Abstract
This study addresses the role of glycogen synthase kinase (GSK)-3β signaling in the tumorigenic behavior of melanoma. Immunohistochemical staining revealed GSK3β to be focally expressed in the invasive portions of 12% and 33% of primary and metastatic melanomas, respectively. GSK3 inhibitors and siRNA knockdown of GSK3β were found to inhibit the motile behavior of melanoma cells in scratch wound, 3D collagen implanted spheroid and modified Boyden chamber assays. Functionally, inhibition of GSK3β signaling was found to suppress N-cadherin expression at the mRNA and protein levels and was associated with decreased expression of the transcription factor Slug. Pharmacological and genetic ablation of GSK3β signaling inhibited the adhesion of melanoma cells to both endothelial cells and fibroblasts and prevented transendothelial migration, an effect rescued by the forced overexpression of N-cadherin. A further role for GSK3β signaling in invasion was suggested by the ability of GSK3β inhibitors and siRNA knockdown to block phosphorylation of FAK and increase the size of focal adhesions. In summary, we have demonstrated a previously unreported role for GSK3β in modulating the motile and invasive behavior of melanoma cells through N-cadherin and FAK. These studies suggest the potential therapeutic utility of inhibiting GSK3β in defined subsets of melanoma.
Introduction
Glycogen synthase kinase-3β is a serine/threonine kinase that sits at the junction of the PI3K/AKT and Wnt signaling pathways (Cohen and Frame, 2001). Its activity is inhibited by AKT, which phosphorylates and inactivates the kinase (Cross et al., 1995). GSK3β plays a critical role in the regulation of canonical Wnt signaling by directly phosphorylating β-catenin leading in turn to its proteasomal targeting and subsequent degradation (Yost et al., 1996). Although increased Wnt/β-catenin signaling has been implicated in oncogenesis (Camilli and Weeraratna, 2010; He et al., 1998), there is currently little evidence that inhibition of GSK3β contributes to melanoma progression (Arozarena et al., 2011; Chien et al., 2009).
In addition to its role in Wnt survival signaling, β-catenin is also expressed at adherens junctions, where it facilitates cell-cell adhesion through an association with E-cadherin and N-cadherin (Harris and Tepass, 2010; Smalley et al., 2005). Adherens junctions have been best studied in epithelial tissues where they are known to be critical for the maintenance of cellular architecture (Lioni et al., 2007). Of relevance to melanoma, both melanocytes and skin keratinocytes express E-cadherin and there is evidence that homotypic E-cadherin signaling constitutes a major regulatory mechanism of the “epidermal melanin unit” in normal skin (Haass et al., 2005; Hsu et al., 2000b). Loss of E-cadherin expression is often viewed as a first step in melanoma progression that allows transformed melanocytes to escape from local keratinocyte control (Li et al., 2001). In melanoma development, E-cadherin loss is typically accompanied by an increase in N-cadherin expression that facilitates tumor cell dissemination by increasing the interaction of melanoma cells with host endothelial cells and fibroblasts as well as increasing melanoma cell survival (Li et al., 2001; Li et al., 2003).
Very little is currently known about the function of GSK3β signaling in melanoma. Here we present new data demonstrating that low constitutive levels of GSK3β signaling contribute to the oncogenic behavior of melanoma by regulating both N-cadherin expression and focal adhesion complexes. For these studies we used a highly potent organometallic kinase inhibitor of GSK3β (IC50 0.3 nM) (Atilla-Gokcumen et al., 2008; Pagano et al., 2007) to show that inhibition of GSK3β signaling limits the motile and invasive behavior of melanoma cells through a mechanism associated with decreased Slug expression. We also provide evidence that inhibition of GSK3β abrogates the interaction of melanoma cells with host fibroblasts and endothelial cells.
Results
GSK3β inhibition blocks the migration and invasion of melanoma cell lines
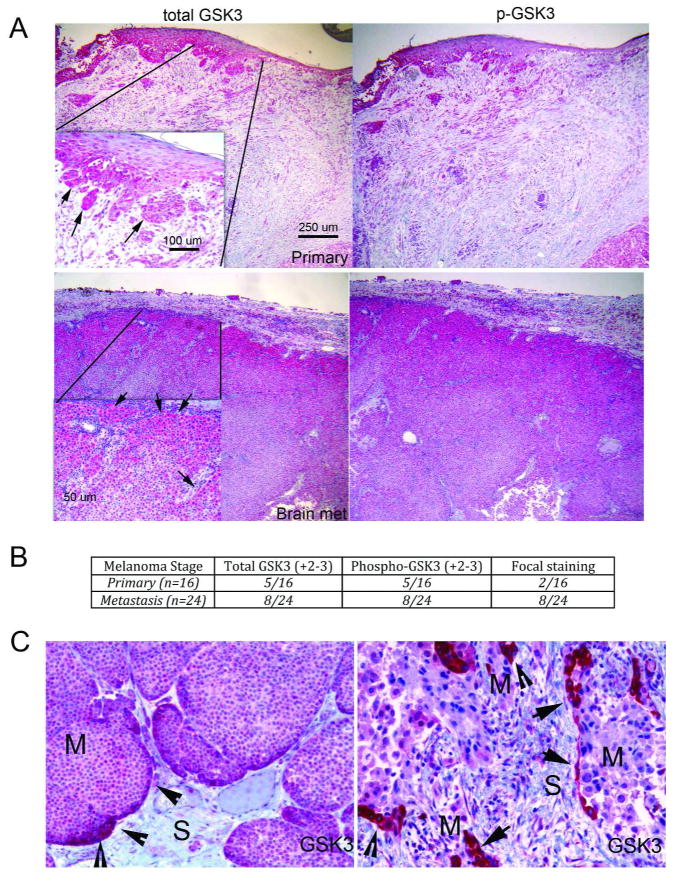
Western blotting revealed GSK3β to be constitutively phosphorylated at Ser9 in nearly all of the melanoma cell lines tested (Supplemental Figure 1A). No correlation was noted between phospho-GSK3β expression and the presence of either BRAF or an NRAS mutation or PTEN expression (Supplemental Table 1 and not shown). Treatment of melanoma cell lines with NP309 (300 nM) and another structurally unrelated GSK3β inhibitor (LiCl) led to increased β-catenin expression (Supplemental Figure 2), demonstrating the presence of an activated GSK3β pool. Analysis of melanoma lesions (n=40) showed GSK3β and phospho-GSK3β to be expressed in both primary (5/16) and metastatic specimens (8/24). The strongest staining was noted to be focal and located to leading edge areas of the tumor, where the tumor and stroma were interacting (Figures 1A–C; Supplemental Figure 3). In primary melanoma, the strongest GSK3β staining was located at the invasive front, with fewer primary samples exhibiting strong focal staining (2/16) than metastatic samples (8/24). As the leading edge is the area where invasion occurs, we next asked whether GSK3β signaling was required for melanoma cell migration and invasion.
