Abstract
In adult rat striatum the dopamine D1-D2 receptor heteromer is expressed selectively in a subset of medium spiny neurons (MSNs) that coexpress the dopamine D1 and D2 receptors (D1R and D2R) as well as dynorphin (DYN) and enkephalin (ENK), with higher coexpression in nucleus accumbens (NAc) and much lower in the caudate putamen (CP). In the present study we showed that in neonatal striatal cultured neurons >90% exhibited the D1R/D2R-DYN/ENK phenotype. Similarly, in the striatum of juvenile rats (age 26-28 days) coexpression of D1R and D2R was also coincident with expression of both DYN and ENK. Quantification of the number of striatal MSNs exhibiting coexpression of D1R and D2R in juvenile rats revealed significantly lower coexpression in NAc shell, but not core, and CP than in adult rats. However, within MSNs that coexpressed D1R and D2R, the propensity to form the D1-D2 receptor heteromer did not differ between age groups. Consistent with reduced coexpression of the D1R and D2R, juvenile rats exhibited subsensitivity to D1-D2 receptor heteromer-induced grooming following activation by SKF 83959. Given the proposed role of D1R/D2R-coexpressing MSNs in the regulation of thalamic output, and the recent discovery that these MSNs exhibit both inhibitory and excitatory capabilities, these findings suggest that the functional regulation of neurotransmission by the dopamine D1-D2 receptor heteromer within the juvenile striatum may be significantly different than in the adult.
Keywords: dopamine D1-D2 receptor heteromer, juvenile, dynorphin, enkephalin, nucleus accumbens, grooming
Dopaminergic signaling within the basal ganglia has classically been thought to occur within two distinct neuronal pathways; the direct striatonigral pathway which contains the dopamine D1 receptor (D1R) and the neuropeptides dynorphin (DYN) and substance P, and the indirect striatopallidal pathway which expresses the dopamine D2 receptor (D2R) and enkephalin (ENK). A number of studies have also shown, however, that D1R and D2R can co-exist within a certain fraction of medium spiny neurons (MSN) (Bertran-Gonzalez et al., 2008, Valjent et al., 2009, Perreault et al., 2010, Lim et al., 2012). Emerging evidence additionally indicates that these D1R/D2R-coexpressing neurons, which also express DYN and ENK (Perreault et al., 2010) as well as the neurotransmitters GABA and glutamate (Perreault et al., 2012), may comprise a distinct neuronal network, as evidenced by a recent report showing D1R and D2R coexpression in the cell bodies and presynaptic terminals of regions of both the striatonigral and striatopallidal projections of the basal ganglia (Perreault et al., 2010). Furthermore, within these coexpressing neurons it has been shown that the D1R and D2R can form a novel and pharmacologically distinct receptor complex, the dopamine D1-D2 receptor heteromer. The D1-D2 heteromer has been shown to exhibit cell signaling properties distinct from its constituent receptors (Lee et al., 2004, Rashid et al., 2007, Hasbi et al., 2009, So et al., 2009, Verma et al., 2010), emphasizing the functionally unique role for these coexpressing MSNs in brain.
Evidence suggests that striatal coexpression of the D1R and D2R, and expression of the D1-D2 receptor heteromer, may be age-dependent thus implicating age as a critical factor in the contribution of these neurons to the regulation of striatal neurotransmission. For example, while it has been shown that the majority of rat neonatal striatal neurons may coexpress D1R and D2R (Aizman et al., 2000, Falk et al., 2006, Iwatsubo et al., 2007, Hasbi et al., 2009) and form D1-D2 receptor heteromers (Hasbi et al., 2009), receptor coexpression is greatly reduced and anatomically circumscribed in adults with approximately 17-35% of NAc MSNs and 5-6% of caudate putamen (CP) MSNs expressing both receptors (Bertran-Gonzalez et al., 2008, Valjent et al., 2009, Perreault et al., 2010). In addition, increased D1-D2 receptor heteromer-induced signaling was more robust in mid-life compared to younger adults (Rashid et al., 2007), an effect that may correspond to increased D1R and D2R coexpression and/or increased D1-D2 receptor heteromer densities.
There have been many reports detailing the differences in behavioural responding to dopamine drugs in immature versus mature rats, such as enhanced responses to pychostimulant-induced reward (Shahbazi et al., 2008, Zakharova et al., 2009a, Zakharova et al., 2009b, Anker and Carroll), but reduced responsiveness to the locomotor activating effects of the drugs (Bolanos et al., 1998, Banerjee et al., 2009, Zakharova et al., 2009a), findings indicative of age-dependent differences in dopamine neurotransmission. Thus, given the evidence of time-dependent changes in striatal D1-D2 receptor heteromer expression, in the present study we sought to compare the expression levels of the D1-D2 heteromer in NAc and CP in juvenile and adult rats, and additionally, to evaluate their grooming responses, a behaviour previously shown to be mediated by the D1-D2 heteromer (Perreault et al., 2012), following activation of the receptor complex. We showed that juvenile animals exhibited reduced coexpression of D1R and D2R in NAc shell and CP, but not in NAc core, suggestive of lower D1-D2 receptor heteromer densities and region-specific decreases in D1-D2 heteromer-induced neurotransmission. Accordingly, these changes in heteromer expression were associated with reduced responsiveness to the induction of grooming induced by activation of the receptor complex.
