Abstract

The alkylation of aryl sulfamates and carbamates using iron catalysis is reported. The method constructs sp2–sp3 carbon–carbon bonds and provides synthetically useful yields across a range of substrates (>35 examples). The directing group ability of sulfamates and carbamates, accompanied by their low reactivity toward conventional cross-couplings, render these substrates useful for the synthesis of polyfunctionalized arenes.
As one of the most abundant, inexpensive, and non-toxic elements on earth, iron has been hailed as an ideal metal for the development of catalytic transformations.1 Various Febased methods to promote C–C bond formation have been reported since the seminal publications of Kharasch2 and Kochi,3 with many key advances being described in the past decade.1 One especially promising area is ironcatalyzed cross-coupling reactions of aryl electrophiles,4,5 largely pioneered by Fürstner,4a,b which nicely complements the most commonly used Pd- and Ni-based methods for forging C–C bonds. The use of iron-mediated coupling reactions in natural product and drug synthesis is testament to the promise of this developing field of research.6
With the aim of discovering iron-promoted reactions for use in synthesis, we explored iron-catalyzed reactions of aryl sulfamate and carbamate substrates. These substrates have recently garnered significant attention in crosscoupling reactions because of their ease of preparation, pronounced stability to a variety of reaction conditions, including conventional transition metal catalysis, and their directing group ability for arene functionalization.7,8 Sulfamates and carbamates have primarily been used in Nicatalyzed arylation,7,8,9 amination,10 and deoxygenation11 reactions, but their use in iron-catalyzed couplings holds much promise for enabling alkylative processes. No examples of iron-catalyzed sulfamate alkylation have been reported, and only a single example of a carbamate alkylation is known.12,13
Herein, we report the alkylations of aryl sulfamates and carbamates using alkyl Grignard reagents14 and iron catalysis to generate sp2–sp3 C–C bonds (Figure 1). The transformation enables the alkylation of a broad range of substrates, including electron-rich arenes, heterocycles, and ortho-substituted aromatics. This promising synthetic tool should prove generally useful for the synthesis of sp2–sp3 C–C bonds,15 and also expands the repertoire of highly sought after iron-catalyzed cross-coupling reactions.
Figure 1.
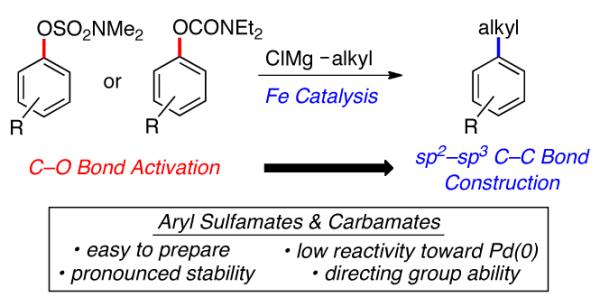
Iron-catalyzed alkylation of aryl sulfamates and carbamates.
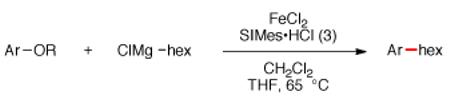
Alkyl Grignard reagents were considered to be ideal coupling partners for the intended alkylation of aryl sulfamates and carbamates. Despite their high reactivity, conventional Grignard reagents are widely available and extensively utilized across a host of modern synthetic applications.14 An extensive survey of reaction conditions was undertaken to effect the alkylative coupling of carbamate 1 with n-hexylmagnesium chloride16 to afford alkylated product 2 (see SI). Although most reaction conditions based upon literature procedures for ironcatalyzed Kumada couplings were unsuccessful, a modification of Shi’s conditions5,12 using the NHC ligand 3,17 delivered the alkylated product (Figure 2). Of note, the addition of substoichiometric quantities of CH2Cl2 was critical in order to obtain good yields and consistent results.18,19 Experiments conducted in the absence of FeCl2 gave <15% product with or without the addition of copper, palladium, or nickel salts (see SI).20,21
Figure 2.
