Abstract
Bariatric surgery alters the gastrointestinal hormonal milieu leading to improved glucose homeostasis, though the mechanism leading to these changes is poorly understood. Ileal transposition (IT) is a procedure that is neither restrictive nor malabsorptive but nevertheless produces profound improvements in glucose regulation. Ileal transposition involves a short segment of distal ileum being transposed to the proximal jejunum in an isoperistaltic direction thereby avoiding any gastric resection or intenstinal bypass.
Methods
Diet-induced obese rats underwent either Ileal Transposition (IT), or Sham procedures. The Sham operated rats were pair fed to the IT surgical group to control for the effects of reduced food intake. Body composition data was recorded at specific time points, and glucose tolerance tests were performed at 5 and 6 weeks both in the presence and absence of Exendin 9–39, a known glucose-like peptide 1 (GLP-1) receptor antagonist. A subset of Naïve rats were also maintained for comparison.
Results
IT and Sham operated rats had no differences in food intake and body weight however, IT rats had a significant decrease in their body fat composition (P<0.05). No difference existed in glucose tolerance when exposed to an intrapertioneal glucose load, however, IT rats showed markedly improved glucose tolerance when submitted to an oral glucose tolerance test (p<0.001). Blocking GLP-1 receptors reversed these important improvements in rats with IT surgery.
Conclusions
The present work recapitulates what is seen in rodents and humans that IT improves glucose tolerance and body composition. The present data provide compelling evidence that these improvements are a product of increased GLP-1 secretion that results from placing the key GLP-1 secreting cells closer to chyme coming from the stomach. Such data support the notion that rather than restriction or malabsorption, the underling molecular mechanisms that mediate the potent improvements produced by bariatric procedures involve increased activation of GLP-1 signaling.
Keywords: Ileal Transposition; Bariatric surgery; Glucose homeostasis; Glucose regulation, Glucagon-like peptide 1 (GLP-1); Exendin 9–39; diabetes; hindgut hypothesis, Roux-en-Y Gastric bypass (RYGB)
Introduction
Along with the continued escalation in rates of obesity has come an increase in the prevalence of obesity related comorbidites such as diabetes, hypertension, hyperlipidemia, and certain cancers1,2. Diabetes is associated with its own comorbidites, namely heart disease, kidney failure, blindness, and peripheral neuropathies3,4,5. Despite numerous pharmaceutical advancements in the management of diabetes, bariatric surgery still remains the most effective therapy to produce sustained weight loss and improved glucose control in obese patients6,7. These findings have sparked much interest to identify the mechanisms that lead to improved glucose regulation after bariatric surgery that might lead to other strategies to manage glucose intolerance that results from obesity.
The procedure that has received the most research attention is the Roux-en-Y Gastric Bypass (RYBG). In a RYGB, a small gastric pouch is made by stapling distal to the esophago-gastric junction. The excluded stomach is left in place, but remains in discontinuity. A gastro-jejunal anastamosis is performed to provide outlet for the newly created gastric pouch. This procedure is often described as both “restrictive” and “malabsorptive”. Often this description is taken as identification of its main mechanisms of action. However, a great deal of evidence makes it unlikely that RYGB exerts its potent actions solely as a result of physical restriction and caloric malabsorption29,30,31.
A number of alternative hypotheses have been proposed to explain the potent effects of RYGB. One possibility is that because less digested chyme is routed closer to the ileum, nutrient activation of key endocrine cells in the ileum would be greater in RYGB. In support of this hypothesis is that RYGB in both people and rodents is associated with much greater secretion of L-cell products such as GLP-1 and PYY8,9,10. GLP-1 acts to reduce glucose excursion by inhibiting glucagon secretion, while simultaneously stimulating insulin secretion in a glucose-dependent manner. Additionally, along with other components of the “ileal brake,” GLP-1 inhibits gastric emptying and reduces food intake11.
