Abstract
Purpose
To compare the effects of osmolality vs. viscosity of radio-contrast media on intra-renal oxygenation as determined by blood oxygenation level dependent (BOLD) magnetic resonance imaging (MRI) in a model of contrast induced nephropathy (CIN).
Materials and Methods
24 Sprague-Dawley rats were divided into five groups. Nitric oxide synthase inhibitor L-NAME (10mg/kg), cycloxygenase inhibitor indomethacin (10mg/kg), or saline, and radio-contrast iodixanol (high viscosity, 784 or 1600 mg I/kg) or iothalamate (high osmolality, 1600 mg I/kg) were administered. BOLD MRI images were acquired on Siemens 3T scanner using a multiple gradient recalled echo sequence at baseline, following L-NAME (or saline), indomethacin (or saline), and radio-contrast agents. R2* (=1/T2*) was used as the BOLD MRI parameter in renal medulla and cortex. Mixed-effects models with first order auto-regressive variance-covariance models were used to analyze the data.
Results
The magnitude of change in medullary R2* (MR2*) with same dose of iodine was larger with iodixanol compared to iothalalmate both in pre-treated groups (303% vs. 225.6%, <0.01) and the control group (191.6% vs. -1.8%, p <0.01). The MR2* change in high dose iodixanol was about twice compared to the low dose (303% vs. 133%, p<0.01).
Conclusion
The viscosity of radio-contrast seems to play a more significant role than osmolality in terms of renal oxygenation changes as evaluated by BOLD MRI. Additionally, iodixanol induced a dose-dependent increase in renal medullary hypoxia.
Keywords: kidney, oxygenation, BOLD, MRI, radio-contrast induced nephropathy, rats
INTRODUCTION
Since the time radio-contrast induced nephropathy (CIN) was recognized more than 50 years ago (1), there have been continued efforts to chemically modify radio-contrast agents to be less nephrotoxic. Significant changes in their composition during the past few decades have been made to potentially reduce the nephrotoxicity, but CIN remains to be the third major source of in-hospital acquired acute renal failure and is associated with long-term morbidity and mortality in elderly patients and those with pre-existing kidney insufficiency and diabetes (2). The physico-chemical properties that have been varied include ionicity, monomer vs. dimer, and osmolality (3). A consequence of reducing the osmolality seems to be an increase in viscosity (4). Reducing osmolality has been shown to have a beneficial effect on direct renal cell toxicity in some(5), but not all (6) in vitro studies. There is increasing evidence supporting negative hemodynamic consequences associated with increased viscosity based on both in vitro (7) and in vivo studies (8). Here it is important to note that the viscosity increases much further in the medulla due to the concentration process and has been demonstrated recently (9). While the relative contributions of the three potential pathways, viz. hemodynamic, direct cellular toxicity and endogenous biochemical disturbances such as oxidative stress have been recognized (10), there is no consensus yet on which pathway is dominant.
The pathophysiology of CIN is not yet fully understood even though it is generally accepted to involve acute tubular necrosis (ATN) resulting from vasoconstriction and consequent renal medullary hypoxemia (4). Other effects include direct cytotoxicity, rheologic alterations, activation of the tubuloglomerular feedback mechanism, regional hypoxia and production of reactive oxygen species (4). Most previous studies do not have a control group that do not get the contrast agent to evaluate causality (11). It may be desirable to have a platform for comparing different contrast media in terms of assessing their relative risk for developing CIN. Animal models can be useful for this purpose.
Here, we present our experience monitoring intra-renal oxygenation changes using Blood Oxygenation Level–Dependent (BOLD) MRI following administration of contrast material in a “functional” model of CIN previously reported (12). It was previously shown that the deleterious effects of high osmolar contrast agent, iothalamate, were observed only when the animals were pretreated with a nitric oxide synthase (NOS) and prostaglandin (PGE) inhibitor (12). This could be viewed as a model of endothelial dysfunction, which is associated with many of the conditions (such as heart disease, diabetes and chronic kidney disease) considered to be risk factors for developing CIN (13). BOLD MRI is gaining acceptance as a viable method of monitoring intra-renal oxygenation status (14-15). A previous study has shown that BOLD MRI can be used to monitor progressive changes in intra-renal oxygenation following administration of the 1st generation, high osmolar radio-contrast iothalamate (16).