EXPERIMENTAL PROCEDURES
Neuronal Cultures
Neonatal rat striata (1 day of age) were trypsinized in Hanks’ balanced salt solution (HBSS) with 0.25% trypsin and 0.05% DNase (Sigma) at 37 °C, and cells were washed three times in HBSS with 12 mM MgSO4. Cells were dissociated in DMEM with 2 mM glutamine and 10% FBS and plated at 2 × 105 cells per poly-L-lysine-coated well (Sigma; 50 g/mL). The next day, media were changed to Neurobasal medium with 50X B27 Supplement and 2 mM glutamine (Invitrogen). On day 3 of culture, 5 μM cytosine arabinoside was added to inhibit glial cell proliferation. Half of the medium was changed every 3 days. Neurons were fixed on day 4, 11 or 25 of culture for use in fluorescence immunocytochemistry.
Animals
Thirty-four experimentally naïve juvenile (aged 26-28 days) or adult male Sprague-Dawley rats (Charles River, Canada), weighing 50-70 g or 400-450 g at the start of the experiment were used. Rats were pair-housed in polyethylene cages in a colony room maintained on a 12-h light–dark cycle with free access to food and water. Following arrival, rats were handled for 2 minutes daily for 3 days before the start of experiments. All treatments were performed during the light phase of the day–night cycle. Animals were housed and tested in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993).
Drugs
SKF 83959 hydrobromide (Tocris Bioscience) was dissolved in physiological saline containing 5% DMSO and administered subcutaneously. SKF 83959 has been previously shown to selectively activate the Gq-coupled D1-D2 receptor heteromer (Rashid et al., 2007). For non-drug injections, an equivalent volume of saline was administered. All injections were administered at a volume of 1.0 ml/kg.
Fluorescence Immunochemistry
Immunochemistry was performed on paraformaldehyde-fixed striatal neurons or floating coronal sections from perfused rat brain that were incubated for 24h or 60h at 4 °C with primary antibodies (1:500 or 1:200; D1R, Sigma-Aldrich; D2R, ENK, Chemicon; DYN, Neuromics) as previously described (Lee et al., 2004, Hasbi et al., 2009). Specificity of the dopamine receptor antibodies for the D1R and D2R have been previously tested and reported (Lee et al., 2004, Perreault et al., 2010)and were tested using the five dopamine receptors (D1-D5) individually expressed in HEK293 cells. The D1R antibody was also shown to have no reactivity in striatal tissue of D1R gene-deleted mice, nor did the D2R antibody exhibit reactivity in striatal tissue of D2R gene-deleted mice. Additional controls were performed in the absence of the primary or secondary antibodies. Antibody dilutions were also used to identify the optimal working concentrations. To minimize background and prevent cross-excitation of the secondary antibody-linked fluorophores, only three primary antibodies were used on the tissue at any given time. Images were obtained using an Olympus Fluoview 1000 confocal microscope with a 60X/1.4 NA objective. Lower magnification images (40X) were obtained for the purpose of cell counting, which was performed using 300 μm2 regions.
Confocal microscopy FRET and data processing
Confocal microscopy FRET was performed as previously described (Hasbi et al., 2009). Paraformaldehyde-fixed striatal neurons or floating sections from rat brain were incubated with primary antibodies to D1R and D2R, and the species-specific secondary antibodies conjugated to Alexa Fluors. The images were acquired with an Olympus Fluoview FV 1000 laser scanning confocal microscope with a 60X/1.4 NA objective. Anti-D2-Alexa 350 was used as the donor dipole, while anti-D1-Alexa 488 was used as the acceptor dipole. The donor was excited with a krypton laser at 405 nm, while the acceptor was excited with an argon laser at 488 nm. The emissions were collected at 430/20 nm and 530/20 nm LP filter. Other FRET pairs (Alexa 488-Alexa 568 and Alexa 568-Alexa 647) were tested and showed comparable results. Eleven images were acquired for each FRET analysis in accordance with an algorithm (Chen et al., 2005), designed to remove both the donor and acceptor spectral bleed-through (SBT) signals and to correct for variation in fluorophore expression level (FEL) associated with FRET imaging. The processed FRET (pFRET) images were generated using a software developed based on the described algorithm (Chen et al., 2005), in which:
where UFRET is uncorrected FRET, ASBT and DSBT are the acceptor and the donor spectral bleed-through signals. The rate of energy transfer efficiency (E) and the distance (r) between the donor (D) and the acceptor (A) molecules were estimated by selecting small regions of interest (ROIs) using the same images and software, based on the following equation:
where IDA is the donor image in the presence of acceptor, ψdd and ψaa are collection efficiency in the donor and acceptor channel, Qd and Qa are the quantum yields of the donor and the acceptor. E is proportional to the 6th power of the distance separating the FRET pair. Thus,
was used to calculate the distance between the D1R and D2R, where Ro is the Förster’s distance.