Alternative procedures provide a way to more directly test the role of the hindgut as a target for the beneficial effects of RYGB. One such procedure is ileal transposition (IT). In an IT, a short segment of ileum is transposed in an iso-peristaltic direction with an intact mesentery to the proximal jejunum, with no resection or bypass of any small bowel. Additionally the stomach is left intact, avoiding any gastric restriction14. Despite this lack of intestinal bypass or gastric restriction, IT results in improved glucose regulation, decreased body weight, and decreased daily food intake resultant from the early delivery of chyme to the ileum. As predicted, IT is associated with much greater secretion of GLP-1 just as is observed in RYGB10,15,34. The current experiments sought to directly test whether the improved glucose regulation seen after IT are the result of the enhanced GLP-1 secretion.
MATERIALS AND METHODS
Animals and Experimental Design
All experiments were done under approved protocol by the Institutional Animal Care and Use Committee of the University of Cincinnati. All animals were adult male Long-Evans rats (Harlan Laboratories, Indianapolis, IN, USA, 200–250gm) which were singly housed, and maintained on a high fat diet (HFD, 41% fat by kcal/g; Research Diets, New Brunswick, NJ, USA) for 8 weeks prior to surgery. They were housed the University of Cincinnati Metabolic Diseases Institute, and kept on a strict 12 hour light/dark cycle, 50–60% humdiity, 25C, and allowed ad libitum access to water. In the initial postoperative period, all animals were kept on a liquid diet in the form of Osmolyte 1Cal (1kcal/ml; Abbott Nutrition) for 5 days, after which the solid high fat diet was resumed. In this time period, they were administered 10ml of Normal Saline once daily, and analgesia (0.1 mg Buprenorphine 0.1mg subcutaneous, Buprenex) daily, and as needed. All Sham animals were pair fed to the average daily food intake of the IT group from the previous.
All rats underwent an oral glucose tolerance test (oGTT) 7 weeks after surgery. This was followed shortly thereafter by repeated oGTT in the presence of a known GLP-1 receptor antagonist, Exendin 9–39 ( Tocris Biosciences, 25nmol/kg IP). An intraperitoneal glucose tolerance test was performed at 10 weeks postoperatively.
Surgeries
The animals were randomized into two surgical groups of 10 animals each prior to the start of surgeries. All animals were weighed prior to surgery, their abdomens clipped of excess hair, and administered Gentamicin (3mg subcutaneous) for preoperative antibiotic coverage. Adequate anesthesia was maintained by inhaled Isoflourane and tested via tail pinch. After induction of anesthesia, all rats underwent a midline laparotomy.
In the IT surgical group, the distal ileum was located by measuring backward from the ileocecal valve after identification of the cecum. A 10cm segment was transected from 12 to 2cm proximal to the ileocecal valve. This segment was maintained on moistened gauze while the two remaining ends of the ileum were anastamosed with 7-0 Vicryl in a running fashion. The small bowel was then run in a retrograde fashion until the Ligament of Treitz was identified. Once located, the jejunum was transected 5cm distal to this location. The previously transected ileum was then interposed in an isoperistaltic fashion, and both ends were anastamosed with 7-0 silk with a running stitch. This resulted in three intestinal transections and anastamoses. Sham animals also underwent three identical intestinal transections however the ileum was not transposed, and instead left in its original anatomic location with three subsequent anastamoses.
Oral Glucose Tolerance Test
The first oral glucose tolerance test was performed at 7 weeks postoperatively. Animals were fasted for 6 hours at the onset of the light cycle, in clean cages to avoid coprophagia. Baseline blood samples were obtained via tail bleed for the measurement of blood glucose and insulin levels. Glucose was administered via oral gavage (1gm, 25% D-Glucose), and blood glucose levels were collected at 15, 30, 45, 60, and 120 minutes respectively via the One Touch Glucometer (LifeScan, CA, USA).
Intraperitoneal glucose tolerance
An intraperitoneal glucose tolerance test was performed at 10 weeks postoperatively. All animals underwent a 6 hour fast at the onset of the light cycle, after which baseline blood samples were obtained via tail bleed, 1.5gm/kg glucose was administered IP as a 25% D-glucose solution. Blood samples were collected via tail bleed at 15, 30, 45, 60, and 120 minutes via the One Touch Glucometer.