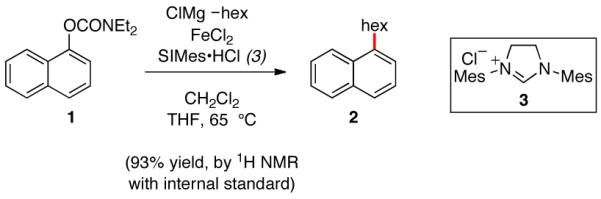
Optimal result obtained after extensive optimization.
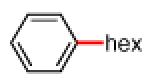
Having discovered conditions for the iron-catalyzed coupling of naphthylcarbamate 1, we examined several other carbamate substrates, in addition to aryl sulfamates (Table 1).22 The parent 1-naphthyl and phenyl systems underwent smooth reaction to give good isolated yields of product (entries 1 and 2). An electron-withdrawing p-trifluoromethyl substituent was examined and was found to be tolerated by the methodology (entry 3). Substrates containing the electron-donating p-methoxy and p-methyl groups also participated in the reaction, although yields obtained from the carbamate substrates were generally higher compared to those from the corresponding sulfamate substrates (entries 4 and 5). These results are notable, as iron-catalyzed alkylation reactions of electron-rich aryl chlorides and tosylates typically do not proceed efficiently.4a,b The use of substrates derived from m-cresol also gave useful yields of coupled product (entry 6).
Table 1.
Iron-catalyzed coupling of aryl carbamates and sulfamates with n-hexylmagnesium chloride.a
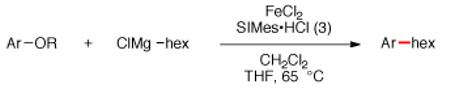
| entry | Ar | OR | product | yieldb |
|---|---|---|---|---|
| 1a 1b |
|
−OSO2NMe2 −OCONEt2 |
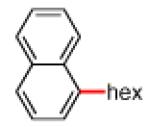
|
84%c 78%c |
| 2a 2b |
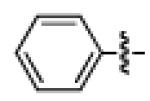
|
−OSO2NMe2 −OCONEt2 |
|
85% 82% |
| 3a 3b |
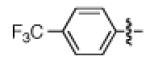
|
−OSO2NMe2 −OCONEt2 |
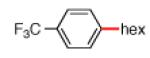
|
67% 66% |
| 4a 4b |
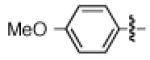
|
−OSO2NMe2 −OCONEt2 |
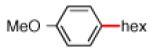
|
67% 90% |
| 5a 5b |
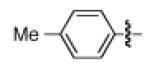
|
−OSO2NMe2 −OCONEt2 |
|
63% 90% |
| 6a 6b |
|
−OSO2NMe2 −OCONEt2 |
|
53% 92% |
Reaction conditions: FeCl2 (5 mol %), 3 (15 mol %), n-hexMgCl (1.5–2.0 equiv), CH2Cl2 (15–60 mol %), 3 h.
Isolated yields.
Isolated with naphthalene; see Supporting Information for details.
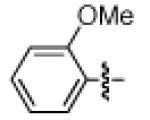
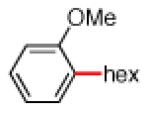
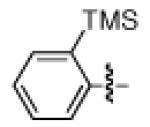
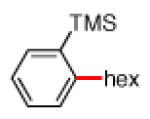
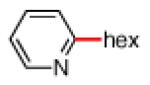
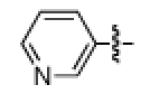
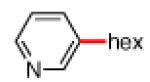
As shown in Table 2, the scope of the methodology includes heterocyclic substrates, in addition to orthosubstituted substrates that are typically not tolerated in related iron-catalyzed couplings.1c,4 Of note, orthosubstituted aryl carbamates and sulfamates are readily accessible using ortho-metallation7c,23 or through transition metal-catalyzed carbamate functionalization processes.24 o-Cresol derivatives underwent the desired coupling (entry 1), as did substrates possessing an ortho phenyl substituent (entry 2). An ortho methoxy group could also be employed (entry 3). Moreover, a bulky trimethylsilyl substituent was tolerated (entry 4). Heterocycles were also examined. Indole and dihydrobenzofuran carbamate derivatives coupled successfully (entries 5b and 6b), although coupling of the corresponding sulfamates proved difficult (entries 5a and 6a). Pyridine-containing substrates also participated in the desired transformation (entries 7a and 8).25
Table 2.