Figure 1. GSK3β is focally expressed in melanoma specimens.
A: Representative immunohistochemical staining of an invasive primary melanoma and a melanoma brain metastases for expression of total GSK3β and phospho-GSK3β. Scale bar: 250 μm. Inset: arrows indicate focal expression of GSK3β. Scale bar: 100μm. B: Number of primary and metastatic melanoma specimens with high levels (+2/3) of focal staining for GSK3β. C: High power images of two melanoma metastases, showing increased levels of total GSK3β staining at the invasive front.
NP309 prevent the migration and invasion of melanoma cell lines
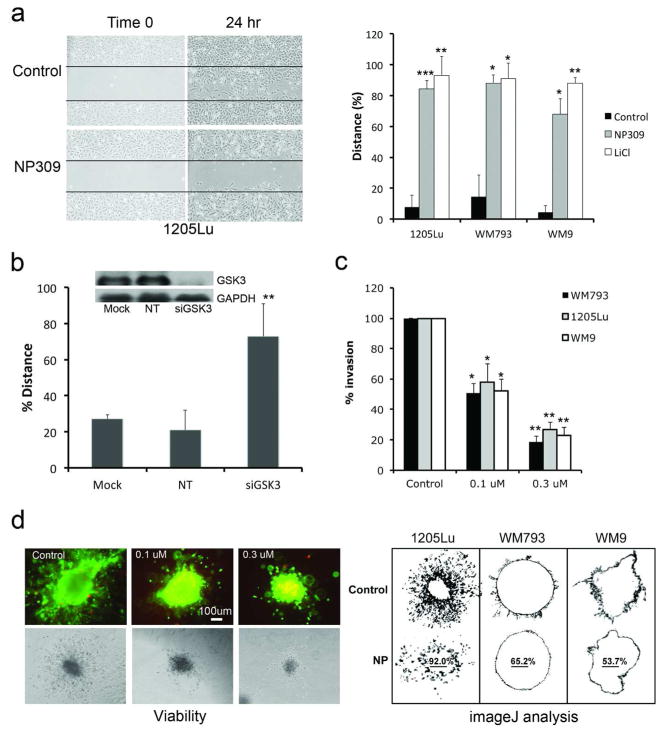
Treatment of the WM793, 1205Lu and WM9 melanoma cell lines with the GSK3β inhibitors NP309, LiCl and siRNA knockdown of GSK3β inhibited the motile behavior of melanoma cells in a scratch wound assay (Figure 2A,B: Supplemental Figure 4). NP309 and LiCl also prevented the invasion of 1205Lu, WM9 and WM793 melanoma cells in a modified Boyden chamber assay as well as the invasion of spheroids into a collagen gel (Figures 2C,D; Supplemental Figure 5). Treatment of melanoma cells with NP309 for 24 hrs did not affect either the growth of the melanoma cells (Supplemental Figure 6), suggesting that the observed effects on invasion were not the result of reduced cell proliferation.
Figure 2. GSK3β inhibition prevents the migration and invasion of melanoma cell lines.
A: NP309 (0.3 μM) and LiCl (50 mM) prevents the movement of melanoma cells into a scratch wound. B: siRNA knockdown of GSK3β prevents the movement of 1205Lu melanoma cells into the scratch. Western blot shows knockdown of GSK3β (Mock, no siRNA; NT: scrambled control and GSK3β siRNA). C: NP309 prevents the invasion of melanoma cells in a modified Boyden Chamber model. D: NP309 (0.3 and 1 μM, 48 hr) prevents the invasion melanoma cells in a 3D collagen implanted spheroid model. Scale bar: 100μm. Images were analyzed using ImageJ. Statistically significant differences from controls are indicated where *P<0.05, **P<0.01, ***P<0.005.