The specific goals for this study were to investigate any differences between the 3rd generation radio-contrast iodixanol and the 1st generation radio contrast iothalamate in terms of their effects on intra-renal oxygenation as evaluated by BOLD MRI and to verify any dose response with iodixanol.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Animal Care and Use Committee. Twenty four male Sprague-Dawley (SD) rats (Harlan Laboratories , Madison, WI USA) weighing 336.4±10.3 gram (mean ± SE) were divided into five groups as shown in Table 1. Three groups received iodixanol, 3rd generation high viscosity contrast agent, (low and high dose with pre-treatment and high dose control) and two groups received iothalamate, 1st generation high osmolality contrast agent, (high dose with pre-treatment and control). Rats were anesthetized using inactin (100 mg/kg i.p., Sigma-Aldrich, St. Louis, MO, USA). A femoral vein was catheterized for the administration of nitric oxide synthase inhibitor (L-NAME, 10mg/kg, Sigma-Aldrich, St. Louis, MO, USA), cyclooxygenase (COX) inhibitor, reducing the production of prostaglandin, (indomethacin, 10 mg/kg, Sigma-Aldrich, St. Louis, MO, USA), or saline (1ml/kg), and radio-contrast agents. Indomethacin was dissolved in saline using potassium hydroxide (KOH) and hydrochloric acid (HCI) to balance the pH to 7.4-8.0.
Table 1.
Experimental groups
| Group | Treatment1 | Treatment2 | Radio-contrast | Dose of RC | Animal # |
|---|---|---|---|---|---|
| 1 | L-NAME | Indomethacin | Iodixanol | 784 mg | n=6 |
| 2 | L-NAME | Indomethacin | Iodixanol | 1600 mg | n=5 |
| 3 | L-NAME | Indomethacin | Iothalamate | 1600 mg | n=6 |
| 4 | Saline | Saline | Iodixanol | 1600 mg | n=3 |
| 5 | Saline | Saline | Iothalamate | 1600 mg | n=4 |
Note: dose is based on mg iodine/kg body weight. Iothalamate: 1st generation contrast (high osmolality); Iodixanol: 3rd generation contrast (high viscosity).
Either iodixanol (Visipaque, iodine concentration 320 mg/ml, GE Healthcare, USA) or iothalamate (Conray, iodine concentration 282 mg/ml, Mallinckrodt, St. Louis, MO, USA) was administrated after pre-treatment. The low dose was 784 mg and high dose was 1600 mg of organic iodine per kilogram body weight. For reference, a contrast enhanced CT delivers ~500 mgI/kg while cardiac angiography and percutaneous coronary intervention (PCI) would deliver 2-3 fold more. The detailed information about the radio-contrast agents is shown in Table 2.
Table 2.
Properties of Radio-contrasts in this Study
| Systemic name | Iodixanol | Iothalamate meglumine (USP 60%) |
|---|---|---|
| Brand name | visipaque | conray |
| Ionicity | nonionic | ionic |
| osmolality | isosmolar | 1000 mOsmol/L |
| iodine concentration | 320 mg/ml | 282 mg/ml |
| Viscosity at 37°C | 11.8 | 4.0 |
Note: based on data from GE Healthcare and Mallinckrodt
Imaging was performed on a 3.0 T scanner (Magnetom Verio, Siemens, Germany) using a multiple gradient recalled echo sequence (TE=3.6-41.3ms; FOV=12x6 cm; TR=69ms; bandwidth=320Hz/pixel; FA=30°; NEX=20; matrix: 256x256; slice thickness=2mm) to acquire 12 T2* weighted images. The rats were placed in a right decubitus position with their kidneys in the middle of a standard extremity coil. One transverse slice was selected in the middle of the kidney. Five sets of baseline BOLD images were acquired before of any chemicals. Then L-NAME (or saline in control groups) and indomethacin (or saline in control groups) were given as pre-treatment. Following each pre-treatment, five sets of BOLD images were acquired. One of the radio-contrast agents was then administered. Further sets of T2*-weighted images after the radio-contrast were obtained every 3 minutes for one hour. Figure 1 shows the timing diagram.