Coupling of ortho-substituted and heterocyclic substrates.a
| entry | Ar | OR | product | yieldb |
|---|---|---|---|---|
| 1a 1b |
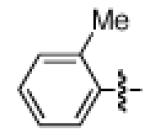
|
−OSO2NMe2 −OCONEt2 |
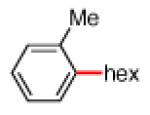
|
82% 84% |
| 2a 2b |
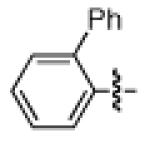
|
−OSO2NMe2 −OCONEt2 |
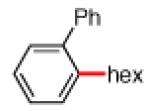
|
85% 82% |
| 3a 3b |
|
−OSO2NMe2 −OCONEt2 |
|
56% 52% |
| 4a 4b |
|
−OSO2NMe2 −OCONEt2 |
|
67% 64% |
| 5a 5b |
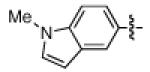
|
−OSO2NMe2 −OCONEt2 |
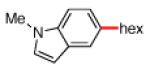
|
<5%e 48% |
| 6a 6b |
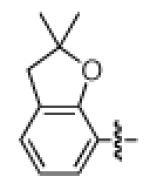
|
−OSO2NMe2 −OCONEt2 |
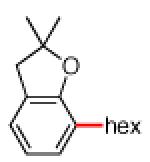
|
<30%e 63% |
| 7ac 7bd |
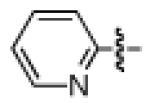
|
−OSO2NMe2 −OCONEt2 |
|
72% – |
| 8ac 8b |
|
−OSO2NMe2 −OCONEt2 |
|
79% 62% |
Reaction conditions: FeCl2 (5 mol %), 3 (15 mol %), n-hexMgCl (1.5–4.0 equiv), CH2Cl2 (15–60 mol %), 3 h.
Isolated yields.
0 °C.
23 °C.
Yield determined by 1H NMR analysis of the crude reaction mixtures using hexamethylbenzene as an internal standard.
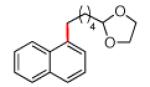
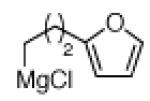
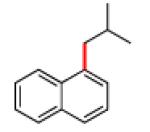
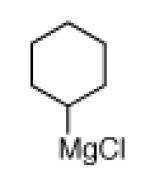
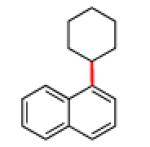
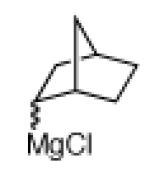
The scope of the methodology with respect to the Grignard reagent is highlighted in Table 3. Alkyl Grignard reagents possessing pendant ether or acetal functional groups were suitable coupling partners (entries 1 and 2). In addition, use of a furan-containing reagent provided the corresponding alkylated product (entry 3). The influence of branching near the Mg center was also evaluated. As demonstrated by the coupling of isobutylmagnesium chloride (entry 4), β-branching was tolerated. Finally, when Grignard reagents with substituents on the α-carbon were tested, coupling proceeded smoothly (entries 5 and 6). These latter two examples showcase the methodology’s utility to construct C–C linkages between sp2 and secondary sp3 centers, which remains a significant challenge in cross-coupling chemistry.26
Table 3.