Inhibition of GSK3β signaling in melanoma cells reduces N-cadherin expression
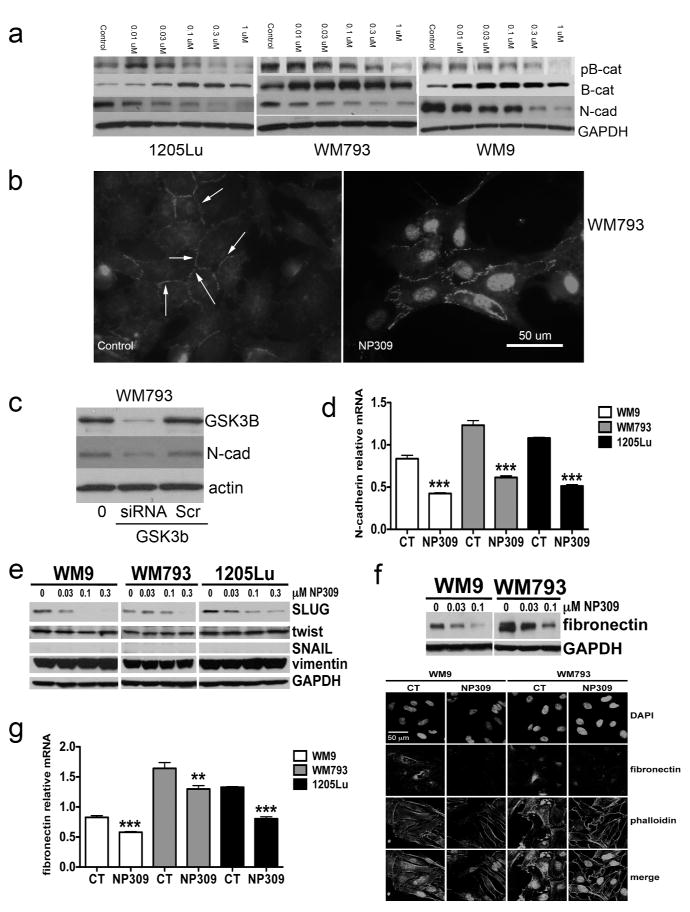
Previous work from our group has shown that increased N-cadherin expression increases the migratory behavior of melanoma cells (Li et al., 2001). Treatment of melanoma cells with increasing concentrations of NP309 or LiCl led to biphasic effects upon the Ser33/Ser37/Thr41 phosphorylation of β-catenin (an increase at lower concentrations followed by a decrease at higher NP309 concentrations), an upregulation of total β-catenin expression (and its localization to the nucleus) and a reduction in N-cadherin expression (Figures 3A,B: Supplemental Figures 7,8). The effects of NP309 upon N-cadherin expression were GSK3β dependent, and could be re-capitulated following siRNA knockdown of GSK3β expression (Figure 3C). We next used mass spectrometry to demonstrate that GSK3β inhibition did not post-translationally modify N-cadherin (no changes were observed in ubiquitination, acetylation, phosphorylation and methylation) (Supplemental Figure 9). Instead, it was noted that NP309 reduced N-cadherin expression at the mRNA level (Figure 3D), and decreased expression of the epithelial-to-mesenchymal-transition (EMT)-associated transcription factor Slug (Figure 3E). In agreement with the GSK3β inhibitor partly reversing the “EMT-like” state of melanoma cells, NP309 also reduced expression of fibronectin at both the RNA and protein levels (Figure 3F,G). No changes were noted in the expression of E-cadherin, Vimentin, Twist or Snail (Figures 3E and not shown).
Figure 3. Inhibition of GSK3β leads to a reduction in N-cadherin expression.
A: Western blot showing NP309 increases β-catenin expression and decreases N-cadherin expression in melanoma cells. B: Immunofluorescence pictures demonstrating the ability of NP309 to increase membrane and nuclear β-catenin expression in WM793 cells. Scale bar: 50μm. C: siRNA knockdown of GSK3β reduces N-cadherin expression in WM793 cells. D: NP309 (0.3 μM, 24 hrs) decreases N-cadherin expression at the mRNA level. E: NP309 decreases expression of Slug. F: (top panel) Western blot showing NP309 (0–0.1μM, 24 hrs) decreases expression of fibronectin (bottom panel) Immunofluorescence staining showing decreased fibronectin expression following NP309 (0.3 μM, 24 hrs) treatment. G: NP309 (0.3 μM, 24 hrs) decreases fibronectin expression at the mRNA level.