Figure 1.
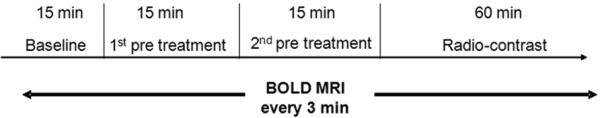
Experimental timing diagram. The vertical lines represent the timing of administration of pretreatment and radio-contrast. Scanning was done every 3 minutes for entire study.
The Ln (signal intensity) vs. echo time data were fit to a linear function to generate T2* map inline. Regions of interest (ROIs) were defined on the anatomic image and copied to T2* maps to obtain T2* reading and then converted into R2* in excel spread sheet. R2* maps were independently generated using custom Matlab (Mathworks, Natick, MA) code where signal intensity vs. echo time data were fit to a single exponential decay function. High R2* value or brighter regions on the R2* map represent higher hypoxic levels.
To allow for a simple graphical representation of the data, a single representative R2* value was generated for each animal by averaging all R2* readings within a time period. The time series of BOLD R2* measurements were classified into four time periods, i.e. baseline (BL) including time points 1 to 5, “pre-treatment-1” (L-NAME or saline for control group) including time points 6 to 10, “pre-treatment-2” (indomethacin or saline for control group) including time points 11 to 15, and “radio-contrast” (iodixanol or iothalamate) including time point 16 and beyond. Then the statistical significance of the R2* differences between one time period and baseline was assessed using the two-tailed paired Student's t-test.
To fully take advantage of the multiple measurements, a mixed effects regression model was used to assess BOLD R2* changes over time within each group and between-group difference. Given this design and data structure, we used a model which included all the 35 continuous time points (at 3 minutes intervals), i.e. time measured as 0, 3, 6, 9, ...., 102 min, and a first order auto-regressive variance-covariance structure was specified. Fixed effects included groups (5-levels), time (continuous), and an interaction of group by time, and random effects included each individual rat. Akaike Information Criterion (AIC, smaller is better) was used to determine the appropriate variance-covariance structure. Normality assumption by group by time point was assessed via Shapiro-Wilke's test and regarded fulfilled. The Bonferroni adjustment was used for multiple comparisons. Statistical analyses were carried out by SAS 9.2 (SAS, Cary, NC), and a p<0.05 was regarded statistically significant.
RESULTS
Figure 2 is a plot describing the temporal changes in average R2* obtained from all rats in Group 1. On the top of the time curve are R2* maps from a representative animal. Note the progressive increase in the level of hypoxia in the renal medulla following administration of each additional chemical agent. The medullary R2* (MR2*) increases steadily after each chemical. The cortical R2* (CR2*) did not show much of a change over time.
Figure 2.
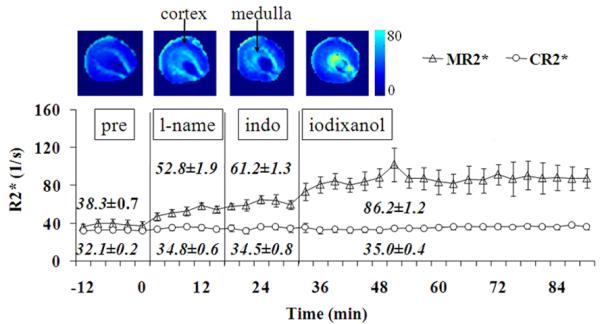
Averaged (mean ± SE) renal BOLD R2* time course from six rats in Group 1 (low dose of iodixanol group) in renal medulla and cortex following the administration of L-NAME, indomethacin, and radio-contrast iodixanol. The vertical lines indicate the time of administration of each of chemicals. Note pre: baseline; lname: L-NAME; indo: indomethacin. Error bars represent standard error among rats. On the top are R2* maps generated using custom Matlab (Mathworks, Natick, MA) code in one representative rat. The relative brightness in renal medulla suggests low oxygenation level compared to cortex. The window settings were the same in all maps. The brightness in renal medulla increases gradually after each chemical suggesting the progressively decreasing of oxygenation. The R2* maps are from baseline, following administration of L-NAME, indomethacin, and iodixanol. The arrows show the renal medulla and cortex where the ROIs were placed. The renal medulla is relatively brighter than renal cortex in baseline R2* map, suggest lower oxygenation level there. Each chemical contributes to the additional increased brightness in R2* maps in the renal medulla, suggesting progressive hypoxia in the renal medulla.