Coupling of 1-naphthyl carbamate and sulfamate substrates with various alkyl Grignard reagents.a
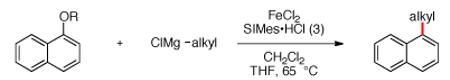
| entry | ClMg alkyl | OR | product | yieldb |
|---|---|---|---|---|
| 1a 1b |
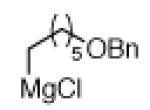
|
−OSO2NMe2 −OCONEt2 |
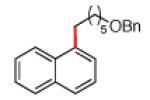
|
59% 78% |
| 2a 2b |
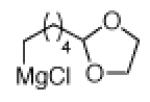
|
−OSO2NMe2 −OCONEt2 |
|
84% 93% |
| 3a 3b |
|
−OSO2NMe2 −OCONEt2 |
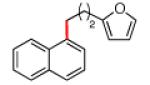
|
58% 52% |
| 4a 4b |
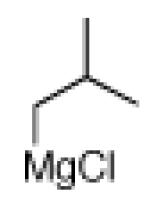
|
−OSO2NMe2 −OCONEt2 |
|
93% 99% |
| 5a 5b |
|
−OSO2NMe2 −OCONEt2 |
|
82% 80% |
| 6ac 6bc |
|
−OSO2NMe2 −OCONEt2 |
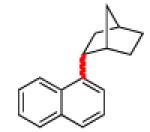
|
54%d 60%d |
Reaction conditions: FeCl2 (5 mol %), 3 (15 mol %), alkyl–MgCl (2.0 equiv), CH2Cl2 (15–60 mol %), 3 h.
Isolated yields.
Grignard reagent exists as a mixture of endo:exo isomers; see SI for details.
Isolated as a mixture of endo:exo isomers; see Supporting Information for details.
The iron-catalyzed sp2–sp3 C–C bond formation using aryl carbamates and sulfamates holds much promise for the synthesis of polyfunctionalized aromatic compounds. A demonstration of this attribute is shown in Figure 3, involving the functionalization of p-chlorophenyl carbamate 4, which, in turn, can be prepared by carbamoylation of p-chlorophenol or through electrophilic chlorination of N,N-diethylphenyl carbamate.27 Regioselective lithiation directed by the carbamate23 and subsequent quenching with iodomethane afforded trisubstituted arene 5 in excellent yield, without disturbing the aryl chloride. Next, a Ni-catalyzed Suzuki–Miyaura coupling28 led to the selective functionalization of the aryl chloride moiety (5→6). It should be emphasized that no evidence of competitive carbamate coupling was detected under these conditions.29 With the o-functionalized carbamate 6 in hand, we tested the iron-catalyzed coupling. Using cyclohexylmagnesium chloride, the desired coupling proceeded smoothly to furnish the alkylated product 7 in 72% yield.
Figure 3.
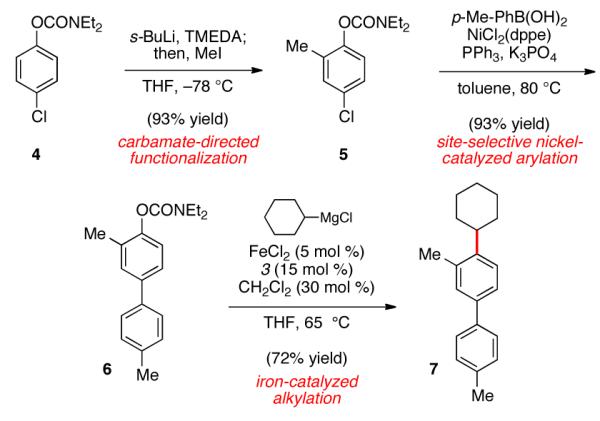
Sequential site-selective couplings and assembly of 7.
In summary, the iron-catalyzed coupling of aryl sulfamates and carbamates with alkyl Grignard reagents provides a versatile means for the construction of sp2–sp3 C–C bonds. The methodology complements the more established methods for aryl sulfamate and carbamate functionalization achieved through arylation7 and amination10 processes. Considering the attractive features of sulfamate and carbamate substrates, the coupling reaction’s broad scope, and the many virtues of ironcatalysis, we expect this methodology will prove useful in multistep synthesis and will further encourage the development of iron-promoted synthetic transformations.