Grooming
The induction of grooming has been previously shown to be induced following activation of the D1-D2 receptor heteromer (Perreault et al., 2010, Perreault et al., 2012). On the day of testing, animals were removed from their home cage, administered SKF 83959 (1.5 mg/kg s.c.) and placed immediately inside a novel cage with no bedding. Grooming activity was then monitored for 60 minutes. The measurement of grooming behavior was modified from a previously described protocol (Culver et al., 2000). During the testing period, the experimenter was present in room to record the grooming activity of each rat using a hand-held timer. The animal was considered to have engaged in grooming when the rat’s mouth and/or tongue made contact with parts of its own body (e.g., nibbling of paws or tail, licking of hindquarters or body fur). The timer was started by the experimenter when the rat’s mouth made contact with its own body, and was stopped when contact had ceased for a duration no less than 1 second. Grooming was scored for 30 seconds every 6-7 minutes, for a total of 4 minutes (2 minutes sampled from the first 30 minutes of testing and 2 minutes sampled from the last 30 minutes of testing). 90 minutes following injection, animals were decapitated and brains were rapidly removed. NAc and CP tissues were dissected, flash frozen, and immediately stored at −80° C until use in immunoblotting.
Data Analysis
All values are reported as mean ± SEM. For the grooming data, the main dependent variable was time (s) and the statistical significance evaluated using an ANOVA, with Age and Drug as the between subjects, factors followed by post-hoc Student’s t tests. Computations were performed using the SPSS/PC+ statistical package. Statistical criteria for significant differences were set at P<0.05.
RESULTS
Neuronal coexpression of the D1R and D2R is linked with DYN and ENK
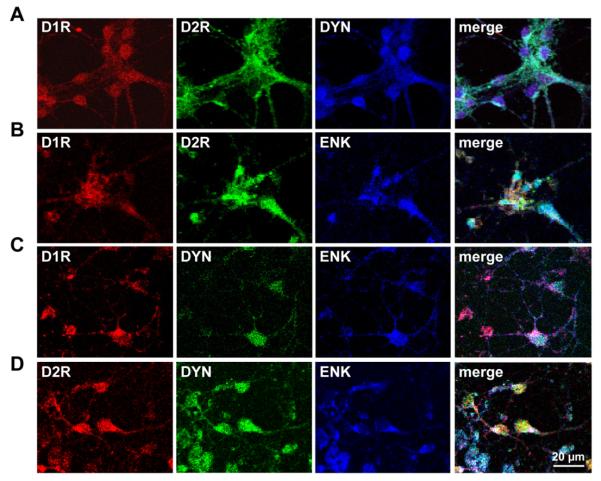
It has been reported that in adult rat striatum coexpression of the D1R and D2R occurs in MSNs also expressing both DYN and ENK, (Perreault et al., 2010). To determine whether the D1R/D2R coexpression was consistently linked to DYN and ENK expression, we first examined the phenotype of D1R/D2R-coexpressing cultured striatal neurons from 1 day old rat pups, and individual and coexpression of D1R and D2R with DYN, ENK or both neuropeptides was assessed (Fig. 1). There was a high degree of colocalization of D1R in D2R in cultured striatal neurons, with greater than 90% showing expression of both receptors (Fig. 1A and B). D1R and D2R were present in DYN neurons and also present in ENK neurons. Coexpression of DYN and ENK was also evident in the majority of neonatal striatal neurons that were also shown to express D1R and D2R (Fig. 1C and D).
Fig. 1.
Colocalization of the D1 and D2 receptor with dynorphin and enkephalin in rat neonatal striatal neurons. (A, B) Confocal images of 11 day old cultured striatal neurons showed D1 and D2 receptor (D1R and D2R) colocalization with dynorphin (DYN) or with enkephalin (ENK). Colocalization of the D1R and D2R was present in the majority (>90%) of neurons examined. (C, D) DYN and ENK showed abundant coexpression in striatal neurons, and were also found to be colocalized with the D1R or D2R.