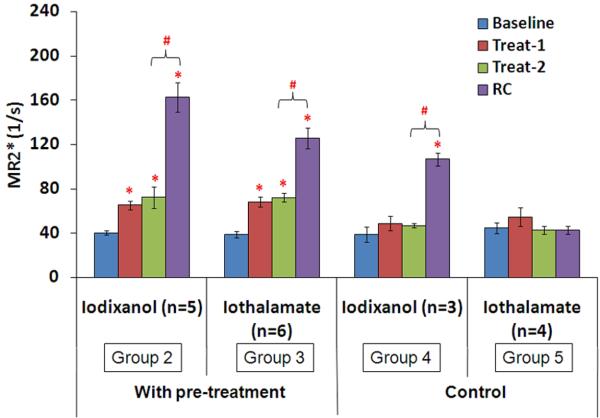
Figures 3 and 4 summarize the data from all animals. Figure 3 illustrates the difference between the effects of iodixanol and iothalamate on renal medullary R2*, while Figure 4 summarizes the dose response with iodixanol in both medulla and cortex. In the pre-treatment groups, both radio-contrasts induced significant R2* response in renal medulla compared to baseline. However, the magnitude of the change was higher with iodixanol compared to iothalamate (303% vs 225.6%, <0.01). In the control groups, only iodixanol induced significant MR2* increase in renal medulla (191.6% vs. -1.8%, p <0.01). There was no significant change in the cortex with either agent in the control groups (data not shown). In the dose dependence study, there was no significant difference in baseline R2* measurements between low and high dose groups (Figure 4). Both MR2* and CR2* showed a dose dependent response with iodixanol. L-NAME, indomethacin and iodixanol each induced a significant increase in MR2* compared to their own baseline in both groups. However, the magnitude of the increase in MR2* after iodixanol in the high dose group was about twice compared to the low dose group (303% vs 133%, p<0.01) against their own baseline. Low dose iodixanol did not change CR2*, whereas, high dose iodixanol increased CR2* significantly from baseline.
Figure 3.
Comparison of MR2* response to iodixanol and iothalamate with and without pre-treatment based on two tailed Student's t-test. Data shown as mean ± SE. With treatment, Treat-1 is L-NAME; Treat-2 is indomethacin. RC is radio-contrast, either iodixanol or iothalamate (1600 mg I/kg). In the control groups, both Treat-1 and Treat-2 were saline. * stands for p<0.05 by Student's t-test compared to baseline; # stands for p<0.05 by Student's t-test compared to the previous phase. Number of rats in each group are shown in parenthesis.
Figure 4.
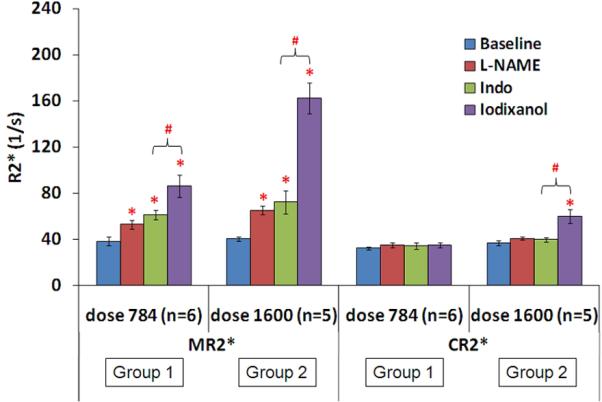
Summary of dose dependence of BOLD R2* response in renal medulla and cortex based on two tailed Student's t-test. Data shown as mean ± SE. The unit for “dose 784” and “dose 1600” is mg iodine per kilogram body weight. Indo stands for indomethacin. There was no significant difference in baseline values between low and high dose groups. * indicates a significant difference (p<0.05) compared to own baseline; # stands for p<0.05 by Student's t-test compare to the previous phase.