GLP-1R antagonism
An oral glucose tolerance test was repeated in a similar fashion to the initial oral glucose tolerance experiment, with a 6 hour fast prior to onset of baseline measurements. After baseline samples were collected, all animals received the highly specific and sensitive peripheral GLP-1 receptor antagonist Exendin 9–39 (25nmol/kg) IP, 10 minutes prior to oral gavage of 1gm of glucose. Glucose measurements were taken at 15, 30, 45, 60, and 120 minutes.
Insulin assay
Blood samples were obtained from all glucose tolerance tests at 15, 30, and 60 minutes. Plasma was isolated after blood was centrifuged for 20 minutes. Plasma samples were then analyzed using the rat insulin Elisa kit (Crystal Chem Inc, IL, USA) via the manufacturer’s instructions.
Body Composition
Body composition analysis was performed preoperatively, then at 30 days and 45 days for fat mass and lean mass quantification with the Echo MRI (Echo MRI Whole Body Composition Analyzer, Echo Medical Systems, TX, USA).
Statistical analysis
All data was assessed using GraphPad (Prism, San Diego, CA, USA) and is expressed as mean +- SEM. At the conclusion of our study, 9 animals remained in each group, with 1 animal from each surgical group lost during the acute postoperative recovery period. These animals were excluded from any statiscal calculation. Body weights, daily food intake, insulin, and glucose measurements were analyzed using repeated measures ANOVA with a Bonferroni post hoc test where appropriate. Body composition data was analyzed using a t-test.
Results
Body Weight
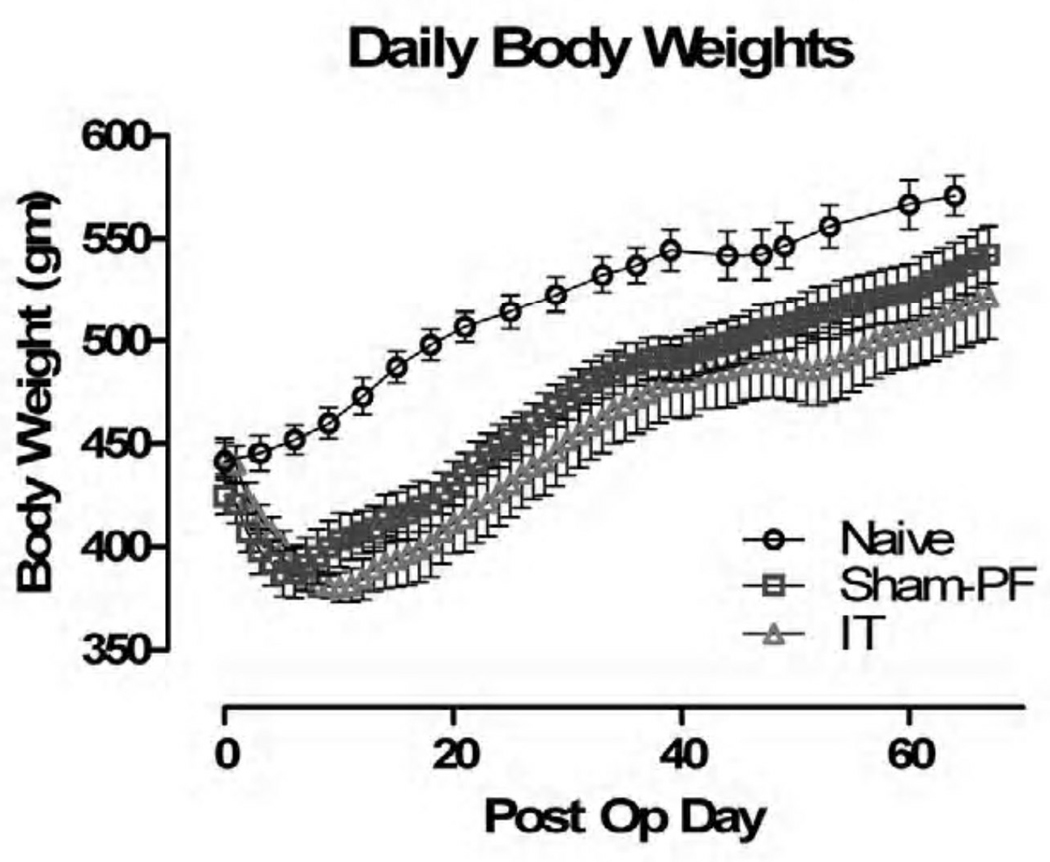
There was no statistically significant difference between IT and Sham-PF animals with regard to body weight, though the Sham-PF animals did recover from surgery earlier as indicated by the quicker recovery to preoperative body weight, and earlier start to their regaining of weight. Compared to Naive animals, both had statistically significant weight changes beginning on POD#4 (Figure I).