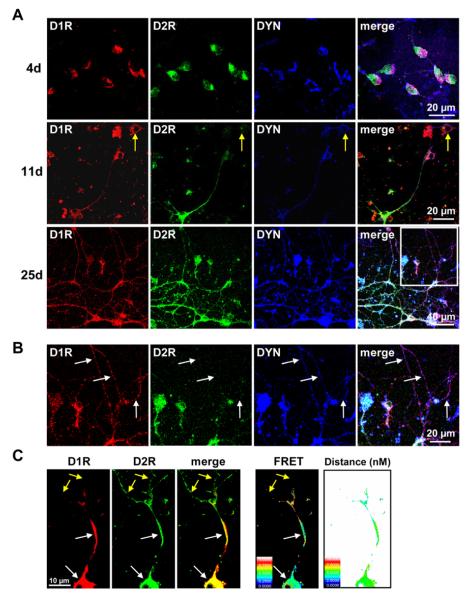
Although these findings showing > 90% coexpression of D1R, D2R, DYN, and ENK in cultured striatal neurons are likely not an accurate depiction of coexpression levels in the developing intact animal at PND1 (Lobo et al., 2006, Agoston et al., 2007, Lobo et al., 2008, Martin-Ibanez et al., 2010, Baydyuk et al., 2011), they can provide insights into the link between dopamine receptors, their neuropeptides, and whether endogenous factors within the individual neurons regulate this link. To further characterize this, we therefore next examined receptor expression in 1 day old neonatal striatum cultured for 4, 11 or 25 days to determine whether endogenous factors within D1R and D2R coexpressing MSNs were sufficient to switch the phenotype to individual receptor expression and whether this was associated also with expression of a specific neuropeptide (Fig. 2). The neurons revealed a similar extent of D1R and D2R coexpression at all 3 time points and cells expressing receptors individually were sporadically observed (Fig. 2A). Very few neuronal processes were observed on day 4, but were in evidence by day 11, with enhanced growth and dendritic sprouting by day 25. At the latter time point, in the dendrites of a few D1R/D2R coexpressing neurons the localization of only a single receptor was observed. In these instances individual expression of D1R and D2R was always in the presence of its associated neuropeptide, such as D1R and DYN (Fig. 2B), or D2R and ENK (not shown). It must be noted that although these findings showing > 90% coexpression of D1R, D2R, DYN, and ENK In cultured striatal neurons FRET analysis revealed D1R and D2R existed in close proximity with a relative distance of 4–7 nm (50–70 Å), indicative of D1-D2 receptor heteromer formation (Fig. 2C). FRET between the D1R and D2R was only evident in regions of receptor colocalization.
Fig. 2.
Neuronal growth and differentiation of rat neonatal striatal D1 and D2 receptor coexpressing neurons. (A) Confocal images showing D1 receptor (D1R), D2 receptor (D2R) and dynorphin (DYN) expression in rat striatal neurons following 4, 11 or 25 days in culture. Neurons showed a progressive increase in dendritic sprouting and growth after 4 days in culture. D1R and D2R showed significant colocalization with DYN in neuronal cell bodies at all time points, and in the dendrites on days 11 and 25. On day 25 of culture, some D1R and D2R coexpressing neurons began to show differential segregation of D1R and D2R to the distal dendrites (box). Neurons showing expression of only one dopamine receptor were in evidence but scarce (yellow arrows) (B) Magnification of the boxed area in (a) depicting neuronal dendrites of a D1R and D2R coexpressing neuron on day 25 of culture. While D1R and DYN exhibited colocalization in these dendrites, D2R was not present (white arrows). (C) Example of a representative neuron showing colocalization and interaction (FRET) of D1R and D2R, and the distance between the two receptors in a striatal cultured neuron. FRET between the D1R and D2R was evident in regions of receptor colocalization (white arrows) but was not present in regions absent of D1R expression (yellow arrows). Scale panel denotes FRET efficiency or distance (nM) accordingly.
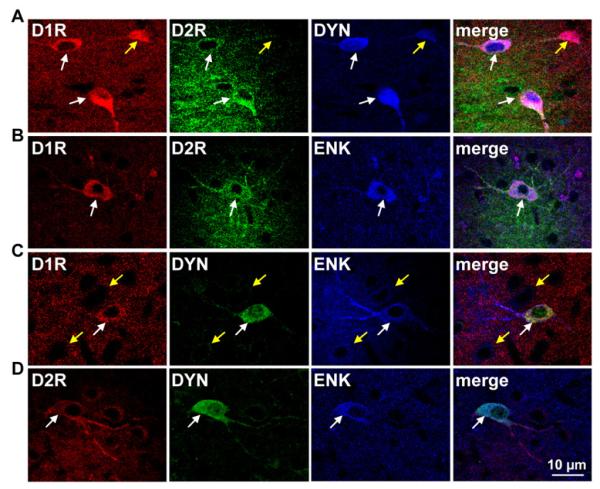
We next assessed the neuropeptide content of D1R/D2R-coexpressing striatal neurons in juvenile rats 26-28 days of age. Unlike the neurons in culture, these young rats exhibited prominent receptor segregation in the cell bodies of neurons in the NAc and CP indicating that the neurons in culture lacked integral endogenous factors that would normally contribute to such neuronal differentiation and phenotypic segregation. However, of the neurons that did show D1R and D2R coexpression in striatum, the phenotype remained consistent with that observed both in culture and in adult animals, having expressed D1R and D2R with expression of DYN and ENK (Fig. 3A-D).
Fig. 3.
Colocalization of the D1 and D2 receptor with dynorphin and enkephalin and D1-D2 receptor heteromer formation in juvenile rat nucleus accumbens. (A, B) Confocal images revealed D1 and D2 receptor (D1R and D2R) colocalization with dynorphin (DYN) or with enkephalin (ENK) (white arrows) in NAc. Neurons that expressed only D1R were positive for DYN and neurons expressing only D2R were positive for ENK (yellow arrows). (C) A subset of neurons coexpressed DYN and ENK (yellow arrows) and these neurons could also express the D1R (white arrows). (D) DYN and ENK coexpressing neurons could also express the D2R (white arrows) in juvenile rat NAc.