The comparison of the change over time between groups based on the mixed effects regression model is shown in Table 3. In the pre-treatment group, both cortex and medulla showed a dose related increase in slope, that is, MR2* and CR2* increased faster (related to rapid decreasing of renal oxygenation) with high dose iodixanol (Group 2 vs. Group 1). When comparing iodixanol vs. iothalamate, there were significant difference in the slopes both in MR2* and CR2* and both in pre-treatment and control groups (Group 2 vs. Group 3 and Group 4 vs. Group 5). When comparing pre-treatment vs. control groups, there were significant differences in the slopes in the medulla (but not cortex) with both agents (Group 2 vs. Group 4 and Group 3 vs. Group 5).
Table 3.
Rate of change in R2* over time based on mixed effects regression model
| MR2* | CR2* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| group comparison | Description | Est. Slope diff | Low | Upper | adj. p | Est. Slope diff | Low | Upper | adj. p |
| 2 vs. 1 | Dose response in pre-treatment groups | 0.95 | 0.73 | 1.17 | <0.01 | 0.36 | 0.24 | 0.47 | <0.01 |
| 2 vs. 3 | 3rd vs. 1st generation in pre-treatment | 0.44 | 0.66 | 0.22 | <0.01 | 0.26 | 0.37 | 0.14 | <0.01 |
| 4 vs. 5 | 3rd vs. 1st generation in control group | 0.77 | 1.04 | 0.49 | <0.01 | 0.26 | 0.41 | 0.12 | <0.01 |
| 2 vs. 4 | 3rd generation, pre-treatment vs. control | 0.71 | 0.98 | 0.45 | <0.01 | 0.14 | 0.28 | 0.01 | 0.16 |
| 3 vs. 5 | 1st generation, pre-treatment vs. control | 1.04 | 1.27 | 0.80 | <0.01 | 0.15 | 0.27 | 0.03 | 0.09 |
DISCUSSION
To our knowledge, this is the first study, which compared the 1st generation (iothalamate) directly with the 3rd generation radio-contrast (iodixanol) on intra-renal oxygenation in a model of CIN. Our results with iothalamate are in good agreement with a previous report using BOLD MRI at 1.5T using the same animal model (16). There were four main findings in this study.
When pretreated with L-NAME and indomethacin, there was a significant increase in MR2* after either contrast agent (Figure 3). However, the magnitude of change was higher with iodixanol compared to iothalamate (for the same dose of iodine). It is not yet clear whether this may influence the final outcome in terms of developing kidney injury and CIN. MR2* increased faster over time with iodixanol compared to iothalamate (Table 3).
In the pretreated rats, iodixanol showed a dose response in both cortex and medulla. CR2* and MR2* values were significantly higher (Figure 4) and increased faster over time (Table 3) at the higher dose (1600 vs. 784 mgI/kg).
In the control groups, only iodixanol resulted in a significant increase in MR2* (Figure 3). This suggests that the direct effect of viscosity on renal hemodynamics may be a significant determinant of renal medullary hypoxia and is consistent with previous reports (8,17-18).
When comparing pre-treatment vs. control groups (Table 3), there was a significant difference in the slope in the medulla with both agents. This suggests that the deleterious effects may be worse in subjects at risk.
Pre-existing conditions, such as defective vasodilation due to insufficient nitric oxide or prostaglandin play a role in the aggravation of intra-renal hypoxia as shown in this study and previous ones (12,16). Since hypoxia is now well accepted to play a key role in the pathogenesis of CIN (19), our results suggest that iodixanol, the 3rd generation radio contrast, may lead to more deleterious effects irrespective of the pre-existing conditions.