Figure I.
Food Intake
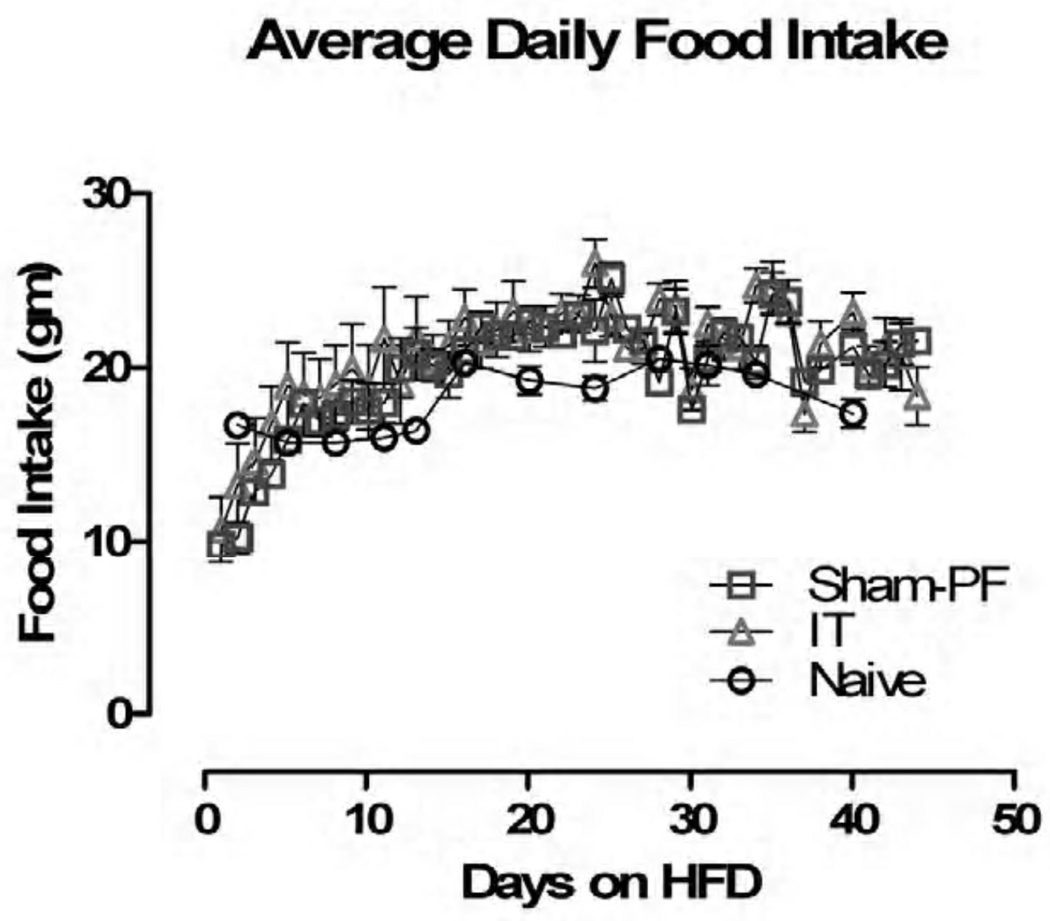
Given that the Sham animals were pair-fed to the IT surgical group, there were no statistically significant differences in their food intake. Both groups compared to the Naive animals did not demonstrate a difference in food intake (Figure II).
Figure II.