Age-dependent changes in striatal dopamine D1R-D2R heteromer expression
The phenotype of neurons coexpressing the D1R and D2R was shown to be consistent across all ages as expressing DYN and ENK. We next sought to determine whether expression of the D1R-D2R heteromer varied between juveniles and adults. As the D1R-D2R heteromer can only be expressed in neurons containing both the D1R and D2R, we first quantified the number of neurons exhibiting receptor coexpression in NAc core and shell and CP. It was found that while the overall expression of the D1R in juveniles was similar to that of adults both in NAc core and shell, as well as in CP (Fig. 4A), a significantly fewer number of D1R-expressing cells showed D2R colocalization in juvenile NAc shell (juvenile: 24.8±1.5%, adult: 33.8±4.5%, P<0.02). Similarly in CP, receptor coexpression in juveniles was significantly reduced (juvenile: 2.8±0.5%, adult: 6.8±0.4%) (Fig. 4B). To examine whether the propensity to form D1-D2 receptor heteromers in any given D1R/D2R-coexpressing neuron differed between ages, we next performed FRET in situ to examine D1-D2 heteromer expression. We found no age-dependent differences in the propensity for the D1R and D2R to form D1-D2 heteromers (Fig. 4C), with relatively high FRET in the NAc and lower FRET values in the CP in both adults and juveniles. Nonetheless, although D1-D2 heteromer densities in any individual coexpressing neuron did not vary, the reduction in the overall number of neurons exhibiting receptor coexpression in juvenile NAc shell and CP would undoubtedly limit the functional contribution of the D1-D2 receptor heteromer in these regions.
Fig. 4.
Reduced D1R and D2R coexpression in NAc shell and caudate putamen of juvenile rats. (A) Quantification of the number of D1R-expressing neurons per 300 μm2 area (N=16-25 sections/region). (B) The proportion of D1R-expressing neurons also expressing the D2R. Adults exhibited a significantly greater proportion of D1R and D2R coexpressing neurons in NAc shell and CP, but not NAc core (C) D1R and D2R formed D1-D2 receptor heteromers as determined by FRET. FRET signal indicated equivalent FRET efficiencies between juvenile and adult rats in NAc and CP (N=25-62 neurons/region). Data expressed as mean ± SEM. *P<0.05 Student’s ttest.
Subsensitivity to D1-D2 receptor heteromer-induced grooming in juvenile rats
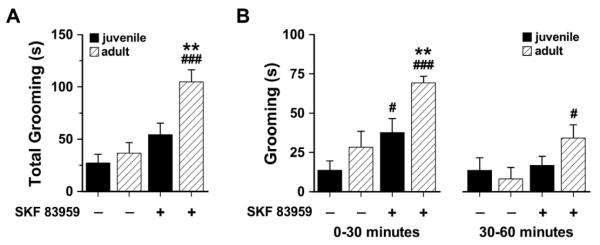
Acute systemic or intra-NAc shell administration of the atypical dopamine agonist SKF 83959 has been reported to result in the induction of grooming, but not locomotor activity, in adult rats (Perreault et al., 2010, Perreault et al., 2012). SKF 83959 does not activate the Gs-coupled dopamine D1R (Jin et al., 2003, Rashid et al., 2007), and has been previously shown to induce grooming behaviour via activation of the dopamine D1-D2 receptor heteromer (Perreault et al., 2012). To determine whether reduced striatal D1R/D2R coexpression in the juveniles would manifest as reduced grooming, juvenile and adult rats were administered a single dose of SKF 83959 or saline and their grooming behavior monitored for 60 minutes (Fig. 5A). There was no difference in basal grooming duration between juvenile and adult rats across the 60 minute testing period. Activation of the D1R-D2R heteromer by SKF 83959 induced a robust increase in the grooming response of adults, while juveniles showed a modest but insignificant increase in grooming. Within the first 30 minutes, however, juveniles did exhibit a significant D1-D2 heteromer-induced increase in the time spent grooming, although the magnitude of the response was still lower than in the adults (Fig. 5B). This effect of SKF 83959 on grooming was lost in the juveniles in the latter half of the 60 minute testing period but was maintained in the adults. Together these findings suggest that the reduced expression of the D1-D2 receptor heteromer in NAc shell likely contribute, at least in part, to the behavioural subsensitivity observed in the grooming responses induced by SKF 83959, and emphasize a role for the D1-D2 receptor heteromer in mediating behavioural output.
Fig. 5.