CIN is defined as an acute impairment of renal function manifested by an absolute increase in serum creatinine of at least 0.5 mg/dl or by relative increase by at least 25% from the baseline levels (4,20). For a number of reasons, creatinine is a poor marker of true injury to the kidney. Peak creatinine typically occurs 3–5 days after contrast administration and returns to baseline (or a new baseline) within 1–3 weeks (11). The renal failure is nonoliguric for the vast majority of patients. In almost all cases, the decline in renal function is mild and transient. The use of serum creatinine for CIN and in general for acute kidney injury (AKI) is being questioned and alternate and potentially early biomarkers are being sought (21). Creatinine is an unreliable indicator during acute changes in kidney function (22). First, the use of serum creatinine to estimate true renal function is now well-recognized to involve inaccuracies and limitations (23). A marked reduction in glomerular filtration rate (GFR, up to 50% of kidney function) may take place before creatinine levels rise. Second, creatinine does not reflect kidney function during acute changes until a steady state has been reached, which can take several days. Moreover, creatinine is a poor biomarker for AKI due principally to its inability to help diagnose early acute renal failure and complete inability to help differentiate among its various causes. The natural delay in rise of serum creatinine makes it difficult to establish causality especially because it has been reported similar level of changes could be observed in the subjects at risk even without receiving any contrast (11). So, there is an acute need for alternate markers for CIN.
Previous studies evaluating hemodynamic consequences of contrast administration primarily studied renal blood flow or vascular resistance. However, the contributions of blood flow to the development of CIN remain unclear (10). Since availability of oxygen ultimately determines tissue viability, direct evaluation of oxygenation status is desirable. This is more true for the kidney where renal blood flow varies significantly between cortex and medulla. While renal function (GFR) is related to renal blood flow, changes in total renal blood flow may not be associated with changes in renal oxygenation, particularly in the medulla (24). For example, administration of furosemide results in a decrease in medullary blood flow (oxygen delivery) but a greater inhibition of sodium reabsorption along the medullary thick ascending limbs (decreasing oxygen demand) resulting in an increase in medullary oxygenation (25). Furthermore, reduction in GFR effectively reduces the amount of sodium to be reabsorbed and hence reduces medullary oxygen consumption. It has been previously suggested that the reduction in GFR may actually be a protective mechanism (26). This may serve as yet another motivation to consider alternate markers for acute renal failure, including CIN. Recent reports suggest a role for neutrophil gelatinase-associated lipocalin (nGAL) (27), which has been shown to demonstrate changes as early as two hours post-contrast in humans (28).
One limitation of the study is the lack of direct evidence of CIN, even though we had utilized a model that was previously validated (12). This was mostly due to the logistics. Since serum creatinine measurements have to be made at least 24 hours post-contrast administration, we were not able to obtain them due to the use of inactin for anesthesia which is not suitable for survival studies. With the availability of alternate injury markers such as nGAL, it is possible that future studies could include them along with BOLD MRI.
In conclusion, our data support that the BOLD MRI technique in concert with the CIN model can provide a useful platform to test different radio-contrast agents and interventions to mitigate them. Our results suggest that viscosity of the contrast agent may play an equal or greater role compared to osmolality in determining the intra-renal hemodynamics. Whether that has any direct impact on the overall outcome, i.e. development of CIN is not yet clear. Future studies should include other biomarkers to document CIN.
ACKNOWLEDGEMENT
Work supported in part by a grant from the National Institutes of Health, RO1- DK-053221.