Supplementary Material
Acknowledgment
We are grateful to Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, AstraZeneca, Roche, the A. P. Sloan Foundation, the University of California, Los Angeles, and the CBI training program (A.L.S., USPHS National Research Service Award GM08496) for financial support. We thank Dr. John Greaves (UC Irvine) for mass spectra, Kyle Quasdorf and Anna Komaromi for experimental assistance, and Materia Inc. for chemicals. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Footnotes
Supporting Information Available: Experimental details and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1a).Sherry BD, Fürstner A. Acc. Chem. Res. 2008;41:1500. doi: 10.1021/ar800039x. [DOI] [PubMed] [Google Scholar]; b) Fürstner A. Angew. Chem. Int. Ed. 2009;48:1364. doi: 10.1002/anie.200805728. [DOI] [PubMed] [Google Scholar]; c) Fürstner A, Martin R. Chem. Lett. 2005;34:624. [Google Scholar]; d) Czaplik WM, Mayer M, Cvengros J, Jacobi von Wangelin A. ChemSusChem. 2009;2:396. doi: 10.1002/cssc.200900055. [DOI] [PubMed] [Google Scholar]; e) Ritter SK. Chem. Eng. News. 2008;86:53. [Google Scholar]
- 2.Kharasch MS, Fields EK. J. Am. Chem. Soc. 1941;63:2316. [Google Scholar]
- 3a).Tamura M, Kochi JK. J. Am. Chem. Soc. 1971;93:1487. [Google Scholar]; b) Smith RS, Kochi JK. J. Org. Chem. 1976;41:502. [Google Scholar]
- 4a).For the iron-catalyzed alkylation of aryl halides, tosylates, and triflates, see: Fürstner A, Leitner A. Angew. Chem. Int. Ed. 2002;41:609. Fürstner A, Leitner A, Méndez M, Krause H. J. Am. Chem. Soc. 2002;124:13856. doi: 10.1021/ja027190t. Fürstner A, Martin R, Krause H, Günter S, Goddard R, Lehmann CW. J. Am. Chem. Soc. 2008;130:8773. doi: 10.1021/ja801466t. For the iron-catalyzed alkylation of heteroaromatic sulfonates and phosphates, see: Gøgsig TM, Lindhardt AT, Skrydstrup T. Org. Lett. 2009;11:4886. doi: 10.1021/ol901975u. For a relevant mechanistic study, see: Kleimark J, Larsson P-F, Emamy P, Hedström A, Norrby P-O. Adv. Synth. Catal. 2012;354:448.
- 5.For the related iron-catalyzed alkylation of styrenyl or electrondeficient vinyl pivalates, see: Li B-J, Xu L, Wu Z-H, Guan B-T, Sun C-L, Wang B-Q, Shi Z-J. J. Am. Chem. Soc. 2009;131:14656. doi: 10.1021/ja907281f.