Inhibition of GSK3β signaling prevents melanoma cell adhesion to fibroblasts and endothelial cells and transendothelial migration
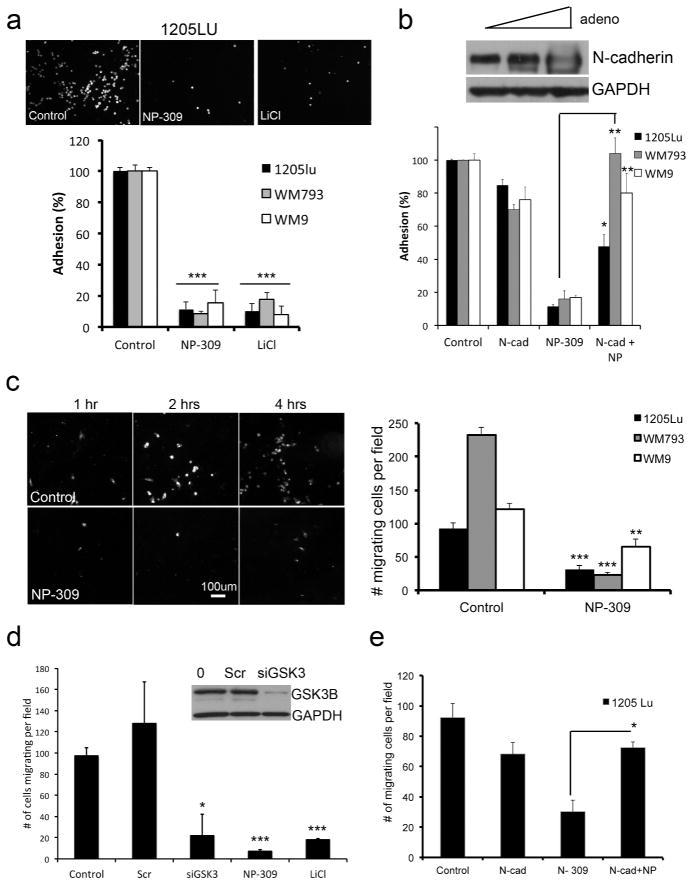
Previous work from our group demonstrated that N-cadherin is important for the interaction of melanoma cells with host fibroblasts and endothelial cells (Li et al., 2001; Smalley et al., 2005). In agreement with these findings, we observed that the pre-treatment of DiI-labeled 1205Lu, WM793 and WM9 cells with NP309 or LiCl prevented adhesion onto a fully confluent monolayer of human skin fibroblasts and endothelial cells (Figure 4A and not shown). These effects were partly N-cadherin mediated and could be recapitulated by siRNA knockdown of N-cadherin expression (Supplemental Figure 10) and partly rescued following the overexpression of N-cadherin (Figure 4B). Pharmacological inhibition and siRNA knockdown of GSK3β also inhibited the migration of 1205Lu, WM793 and WM9 melanoma cells through an activated, confluent endothelial cell monolayer (Figure 4C, D). Again, these effects were N-cadherin-dependent and could be rescued following the overexpression of N-cadherin (Figure 4E).
Figure 4. GSK3β inhibition prevents the interaction of melanoma cells with fibroblasts and endothelial cells.
A: 24 hr NP309 (0.3 μM) and LiCl (50 mM) pre-treatment reduced the adhesion of melanoma cells to a fibroblast monolayer. B: Overexpression of N-cadherin reverses the effects of NP309 upon the adhesion of 1205Lu melanoma cells to a fibroblast monolayer C: NP309 prevents the transendothelial migration of melanoma cells. Scale bar: 100μm. Data shows quantification of cells migrating through the HUVEC layer. D: NP309, LiCl and siRNA knockdown of GSK3β prevents the transendothelial cell migration of 1205Lu melanoma cells. E: Overexpression of N-cadherin reverses the anti-transendothelial cell migratory effects of NP309 on 1205Lu cells. Statistically significant differences from controls are indicated where *P<0.05, **P<0.01, ***P<0.005.
Inhibition of GSK3β increases the size of focal adhesions
Studies in colorectal and pancreatic cancer suggest that GSK3β modulates cell migration and invasion through the regulation of focal adhesion assembly (Kobayashi et al., 2006). We next determined whether inhibition of GSK3β also regulated FAK activity. Using, WM793 melanoma cells expressing EGFP-FAK, and 1205Lu cells stained for total FAK, we demonstrated that NP309 treatment and siRNA knockdown of GSK3β increased the number of large focal adhesions as shown by increased staining for FAK (Figure 5), paxillin and vinculin (Supplemental Figure 11).
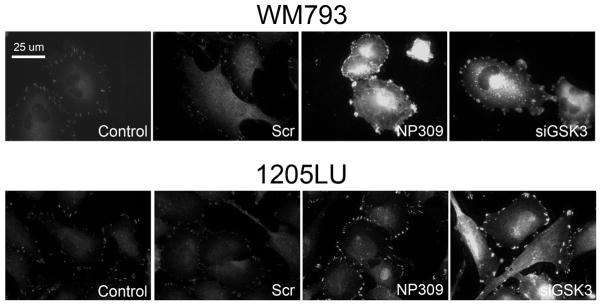
Figure 5. GSK3β regulates focal adhesions in melanoma cells.
Inhibition of GSK3β and siRNA knockdown of GSK3β increases the size of focal adhesions in WM793 and 1205Lu melanoma cells. Doxycycline-inducible EGFP-FAK expressing WM793 and parental 1205Lu cells were treated with vehicle (control), scrambled siRNA control (Scr), NP309 (0.3 μM) or an siRNA against GSK3β. WM793 were imaged directly, and 1205Lu cells were fixed and stained for FAK expression. Scale bar: 25 μm.