Effect of acute D1-D2 receptor heteromer activation on grooming behavior in juvenile and adult rats. (A) In adult rats, SKF 83959 (1.5 mg/kg, s.c.) significantly increased the total amount of time spent grooming within the 60 minute testing period compared to saline controls, but had no significant effect on grooming in juvenile animals {Drug, F(1,22)=8.0, P<0.01; Age, F(1,22)=20.4, P<0.0001; Drug × Age, F(1,22)=3.8, P<0.06, n.s.} (B) Effect of SKF 83959 on grooming in the first and last 30 minutes of the testing period. SKF 83959 significantly increased grooming in both juvenile and adult rats in the first 30 minutes following injection {Drug, F(1,22)=12.2, P<0.002; Age, F(1,22)=23.8, P<0.0001}, an effect that was lost in the juvenile rats in the latter 30 minutes of the testing period {Drug, F(1,22)=3.8, P<0.06, n.s.}. Data are expressed as mean ± SEM. **P<0.01 compared to juveniles of the same treatment; #P<0.05, ###P<0.001 compared to saline controls of the same age, Student’s t test.
DISCUSSION
The present study showed that in juvenile striatum the D1R and D2R were coexpressed solely in unique subset of neurons that also contained the neuropeptides DYN and ENK. Similarly in cultured striatal neurons, the majority of which coexpress the D1R and D2R, an identical phenotype was observed. Furthermore, we showed that the number of MSNs that exhibited D1R/D2R coexpression, and therefore the ability to express the D1-D2 receptor heteromer, showed an age-dependent variation, with reduced levels in juvenile NAc shell and CP compared to adults. These reduced levels in D1-D2 heteromer densities were associated with subsensitivity to the induction of grooming following D1-D2 heteromer activation.
That there exists a D1R/D2R/DYN/ENK coexpressing neuronal phenotype in a subset of striatal MSNs in young and adult animals suggests an important physiological function for these neurons. The interplay between the dopamine and opioid systems has been widely studied; yet the molecular mechanisms that underlie the linkage between these specific dopamine receptors and their neuropeptides remain entirely unknown. The present evidence does suggest that the gene expression of these receptors and neuropeptides can be mutually turned “off” for the purpose of differentiating D1R and D2R coexpressing neostriatal neurons to their D1R/DYN-only or D2R/ENK-only phenotypes. Conversely, the increase in NAc shell and CP D1R and D2R coexpression observed in adults suggests that the reverse is also true; that neurons expressing a D1R/DYN- or D2R/ENK-only phenotype also have the ability to induce gene expression of the other dopamine receptor and its neuropeptide. Together these findings suggest that MSNs along the major pathways may have the potential to express both of the dopamine receptors, as well as DYN and ENK. Given the number of psychopathologies that arise through altered basal ganglia dopamine and/or opioid signaling, the idea that atypical neurotransmission along the striatonigral or striatopallidal pathways could be alleviated via activation of dormant genes has great potential. However, further research into the transcriptional regulators of dopamine receptors, their neuropeptides, and how their gene expression is linked, is first required to determine the mechanisms by which the phenotype of these neurons are regulated. BDNF, for example, has been shown to contribute to the phenotypic differentiation of striatal neurons to their spiny or aspiny subtypes (Mizuno et al., 1994, Ventimiglia et al., 1995), and additionally influence the striatal expression of both DYN (Logrip et al., 2008, Saylor and McGinty, 2008) and ENK mRNA (Sauer et al., 1994, Saylor and McGinty, 2008), possibly via TrkB-induced phosphorylation of CREB (Finkbeiner et al., 1997, Arthur et al., 2004).
In addition to coexpressing DYN and ENK, D1R/D2R-coexpressing neurons are distinct from other dopamine receptor expressing neurons in that they also express the D1R-D2R heteromer, a receptor complex that exhibits unique signaling properties and has been shown to link dopamine to calcium (Lee et al., 2004, Rashid et al., 2007) and BDNF signaling in striatal neurons (Hasbi et al., 2009). The relative densities of the D1-D2 receptor heteromer are dependent upon two factors; the total number of coexpressing neurons present within a specific brain region and the propensity of D1R and D2R to form the receptor complex within a given neuron coexpressing the receptors. We found that the degree of expression of the D1-D2 heteromer in neurons coexpressing the D1R and D2R in NAc or CP did not differ between juveniles and adults. However, the quantity of D1R/D2R-coexpressing neuronal cell bodies in both NAc shell and CP did differ between the two age groups, with adults showing a significantly higher number of coexpressing neurons in these regions compared to their younger counterparts. Thus although the D1-D2 heteromer was equally well expressed in any given neuron coexpressing the D1R and D2R in either age group, the overall reduction in the numbers of neurons coexpressing the receptors would be indicative of reduced D1-D2 receptor heteromer expression in juvenile animals.