REFERENCE
- 1.Bartels ED, Brun GC, Gammeltoft A, Gjorup PA. Acute anuria following intravenous pyelography in a patient with myelomatosis. Acta Med Scand. 1954;150(4):297–302. doi: 10.1111/j.0954-6820.1954.tb18632.x. [DOI] [PubMed] [Google Scholar]
- 2.Abu Jawdeh BG, Kanso AA, Schelling JR. Evidence-based approach for prevention of radiocontrast-induced nephropathy. J Hosp Med. 2009;4(8):500–506. doi: 10.1002/jhm.477. [DOI] [PubMed] [Google Scholar]
- 3.Katzberg RW, Haller C. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int Suppl. 2006;(100):S3–7. doi: 10.1038/sj.ki.5000366. [DOI] [PubMed] [Google Scholar]
- 4.Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005;68(1):14–22. doi: 10.1111/j.1523-1755.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Kuhlmann MK, Grgic A, Heckmann M, Kramann B, Uder M. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology. 2005;235(3):843–849. doi: 10.1148/radiol.2353040726. [DOI] [PubMed] [Google Scholar]
- 6.Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29(20):2569–2576. doi: 10.1093/eurheartj/ehn197. [DOI] [PubMed] [Google Scholar]
- 7.Sendeski M, Patzak A, Persson PB. Constriction of the vasa recta, the vessels supplying the area at risk for acute kidney injury, by four different iodinated contrast media, evaluating ionic, nonionic, monomeric and dimeric agents. Invest Radiol. 2010;45(8):453–457. doi: 10.1097/RLI.0b013e3181d77eed. [DOI] [PubMed] [Google Scholar]
- 8.Seeliger E, Flemming B, Wronski T, Ladwig M, Arakelyan K, Godes M, Mockel M, Persson PB. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol. 2007;18(11):2912–2920. doi: 10.1681/ASN.2006111216. [DOI] [PubMed] [Google Scholar]
- 9.Seeliger E, Becker K, Ladwig M, Wronski T, Persson PB, Flemming B. Up to 50-fold increase in urine viscosity with iso-osmolar contrast media in the rat. Radiology. 2010;256(2):406–414. doi: 10.1148/radiol.10091485. [DOI] [PubMed] [Google Scholar]
- 10.Katzberg RW. Contrast medium-induced nephrotoxicity: which pathway? Radiology. 2005;235(3):752–755. doi: 10.1148/radiol.2353041865. [DOI] [PubMed] [Google Scholar]
- 11.Newhouse JH, Kho D, Rao QA, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376–382. doi: 10.2214/AJR.07.3280. [DOI] [PubMed] [Google Scholar]
- 12.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94(3):1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon R. Contrast-induced acute kidney injury (CIAKI). Radiol Clin North Am. 2009;47(5):783–788. v. doi: 10.1016/j.rcl.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94(12):3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 15.Kone BC. A ‘BOLD’ new approach to renal oxygen economy. Circulation. 1996;94(12):3067–3068. doi: 10.1161/01.cir.94.12.3067. [DOI] [PubMed] [Google Scholar]
- 16.Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13(5):744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancelot E, Idee JM, Couturier V, Vazin V, Corot C. Influence of the viscosity of iodixanol on medullary and cortical blood flow in the rat kidney: a potential cause of Nephrotoxicity. J Appl Toxicol. 1999;19(5):341–346. doi: 10.1002/(sici)1099-1263(199909/10)19:5<341::aid-jat584>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Lancelot E, Idee JM, Lacledere C, Santus R, Corot C. Effects of two dimeric iodinated contrast media on renal medullary blood perfusion and oxygenation in dogs. Invest Radiol. 2002;37(7):368–375. doi: 10.1097/00004424-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3(1):288–296. doi: 10.2215/CJN.02600607. [DOI] [PubMed] [Google Scholar]
- 20.Gami AS, Garovic VD. Contrast nephropathy after coronary angiography. Mayo Clin Proc. 2004;79(2):211–219. doi: 10.4065/79.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Malyszko J. Biomarkers of acute kidney injury in different clinical settings: a time to change the paradigm? Kidney Blood Press Res. 2010;33(5):368–382. doi: 10.1159/000319505. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30(1):33–37. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 23.Rahn KH, Heidenreich S, Bruckner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17(3):309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen M, Laustsen C, Perot V, Basseau F, Moonen C, Grenier N. Renal hemodynamics and oxygenation in transient renal artery occluded rats evaluated with iron-oxide particles and oxygenation-sensitive imaging. Z Med Phys. 2010;20(2):134–142. doi: 10.1016/j.zemedi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267(6 Pt 2):F1059–1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 26.Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transplant. 2005;20(Suppl 1):i6–11. doi: 10.1093/ndt/gfh1069. [DOI] [PubMed] [Google Scholar]
- 27.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15(4):419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 28.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31(10):910–919. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]