NP309 inhibits FAK signaling in melanoma cells leading to inhibition of motility and invasion
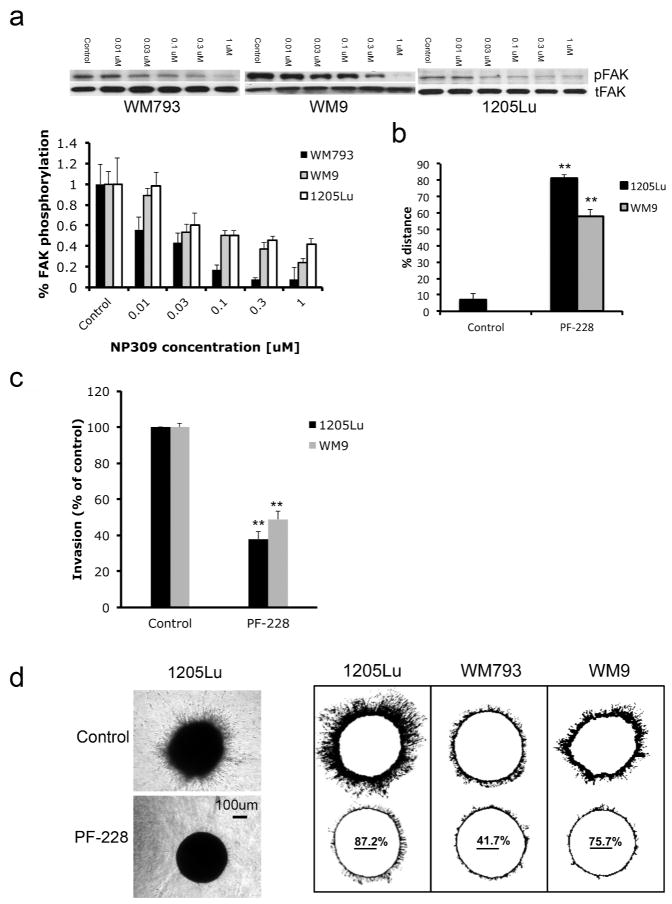
Treatment of WM9, WM793 and 1205Lu melanoma cell lines with increasing concentrations of NP309 inhibited FAK phosphorylation at the Tyr397 activation site (Figure 6A). A comprehensive mass spectrometry-based analysis of FAK phosphorylation demonstrated that GSK3β inhibition increased the phosphorylation of FAK at Ser843, an inhibitory phosphorylation site (Jacamo et al., 2007)(Supplemental Figure 12). The requirement for FAK in the motile and invasive behavior of melanoma cells was demonstrated by the ability of a FAK inhibitor (PF-228, 10μM) to inhibit the movement of cells into a scratch wound and to reduce the invasion of 1205Lu, WM793 and WM9 melanoma cells in modified Boyden Chamber and 3D collagen implanted spheroid assays (Figures 6B–D).
Figure 6. Inhibition of FAK prevents melanoma cell invasion and migration.
A: The expression of phospho-FAK (Tyr397: pFAK), total FAK (tFAK) and GAPDH following NP309 treatment (0–1 μM, 24 hrs). B: The FAK inhibitor PF-228 (10 μM) prevents the scratch wound closure of 1205Lu and WM9 melanoma cells. Data shows % wound closure relative to control scratch wound. C: PF-228 (10 μM) prevents the invasion of melanoma cells in a modified Boyden Chamber assay. D: Inhibition of FAK (PF-228, 10 μM, 72 hrs) prevents the invasion of melanoma cells in a spheroid assay. Left panel shows representative experiment for 1205Lu cells. Scale bar: 100μm. Right panel shows the ImageJ analysis indicating % inhibition of collagen invasion. Statistically significant differences from controls are indicated where **P<0.01.
Discussion
Strategies to prevent the metastatic spread of melanoma are currently lacking. Here we demonstrate that inhibition of GSK3β signaling limits the motile and invasive behavior of melanoma cells and prevents some of the host/tumor interactions required for the transit of melanoma through the dermal microenvironment and into the vasculature.
Since its initial identification as an enzyme involved in the regulation of glycogen synthesis in response to insulin signaling, GSK3β has been implicated in a wide range of physiological processes ranging from protein synthesis to subcellular protein localization (Cohen and Frame, 2001; Frame and Cohen, 2001). A role for GSK3β signaling in cancer development and progression is suggested by its role in the phosphorylation of cyclin D1 and its ability to control Wnt signaling through the phosphorylation and degradation of β-catenin (Diehl et al., 1998). GSK3β is negatively regulated by the serine/threonine kinase AKT through its phosphorylation at Ser9 (Cross et al., 1995). Most melanomas are known to have constitutive activity in AKT that results from increased expression of AKT3, constitutive activation of receptor tyrosine kinase (RTK) signaling or through deregulation of the negative pathway regulator PTEN (Davies et al., 2008; Paraiso et al., 2011; Stahl et al., 2004; Tsao et al., 2004). Given that AKT inactivates GSK3β signaling, the potentially oncogenic role of GSK3β in melanoma has been little considered.
Immunostaining of melanoma tissue specimens showed that GSK3β was focally expressed and mostly localized at the leading edge, suggesting a role for this pathway in melanoma invasion. Although high, focal GSK3β expression was not restricted to melanoma metastases, it was noted that the strongest GSK3β staining observed in primary melanomas was located in nests of cells at the periphery of the tumor, where dermal invasion was occurring. In other tissue systems there is already good evidence for the involvement of GSK3β in the control of cellular polarity and cytoskeletal architecture. In neuronal growth cones, GSK3β localizes to the invasive front and regulates the microtubule assembly required for cell motility and polarity (Eickholt et al., 2002; Gartner et al., 2006). GSK3β signaling is also required for the formation of lamellipodia in migrating keratinocytes, as well as the directional motility of skin stem cells during wound healing (Koivisto et al., 2003; Wu et al., 2011).