- 6a).Fürstner A, Leitner A. Angew. Chem. Int. Ed. 2003;42:308. doi: 10.1002/anie.200390103. [DOI] [PubMed] [Google Scholar]; b) Seidel G, Laurich D, Fürstner A. J. Org. Chem. 2004;69:3950. doi: 10.1021/jo049885d. [DOI] [PubMed] [Google Scholar]; c) Scheiper B, Glorius F, Leitner A, Fürstner A. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11960. doi: 10.1073/pnas.0401322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a).Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. J. Org. Chem. 1992;57:4066. [Google Scholar]; b) Dallaire C, Kolber I, Gingras M. Org. Synth. 2002;78:42. [Google Scholar]; c) Macklin TK, Snieckus V. Org. Lett. 2005;7:2519. doi: 10.1021/ol050393c. [DOI] [PubMed] [Google Scholar]; d) When PM, Du Bois J. Org. Lett. 2005;7:4685. doi: 10.1021/ol051896l. [DOI] [PubMed] [Google Scholar]; e) Quasdorf KW, Riener M, Petrova KV, Garg NK. J. Am. Chem. Soc. 2009;131:17748. doi: 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Antoft-Finch A, Blackburn T, Snieckus V. J. Am. Chem. Soc. 2009;131:17750. doi: 10.1021/ja907700e. [DOI] [PubMed] [Google Scholar]; g) Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J. Am. Chem. Soc. 2011;133:6352. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Xu L, Li B-J, Wu Z-H, Lu X-Y, Guan B-T, Wang B-Q, Zhao K-Q, Shi Z-J. Org. Lett. 2010;12:884. doi: 10.1021/ol9029534. [DOI] [PubMed] [Google Scholar]; i) Baghbanzadeh M, Pilger C, Kappe CO. J. Org. Chem. 2011;76:1507. doi: 10.1021/jo1024464. [DOI] [PubMed] [Google Scholar]
- 8a).For reviews, see: Knappke CEI, Jacobi von Wangelin A. Angew. Chem. Int. Ed. 2010;49:3568. doi: 10.1002/anie.201001028. Yu D-G, Li B-J, Shi Z-J. Acc. Chem. Res. 2010;43:1486. doi: 10.1021/ar100082d. Li B-J, Yu D-G, Sun C-L, Shi Z-J. Chem. Eur. J. 2011;17:1728. doi: 10.1002/chem.201002273. Rosen BM, Quasdorf KW, Wilson DA, Zhang B, Resmerita A-M, Garg NK, Percec V. Chem. Rev. 2011;111:1346. doi: 10.1021/cr100259t.
- 9.Although most C–C bond forming reactions of aryl sulfamates and carbamates construct sp2–sp2 linkages, there are a few examples of sp2–sp3 couplings using nickel, rather than iron catalysis; see reference 7a for the coupling of aryl carbamates with TMSCH2MgCl and reference 7d for the coupling of cyclic sulfamate substrates. Previous attempts to couple aryl carbamates with n-BuMgCl using Ni-catalysis were unsuccessful because of competitive aryl C–O bond reduction (see reference 7a). No general methodology for the alkylation of aryl sulfamates or carbamates is known.
- 10a).Ramgren SD, Silberstein AL, Yang Y, Garg NK. Angew. Chem. Int. Ed. 2011;50:2171–2173. doi: 10.1002/anie.201007325. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mesganaw T, Silberstein AL, Ramgren SD, Fine Nathel NF, Hong X, Liu P, Garg NK. Chem. Sci. 2011;2:1766. doi: 10.1039/c1sc00230a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesganaw T, Fine Nathel NF, Garg NK. Org. Lett. 2012;14:2918. doi: 10.1021/ol301275u. [DOI] [PubMed] [Google Scholar]
- 12.An Fe-catalyzed alkylation of a 2-naphthyl carbamate substrate was reported by Shi (see reference 5), however, attempts to apply these reaction conditions to other carbamate substrates led to low yields (see the Supporting Information). No other examples of Fe-catalyzed aryl carbamate couplings are known.
- 13.For an iron-catalyzed arylation of a sulfamate substrate, see reference 4c.
- 14a).Silverman GS, Rakita PS. Handbook of Grignard Reagents. CRC Press; New York: 1996. [Google Scholar]; b) Inoue A, Oshima K. In: Main Group Metals in Organic Synthesis. Yamamoto H, Oshima K, editors. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Berlin: 2005. pp. 51–154. [Google Scholar]
- 15.sp2–sp3 carbon-carbon bonds are commonly observed in natural products, pharmaceuticals, agrochemicals, ligands for catalysis, and materials.
- 16.The use of N-hexylmagnesium bromide in THF gave comparable yields of coupled product 2.
- 17a).For the preparation and characterization of iron–NHC complexes, see: Przyojski JA, Arman HD, Tonzetich Z. J. Organometallics. 2012;31:3264. Danopoulos AA, Braunstein P, Wesolek M, Monakhov KY, Rabu P, Robert V. Organometallics. 2012;31:4102.