Coincident with reduced D1-D2 receptor heteromer expression, activation of the receptor complex in juvenile rats elicited a blunted grooming response compared to the adults, an effect that may have been attributed to the reduced NAc D1-D2 heteromer densities as activation of the complex in NAc shell has been recently shown to result in orofacial movements and grooming responses in rats (Perreault et al., 2012). It is noteworthy that although grooming has been traditionally linked to D1R activation, SKF 83959 does not activate the Gs-linked D1R, but is associated with PLC activation and phosphoinositide hydrolysis in brain (Panchalingam and Undie, 2001, Jin et al., 2003, Zhen et al., 2005, Rashid et al., 2007), a pathway also activated by a number of D1R agonists in brain tissue (Undie et al., 1994, Desai et al., 2005) but, interestingly, not in cells expressing only the D1R (Lin et al., 1995). Given the ability of D1R agonists to directly induce PI hydrolysis in brain, but not in D1R-only expressing cells, this suggests that D1R agonists that stimulate this pathway do so through agonist activity at the D1-D2 receptor heteromer. A number of reports have shown, for example, that the “selective” D1R agonists SKF 81297 and SKF 38393 induce behavioural and neurochemical effects characteristic of dopamine D1-D2 heteromer activation, such as grooming and activation of PLC leading to intracellular calcium release (Molloy and Waddington, 1987, Undie and Friedman, 1990, Undie et al., 1994, Rashid et al., 2007). In addition, the D1R agonist SKF 83822, which activates the Gs-linked D1 receptor but not the D1-D2 heteromer (Rashid et al., 2007), actually suppresses grooming in adult rats (Perreault et al., 2010). As such, together the evidence indicates that grooming is likely mediated by the D1-D2 receptor heteromer via a PLC-linked pathway, and we therefore suggest that the appropriate selection of dopaminergic drugs is essential to effectively target the specific receptor complex, or signaling pathway, under investigation.
The reduction in grooming responses observed in the juvenile rats is consistent with a number of other reports documenting behavioural subsensitivity in response to psychostimulant drugs in younger animals (Bolanos et al., 1998, Featherby et al., 2008, Banerjee et al., 2009, Zakharova et al., 2009a). The underlying mechanisms contributing to this subsensitivity have remained elusive, and are further complicated by evidence showing that adolescents exhibit enhanced, and not diminished, dopamine release following amphetamine or cocaine administration (Laviola et al., 2001, Walker and Kuhn, 2008) and increased sensitivity to dopamine transporter blockade by cocaine (Bolanos et al., 1998). In keeping with this, it has been hypothesized that increased dopamine transmission could lead to an inhibition of striatal cholinergic interneurons, followed by an upregulation of postsynaptic striatal cholinergic receptors, increased overall cholinergic transmission and behavioural subsensitivity (Bolanos et al., 1998). However, more recent studies have shown that blockade of nicotinic receptors actually attenuate grooming in adult rats (Wooters and Bardo, 2009) and psychostimulant-induced behavioral sensitization was shown to be dependent upon nicotinic receptor activation induced by endogenously released acetylcholine (Schoffelmeer et al., 2002, de Rover et al., 2004). A further confounding factor is the demonstration that periadolescent rats exhibit increased sensitivity to psychostimulant-induced reward (Shahbazi et al., 2008, Zakharova et al., 2009a, Zakharova et al., 2009b, Anker and Carroll). Although it has been suggested that this may be attributed to enhanced ventral striatal activity (Chambers et al., 2003), possibly as a result of the overall elevated D1R and D2R expression seen during adolescence (Tarazi and Baldessarini, 2000, Andersen, 2002), enhanced D1R and D2R transmission is also involved in grooming and locomotion and is thus insufficient to explain the observed age-dependent discrepancies in behavioral responding.
In summary, the present findings showed that the phenotype of striatal neurons shifted in an age-dependent manner, with reduced coexpression of D1 and D2 receptors in juvenile rats, followed by a rise in coexpression in NAc shell and CP at maturity. Consequently D1-D2 receptor heteromer expression was also lower in juvenile NAc shell and CP compared to adults, and this manifested as subsensitivity to the induction of grooming responses following activation of the heteromeric complex, a behavioural response that has been previously attributed to NAc shell activation of D1-D2 heteromers (Perreault et al., 2012). Given the proposed role for these D1R/D2R-coexpressing neurons in balancing neurotransmission within the mesolimbic, striatopallidal and striatonigral pathways (Perreault et al., 2010), and recent evidence showing that these neurons exhibit both inhibitory and excitatory capabilites (Perreault et al., 2012), these findings indicate that there are likely significant age-dependent differences in neurotransmission within these pathways that contribute to the reported incongruities in behavioural output following psychostimulants. Further characterization of these differences will not only provide insights into how neuronal connectivity changes with age but may help clarify the underlying mechanisms involved in the increased propensity of adolescents to become addicted to drugs of abuse.
Highlights.
Dopamine D1 and D2 receptors are coexpressed with dynorphin and enkephalin.
Striatal coexpression of D1 and D2 receptors is age-dependent.
Juvenile rats have lower striatal D1-D2 receptor heteromer expression than adults.
Juvenile rats are subsensitive to D1-D2 receptor heteromer-induced grooming.