Tumor development and metastasis requires the continual bi-directional interaction between host and tumor cells (Bhowmick et al., 2004a; Bhowmick et al., 2004b; Gaggioli et al., 2007). The progression of melanoma is often associated with a cadherin switch, in which E-cadherin expression is downregulated allowing nascent melanoma cells to escape from the control of the skin keratinocytes (Hsu et al., 2000a; Hsu et al., 2000b). Melanoma cells then change binding partners and associate instead with stromal fibroblasts and endothelial cells (Li et al., 2003). The interaction of melanoma cells with endothelial cells is likely to be particularly important when melanomas disseminate through the vasculature, such as during the seeding of metastases into the brain. Previous studies have indicated that the adhesion of melanoma cells to endothelial cells and their subsequent transendothelial migration is dependent upon homotypic N-cadherin binding (Li et al., 2001; Qi et al., 2005). In agreement with a role for GSK3β signaling in the regulation of N-cadherin expression, NP309 treatment blocked the adhesion of melanoma cells onto a confluent endothelial cell layer and prevented the transendothelial migration of melanoma cells. The role for N-cadherin in both of these processes was demonstrated by the ability of forced N-cadherin expression to rescue the inhibitory effects of NP309 upon adhesion and transendothelial cell migration. From a mechanistic standpoint GSK3β was found to regulate N-cadherin expression at the mRNA level, and GSK3β inhibition was associated with the decreased expression of Slug, a Snail-family transcription factor implicated in the metastatic behavior of melanoma cells (Gupta et al., 2005). The potential role of Slug downregulation in the anti-migratory effects of GSK3β inhibition is supported by recent studies showing that overexpression of Slug in melanocytes increases N-cadherin expression, leading to an enhancement of cell motility (Shirley et al., 2012). Taken together, these data suggest a potential role for GSK3β signaling in the transcriptional program required for melanoma dissemination.
The disruption of homotypic N-cadherin signaling following GSK3β inhibition is likely to have other beneficial effects. The engagement of N-cadherin signaling in melanoma cells is known to activate the AKT pathway leading to increased cell survival (Li et al., 2001). It is possible that the interaction of melanoma cells with fibroblasts through N-cadherin contributes to this survival as demonstrated by the reduced cisplatin-induced apoptotic response observed in melanoma cells following their adhesion to fibroblasts (Flach et al., 2011). The potential clinical relevance of these findings has already been suggested by the observation that inhibition of N-cadherin signaling enhances chemotherapy sensitivity in melanoma patients undergoing isolated limb infusion (Beasley et al., 2009; Li et al., 2001).
The coordinated movement of invading cells involves adhesion and membrane protrusion at the leading edge and detachment and retraction at the trailing edge. As GSK3β was primarily expressed at the invasive front of melanoma specimens, we asked whether GSK3β was involved in melanoma cell adhesion. As our focus we studied FAK, a kinase known to occupy a central position linking integrin mediated adhesion to the downstream activation of key signaling pathways involved in cytoskeletal re-arrangement and survival. It was observed that NP309 treatment inhibited phosphorylation of FAK at its activating autophosphorylation (Tyr397) site and that a small molecule FAK inhibitor (PF-228) also prevented both the migration of melanoma cells in a scratch wound assay and the invasion of the highly invasive 1205Lu and WM9 melanoma cell lines in Boyden chamber and 3D spheroid assays. Although our studies did not demonstrate a direct link between GSK3β inhibition and inhibition of FAK phosphorylation at Tyr397, a detailed mass spectrometry analysis of FAK phosphorylation sites did reveal NP309 treatment to enhance FAK phosphorylation at Ser843, a site known to be associated with dephosphorylation of FAK at Tyr397 (Jacamo et al., 2007). Studies are ongoing to identify the candidate serine/threonine kinase that directly phosphorylates FAK at Ser843 upon GSK3β inhibition.
GSK3β inhibition also led to an increase in the size of focal adhesions. Our results mirrored those observed in HeLa cells where inhibition of GSK3β using both siRNA and pharmacological inhibitors impaired cell motility and increased the size and number of focal adhesions (Kobayashi et al., 2006). Links between inhibition of FAK and impaired motility have been reported by a number of other groups with FAK-null fibroblasts being shown to have an impaired migratory response associated by an increase in adhesion strength and the size and number of focal adhesions and that constitutively activated FAK can partially rescue the motile behavior of cells in which GSK3β has been depleted (Ilic et al., 1995; Kobayashi et al., 2006).
To our knowledge, the role of GSK3β in both the motile behavior of melanoma cells and the interaction of melanoma cells with host fibroblasts and endothelial cells has never previously been reported. Further studies will be required to determine whether GSK3β inhibition is a viable strategy to limit the metastatic phenotype of melanoma in vivo.
Materials & Methods
Cell culture and growth inhibition
Melanoma cells lines were grown as described in (Paraiso et al., 2010). The identity of the cell lines was confirmed using the Coriell Institute (Camden, NJ) cell identity mapping kit.
Drugs and inhibitors
NP309 was synthesized and characterized for its anti-GSK3β activity in (Pagano et al., 2007). PF-228 and LiCl were purchased from Sigma-Aldrich.