- 18.The use of CH2Cl2 as solvent returned unreacted substrate. The unique beneficial role of CH2Cl2 as an additive is not presently understood (see Supporting Information for additional additive studies).
- 19.For the use of a dichloroalkane (i.e., 1,2-dichloro-2-methylpropane) as an additive in an unrelated Fe-catalyzed coupling, see: Yoshikai N, Asako S, Yamakawa T, Ilies L, Nakamura E. Chem. Asian J. 2011;6:3059. doi: 10.1002/asia.201100470.
- 20a).For discussions of metal contaminants in iron-catalyzed reactions, see: Buchwald SL, Bolm C. Angew. Chem. Int. Ed. 2009;48:5586. doi: 10.1002/anie.200902237. Thomé I, Nijs A, Bolm C. Chem. Soc. Rev. 2012;41:979. doi: 10.1039/c2cs15249e. Leadbeater NE, Marco M. Angew. Chem. Int. Ed. 2003;42:1407. doi: 10.1002/anie.200390362. Arvela RK, Leadbeater NE, Sangi MS, Williams VA, Granados P, Singer RD. J. Org. Chem. 2005;70:161. doi: 10.1021/jo048531j.
- 21.>98.0% anhydrous FeCl2 (Strem, 25 g or 0.2 mol = $66 USD) was employed in all experiments, although the use of >99.998% anhydrous FeCl2 (Sigma Aldrich) gave comparable results.
- 22.For the subsequent data shown in Tables 1–3, reaction times are 3 h (non-optimized for individual substrates).
- 23a).Snieckus V. Chem. Rev. 1990;90:879. [Google Scholar]; b) Hartung CG, Snieckus V. In: Modern Arene Chemistry. Astruc D, editor. Wiley-VCH; New York: 2002. pp. 330–367. [Google Scholar]; c) Macklin T, Snieckus V. In: Handbook of C-H Transformations. Dyke G, editor. Wiley-VCH; New York: 2005. pp. 106–119. [Google Scholar]
- 24a).Bedford RB, Webster RL, Mitchell C. J. Org. Biomol. Chem. 2009;7:4853. doi: 10.1039/b916724m. [DOI] [PubMed] [Google Scholar]; b) Zhao X, Yeung CS, Dong VM. J. Am. Chem. Soc. 2010;132:5837. doi: 10.1021/ja100783c. [DOI] [PubMed] [Google Scholar]; c) Nishikata T, Abela AR, Huang S, Lipshutz BH. J. Am. Chem. Soc. 2010;132:4978. doi: 10.1021/ja910973a. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yamazaki K, Kawamorita K, Ohmiya H, Sawamura M. Org. Lett. 2010;12:3978. doi: 10.1021/ol101493m. [DOI] [PubMed] [Google Scholar]; e) Feng C, Loh T-P. Chem. Commun. 2011;47:10458. doi: 10.1039/c1cc14108b. [DOI] [PubMed] [Google Scholar]; f) Gong T-J, Xiao B, Liu Z-J, Wan J, Xu J, Luo D-F, Fu Y, Liu L. Org. Lett. 2011;13:3235. doi: 10.1021/ol201140q. [DOI] [PubMed] [Google Scholar]
- 25.Attempts to couple the 2-carbamoylpyridine derivative (Table 3, entry 7b) led to cleavage of the carbamate.
- 26.Valente C, Çalimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG. Angew. Chem. Int. Ed. 2012;51:3314. doi: 10.1002/anie.201106131. [DOI] [PubMed] [Google Scholar]
- 27.Chlorination of N,N-diethylphenyl carbamate using trichloroisocyanuric acid gave 4 in 71% yield (1H NMR with internal standard), albeit with formation of the corresponding ortho substituted isomer in 29% yield.
- 28.Percec V, Golding GM, Smidrkal J, Weichold O. J. Org. Chem. 2004;69:3447. doi: 10.1021/jo049940i. [DOI] [PubMed] [Google Scholar]
- 29.The analogous coupling using the des-methyl derivative of 5 also led to selective coupling of the aryl chloride in high yield.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


