH, McAdams LM, Leon J, Jiang H, Piatt D. Management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: physician practices in the community hospital setting. J Okla State Med Assoc. 2004;97(6):227–232. [PubMed] [Google Scholar]
- 15.Heffner JE, Mularski RA, Calverley PM. COPD performance measures: missing opportunities for improving care. Chest. 2010;137(5):1181–1189. doi: 10.1378/chest.09-2306. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Institute of Medicine. Initial National Priorities For Comparative Effectiveness Research. National Academies Press; Washington, DC, USA: 2009. Provides both the background for the evolving comparative effectiveness research (CER) field and clarification of its goals and approaches. [Google Scholar]
- 17.Federal Coordinating Counsel for Comparative Effectiveness Research. Report to the President and Congress. Department of Health and Human Services; Washington, DC, USA: 2009. pp. 1–73. [Google Scholar]
- 18▪.Lieu TA, Au D, Krishnan JA, et al. The Comparative Effectiveness Research in Lung Diseases Workshop Panel. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the national heart, lung and blood institute workshop. Am J Respir Crit Care Med. 2011;184:848–856. doi: 10.1164/rccm.201104-0634WS. Recent summary of CER and US governmental perspectives regarding lung diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iglehart JK. Prioritizing comparative-effectiveness research – IOM recommendations. N Engl J Med. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 20▪.Lauer MS, Collins FS. Using science to improve the nation’s health system: NIH’s commitment to comparative effectiveness research. JAMA. 2010;303(21):2182–2183. doi: 10.1001/jama.2010.726. Provides a summary of and priorities for CER within the NIH. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Clancy C, Collins FS. Patient-Centered Outcomes Research Institute: the intersection of science and healthcare. Sci Transl Med. 2010;2(37):37cm18. doi: 10.1126/scitranslmed.3001235. Provides a summary of and priorities for the Patient-Centered Outcomes Research Institute. [DOI] [PubMed] [Google Scholar]
- 22.Selker HP, Strom BL, Ford DE, et al. White paper on CTSA consortium role in facilitating comparative effectiveness research: September 23, 2009 CTSA consortium strategic goal committee on comparative effectiveness research. Clin Transl Sci. 2010;3(1):29–37. doi: 10.1111/j.1752-8062.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty D, Conway PH. The ‘3T’s’ road map to transform US healthcare: the ‘how’ of high-quality care. JAMA. 2008;299(19):2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- 24.Kahn JM, Scales DC, Au DH, et al. An official American Thoracic Society policy statement: pay-for-performance in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2010;181(7):752–761. doi: 10.1164/rccm.200903-0450ST. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan JA, Mularski RA. Acting on comparative effectiveness research in COPD. JAMA. 2010;303(23):2409–2410. doi: 10.1001/jama.2010.807. [DOI] [PubMed] [Google Scholar]
- 26.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-experimental Designs for Generalized Causal Inference. Houghton-Mifflin; Boston, MA, USA: 2002. [Google Scholar]
- 27.Mark MM, Rechardt CS. Quasi-experimental and correlational designs: methods for the real world when random assignment isn’t feasible. In: Sansone C, Morf CC, Panter AT, editors. Handbook of Methods in Social Psychology. Sage Publications; Thousand Oaks, CA, USA: 2004. pp. 265–286. [Google Scholar]
- 28.Mercer SL, DeVinney BJ, Fine LJ, Green LW, Dougherty D. Study designs for effectiveness and translation research: identifying trade-offs. Am J Prev Med. 2007;33(2):139–154. doi: 10.1016/j.amepre.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Assignment to treatment group on the basis of a covariate. J Educat Stat. 1977;2:1–26. [Google Scholar]
- 30.Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci. 2000;1(1):31–49. doi: 10.1023/a:1010024016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West SG, Biesanz JC, Pitts SC. Causal inference and generalization in field settings: experimental and quasi-experimental designs. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. Cambridge University Press; NY, USA: 2000. pp. 40–84. [Google Scholar]
- 32.Hawkins NG, Sanson-Fisher RW, Shakeshaft A, D’Este C, Green LW. The multiple baseline design for evaluating population-based research. Am J Prev Med. 2007;33(2):162–168. doi: 10.1016/j.amepre.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340(25):1941–1947. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2(3):i–iv. 1–88. [PubMed] [Google Scholar]
- 35.Vaidya OS, Kumar S. Analytic hierarchy process. Eur J Oper Res. 2006;169(1):1–29. [Google Scholar]
- 36.Saaty TL. Relative measurement and its generalization in decision making. Why pairwise comparisons are central in mathematics for the measurement of intangible factors The analytic hierarchy/network process. Rev R Acad Cien Serie A Mat. 2008;102(2):251–318. [Google Scholar]
- 37.Saaty TL. Decision making with the analytic hierarchy process. Int J Serv Sci. 2008;1(1):83–98. [Google Scholar]
- 38.Mularski RA, McBurnie MA, Lindenauer PK, et al. Establishment of an integrated clinical network and comprehensive data warehouse for the conduct of comparative effectiveness research in COPD. Presented at: European Respiratory Society Annual Congress; Amsterdam, The Netherlands. 24–28 September 2011; p. Abstract 4871. [Google Scholar]
- 39.Lewis S, Krishnan JA, Au DH, et al. Novel multi-institutional data infrastructure for comparative effectiveness research in COPD (COPD DataHub) Am J Resp Crit Care Med. 2011;183:A6437. [Google Scholar]
Websites
- 101. [Accessed 27 August 2011];NHLBI morbidity and mortality chartbook. www.nhlbi.nih.gov/resources/docs/cht-book.htm.
- 102. [Accessed 27 August 2011];FASTSTATS: chronic lower respiratory disease. www.cdc.gov/nchs/fastats/copd.htm.
- 103.HCUP nationwide inpatient sample (NIS) Healthcare cost and utilization project (HCUP) 2000–2006. Agency for Healthcare Research and Quality; Rockville, MD, USA: www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 104. [Accessed 21 June 2011];HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. http://hcupnet.ahrq.gov.
- 105. [Accessed 21 June 2011];Global strategy for the diagnosis, management and prevention of COPD: global initiative for chronic obstructive lung disease (GOLD) www.goldcopd.org.
- 106. [Accessed 21 June 2011];NICE clinical guidance: chronic obstructive pulmonary disease. http://guidance.nice.org.uk/CG12/NICEGuidance/pdf/English.
- 107▪▪. [Accessed 25 July 2011];AHRQ: What is comparative effectiveness research. www.effectivehealthcare.ahrq.gov/index.cfm/what-is-comparative-effectiveness-research1/ Provides a summary and priorities for CER within the Agency for Healthcare Research and Quality.
- 108. [Accessed 4 August 2011];COPD Outcomes-based Network for Clinical Effectiveness and Research Translation (CONCERT) investigators. www.kpchr.org/concert.
- 109.NIH. COPD: learn more, breathe better campaign. National Heart Lung Blood Institute; Washington, DC, USA: [Accessed 4 August 2011]. www.nhlbi.nih.gov/health/public/lung/copd/lmbb-campaign/index.htm. [Google Scholar]