Immunohistochemical staining of melanoma specimens
De-identified formalin-fixed paraffin-embedded tissue samples were obtained from the Moffitt Pathology archives under a written informed consent protocol approved by the Institutional Review Board of the University of South Florida under the Declaration of Helsinki Protocols and stained using the Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) (Paraiso et al., 2011). The rabbit primary antibody for p-GSK3β was from Cell Signaling Technology and the antibody for total GSK3β was from Epitomics (Burlingame, CA). Staining was visualized using the Ventana Chromomap Redkit. Slides were analyzed by two independent observers and consensus scored on a scale from (0 to +3).
Western blotting
Proteins were extracted and blotted for as described in (Smalley et al., 2007). The antibodies to phospho-GSK3β (Ser9), total GSK3β, phospho-FAK (Tyr 397), total FAK, phospho-β-catenin (Ser33/37/Thr41) and vimentin were from Cell Signaling Technology (Beverly, MA). The antibodies for fibronectin, N-cadherin and total β-catenin were from BD Biosciences. The antibodies for Twist and SLUG were from Santa Cruz (Santa Cruz, CA) while the antibody for Snail was from abcam (Cambridge, MA). In all cases, Western blots were stripped once and probed for GAPDH or actin (Sigma-Aldrich) to confirm even protein loading.
RNA interference
RNAi experiments were performed as described in (Paraiso et al., 2011). Cells were treated with 25nM N-cadherin (Dharmacon), 20nM GSK3β (Cell Signaling Technologies). In addition, scrambled siRNA’s at each concentration were also added as non-targeting controls.
3D spheroid assays
Collagen implanted spheroids were prepared using the liquid overlay method (Smalley et al., 2006) and were treated with 0.3μM NP309 or LiCl (50mM) for 24–72hr before being analyzed using a Nikon-TS100 inverted fluorescence microscope and analyzed using ImageJ.
Fibroblast/endothelial cell adhesion assays
Human skin fibroblasts (FF2554) or human vascular endothelial cells (HUVECS) were seeded out to 100% confluency and left to grow overnight. 1205Lu, WM793 and WM9 melanoma cells were labeled with DiI before treatment with vehicle, NP309 (0.3 μM), GSK3 siRNA or LiCl (50 mM) for 24 hrs. Equal numbers of cells were then added to the confluent fibroblast/endothelial cell monolayers and allowed to adhere for 20 minutes before being washed four times with fresh media and quantified by counting five 20X fields.
Transendothelial cell migration assays
HUVECs were plated onto transwell inserts and allowed to grow to confluence over 48 hours before being activated with TNF-α (10ng/ml 24 hrs). 1205Lu, WM793 and WM9 cells were pretreated with NP309, LiCl or siRNA to GSK3β or scrambled control for 48 hrs before being counted and tested for viability by trypan blue staining. 25,000 melanoma cells were then DiI labeled and plated on top of the HUVEC layer in serum free media with FBS containing media added to the lower chamber. Following 1–4 hr incubation, cotton swabs were used to remove non-migratory cells before fixation (4% paraformaldehyde) and imaging with a Nikon Eclipse TS100 microscope.
Adenoviral vector infections
Cells were infected with 10–100 PFUs of adenovirus encoding for N-cadherin as described in (Li et al., 2001).
Scratch wound assays
Confluent monolayers of melanoma cells (1205Lu, WM793, WM9) were allowed to grow to confluence before being scratched with a p10 pipette tip. Cultures were treated with vehicle, NP309, LiCl (concentrations as above) or GSK3β siRNA (48 hrs pretreatment) before being imaged (1–24 hrs). Percentage wound closure was calculated using ImageJ.
Modified Boyden chamber assays
Invasion was measured in Chemotaxis Chambers (96 well format from NeuroProbe (Gaithersburg, MD, USA) following coating with Matrigel (BD). Briefly, cells were trypsinized, rinsed twice with PBS, resuspended in serum-free media, and were loaded on upper chamber and allowed to invade through the Matrigel towards 10% FBS for 20 hours. Non-invasive cells were removed and remaining cells were fixed and stained with Crystal Violet with absorbance being read at OD 560nm. For loading control and to normalize for differences in cell proliferation, cells were allowed to grow for the same time as the incubation in the Boyden chambers after which cells were stained with Crystal Violet. Cells were quantified at OD 560nm after dye extraction and this number was used to normalize the invasion value.
EGFP-FAK expressing cells
The EGFP coding region and multiple cloning site was amplified from pEGFP-C1 (Clontech) and TOPO® cloned into pENTR/D-TOPO (Invitrogen). Chicken FAK was subcloned from pBluescript-FAK (kindly provided by Dr. Jihe Zhao, University of Central Florida Burnett School of Biomedical Sciences) into pENTR/D-TOPO/EGFP-MCS. The resulting pENTR/D-TOPO/EGFP-FAK was shuttled into pLenti4/TO/V5-DEST (Invitrogen) using Gateway® LR Clonase® II (Invitrogen), which put the coding region in frame with the vectors c-terminal V5-epitope and stop codons. pLenti4/TO/V5-GW/EGFP-FAK was packaged in HEK293FT cells using Invitrogen’s ViraPower™ Lentiviral Packaging Mix and protocol, with the substitution of 36μl FuGENE® HD (Roche) for Lipofectamine™ 2000 for the transfection. Media containing lentivirus particles was collected 72 hours post transfection and added to WM793TR cells (Abel and Aplin, 2010) for 72 hours followed by selection in Zeocin™ (100μg/ml).