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse (to S.R.G. and B.F.O.), and a Canadian Institute of Health Research Postdoctoral Fellowship (to M.L.P.). S.R.G. holds a Canada Research Chair in Molecular Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agoston DV, Szemes M, Dobi A, Palkovits M, Georgopoulos K, Gyorgy A, Ring MA. Ikaros is expressed in developing striatal neurons and involved in enkephalinergic differentiation. J Neurochem. 2007;102:1805–1816. doi: 10.1111/j.1471-4159.2007.04653.x. [DOI] [PubMed] [Google Scholar]
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, Impey S. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J Neurosci. 2004;24:4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PS, Aston J, Khundakar AA, Zetterstrom TS. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Eur J Neurosci. 2009;29:465–476. doi: 10.1111/j.1460-9568.2008.06601.x. [DOI] [PubMed] [Google Scholar]
- Baydyuk M, Russell T, Liao GY, Zang K, An JJ, Reichardt LF, Xu B. TrkB receptor controls striatal formation by regulating the number of newborn striatal neurons. Proc Natl Acad Sci U S A. 2011;108:1669–1674. doi: 10.1073/pnas.1004744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Elangovan M, Periasamy A. FRET data analysis: the algorithm. In: Periasamy A, Day RN, editors. Molecular Imaging: FRET Microscopy and Spectroscopy. Oxford University Press; New York: 2005. pp. 126–145. [Google Scholar]
- Culver KE, Rosenfeld JM, Szechtman H. A switch mechanism between locomotion and mouthing implicated in sensitization to quinpirole in rats. Psychopharmacology (Berl) 2000;151:202–210. doi: 10.1007/s002139900346. [DOI] [PubMed] [Google Scholar]
- de Rover M, Mansvelder HD, Lodder JC, Wardeh G, Schoffelmeer AN, Brussaard AB. Long-lasting nicotinic modulation of GABAergic synaptic transmission in the rat nucleus accumbens associated with behavioural sensitization to amphetamine. Eur J Neurosci. 2004;19:2859–2870. doi: 10.1111/j.0953-816X.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. A comparison of the locomotor stimulant effects of D1-like receptor agonists in mice. Pharmacol Biochem Behav. 2005;81:843–848. doi: 10.1016/j.pbb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Falk T, Zhang S, Erbe EL, Sherman SJ. Neurochemical and electrophysiological characteristics of rat striatal neurons in primary culture. J Comp Neurol. 2006;494:275–289. doi: 10.1002/cne.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherby T, van den Buuse M, Lubman DI, Lawrence AJ. Persistent downregulation of hippocampal CREB mRNA parallels a Y-maze deficit in adolescent rats following semi-chronic amphetamine administration. Br J Pharmacol. 2008;154:417–428. doi: 10.1038/bjp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo K, Suzuki S, Li C, Tsunematsu T, Nakamura F, Okumura S, Sato M, Minamisawa S, Toya Y, Umemura S, Ishikawa Y. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am J Physiol Cell Physiol. 2007;293:C1498–1508. doi: 10.1152/ajpcell.00088.2007. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Miller TR, Witte DG, Bianchi BR, Stashko M, Manelli AM, Frail DE. Characterization of cloned human dopamine D1 receptor-mediated calcium release in 293 cells. Mol Pharmacol. 1995;47:131–139. [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Yeh C, Yang XW. Pivotal role of early B-cell factor 1 in development of striatonigral medium spiny neurons in the matrix compartment. J Neurosci Res. 2008;86:2134–2146. doi: 10.1002/jnr.21666. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. Faseb J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Martin-Ibanez R, Crespo E, Urban N, Sergent-Tanguy S, Herranz C, Jaumot M, Valiente M, Long JE, Pineda JR, Andreu C, Rubenstein JL, Marin O, Georgopoulos K, Mengod G, Farinas I, Bachs O, Alberch J, Canals JM. Ikaros-1 couples cell cycle arrest of late striatal precursors with neurogenesis of enkephalinergic neurons. J Comp Neurol. 2010;518:329–351. doi: 10.1002/cne.22215. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- Molloy AG, Waddington JL. Assessment of grooming and other behavioural responses to the D-1 dopamine receptor agonist SK & F 38393 and its R- and S-enantiomers in the intact adult rat. Psychopharmacology (Berl) 1987;92:164–168. doi: 10.1007/BF00177909. [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS. SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology. 2001;40:826–837. doi: 10.1016/s0028-3908(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. Dopamine D1-D2 Receptor Heteromer in Dual Phenotype GABA/Glutamate-Coexpressing Striatal Medium Spiny Neurons: Regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012;7:e33348. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Campbell K, Wiegand SJ, Lindsay RM, Bjorklund A. Brain-derived neurotrophic factor enhances striatal neuropeptide expression in both the intact and the dopamine-depleted rat striatum. Neuroreport. 1994;5:609–612. doi: 10.1097/00001756-199401000-00019. [DOI] [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes Brain Behav. 2008;7:906–914. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O’Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995;7:213–222. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Verma V, Hasbi A, O’Dowd BF, George SR. Dopamine D1-D2 receptor Heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285:35092–35103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. Nicotinic receptors differentially modulate the induction and expression of behavioral sensitization to methylphenidate in rats. Psychopharmacology (Berl) 2009;204:551–562. doi: 10.1007/s00213-009-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009b;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Friedman E. The role of the phosphatidyinositol-linked D1 dopamine receptor in the pharmacology of SKF83959. Pharmacol Biochem Behav. 2005;80:597–601. doi: 10.1016/j.pbb.2005.01.016. [DOI] [PubMed] [Google Scholar]