Immunofluorescence staining
Cells (WM793 or WM793-FAK), 1205Lu were plated onto coverslips and treated with NP309 for 24hrs before being fixed and permeabilized as previously described (Smalley et al., 2007) before being imaged with a Leica confocal microscope at 63X. In some cases slides were stained for paxillin, vinculin or fibronectin (BD Pharmingen).
Quantitative real-time PCR
Cells were treated for 24 hours with 300nM NP309 prior to RNA isolation. Total RNA was isolated using Qiagen’s RNeasy mini kit. The following TaqMan® Gene Expression Assays primer/probes were used: Hs00983056_m1 (N-cadherin), Hs00365052_m1 (fibronectin), and P/N 4319413E (18S). The 18S data were used for normalizing BIM. Q-RT-PCR reactions were performed as previously described (3).
Statistical analysis
Unless otherwise stated, all experiments were performed at least three times. Data shows mean values +/− S.E.M. Significance was analyzed using a Student’s T-test with P<0.05 being considered significant.
Supplementary Material
Acknowledgments
This work was funded by the NIH/National Cancer Institute (R01 CA161107-01), the Harry Lloyd Trust (KSMS), NIH (P01 CA 114046-01) (MH and EM) and The Joanna M. Nicolay Melanoma Foundation (EVA and KHT). The Moffitt Proteomics Core Facility is supported by the US Army Medical Research and Materiel Command (DAMD17-02-2-0051 and W81XWH-08-2-0101), the National Cancer Institute (P30-CA076292) as a Cancer Center Support Grant, and the Moffitt Foundation.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- Abel EV, Aplin AE. FOXD3 is a mutant B-RAF-regulated inhibitor of G(1)-S progression in melanoma cells. Cancer Research. 2010;70:2891–900. doi: 10.1158/0008-5472.CAN-09-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arozarena I, Bischof H, Gilby D, Belloni B, Dummer R, Wellbrock C. In melanoma, beta-catenin is a suppressor of invasion. Oncogene. 2011 doi: 10.1038/onc.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilla-Gokcumen GE, Pagano N, Streu C, Maksimoska J, Filippakopoulos P, Knapp S, et al. Extremely tight binding of a ruthenium complex to glycogen synthase kinase 3. Chembiochem. 2008;9:2933–6. doi: 10.1002/cbic.200800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley G, Sanders G, Zager JS, Hochwald SN, Grobmyer S, Andtbacka RH, et al. A prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion (M-ILI) in patients with advanced extremity melanoma. Journal of Clinical Oncology. 2009;27 doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004a;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004b;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli TC, Weeraratna AT. Striking the target in Wnt-y conditions: intervening in Wnt signaling during cancer progression. Biochem Pharmacol. 2010;80:702–11. doi: 10.1016/j.bcp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–8. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–8. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. The Journal of cell biology. 2002;157:211–7. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach E, Rebecca VW, Herlyn M, Smalley KSM, Anderson AR. Fibroblasts contribute to melanoma tumor growth and drug resistance. Molecular Pharmaceutics. 2011 doi: 10.1021/mp200421k. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Gartner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. Journal of Cell Science. 2006;119:3927–34. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature genetics. 2005;37:1047–54. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–9. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Andl T, Li G, Meinkoth JL, Herlyn M. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. Journal of Cell Science. 2000a;113:1535–42. doi: 10.1242/jcs.113.9.1535. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. American Journal of Pathology. 2000b;156:1515–25. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jacamo R, Jiang X, Lunn JA, Rozengurt E. FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. Journal of Cellular Physiology. 2007;210:436–44. doi: 10.1002/jcp.20870. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hino S, Oue N, Asahara T, Zollo M, Yasui W, et al. Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions. Mol Cell Biol. 2006;26:898–911. doi: 10.1128/MCB.26.3.898-911.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto L, Alavian K, Hakkinen L, Pelech S, McCulloch CA, Larjava H. Glycogen synthase kinase-3 regulates formation of long lamellipodia in human keratinocytes. Journal of Cell Science. 2003;116:3749–60. doi: 10.1242/jcs.00693. [DOI] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Research. 2001;61:3819–25. [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22:3162–71. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- Lioni M, Brafford P, Andl C, Rustgi A, El-Deiry W, Herlyn M, et al. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol. 2007;170:709–21. doi: 10.2353/ajpath.2007.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano N, Maksimoska J, Bregman H, Williams DS, Webster RD, Xue F, et al. Ruthenium half-sandwich complexes as protein kinase inhibitors: derivatization of the pyridocarbazole pharmacophore ligand. Org Biomol Chem. 2007;5:1218–27. doi: 10.1039/b700433h. [DOI] [PubMed] [Google Scholar]
- Paraiso KH, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Chen N, Wang J, Siu CH. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Molecular Biology of the Cell. 2005;16:4386–97. doi: 10.1091/mbc.E05-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley SH, Greene VR, Duncan LM, Torres Cabala CA, Grimm EA, Kusewitt DF. Slug Expression during Melanoma Progression. The American journal of pathology. 2012 doi: 10.1016/j.ajpath.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–54. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Contractor R, Haass NK, Lee JT, Nathanson KL, Medina CA, et al. Ki67 expression levels are a better marker of reduced melanoma growth following MEK inhibitor treatment than phospho-ERK levels. Br J Cancer. 2007;96:445–9. doi: 10.1038/sj.bjc.6603596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–41. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, et al. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell. 2011;144:341–52. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.