Body Composition
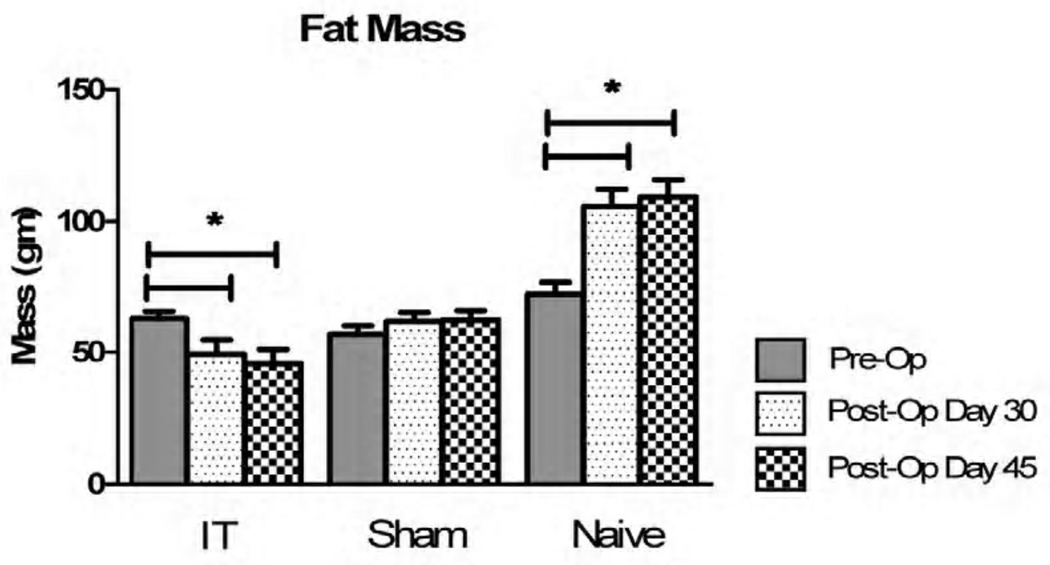
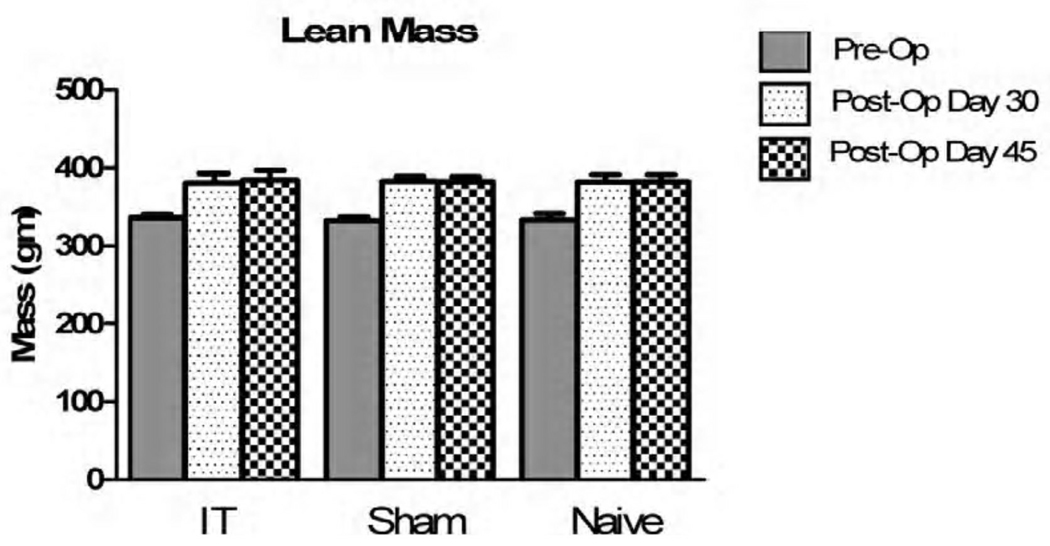
There were no statistically significant changes seen between all groups (IT, Sham-PF, and Naive) prior to the onset of surgical interventions. At 5 weeks post operatively, the IT surgical group demonstrated a significant decrease in fat mass, which persisted at repeat analysis in 7 weeks (Figure III). Naive animals showed a significant increase in fat mass, and no animals had a significant change in their lean mass (Figure IV).
Figure III.
Figure IV.
Glucose Tolerance
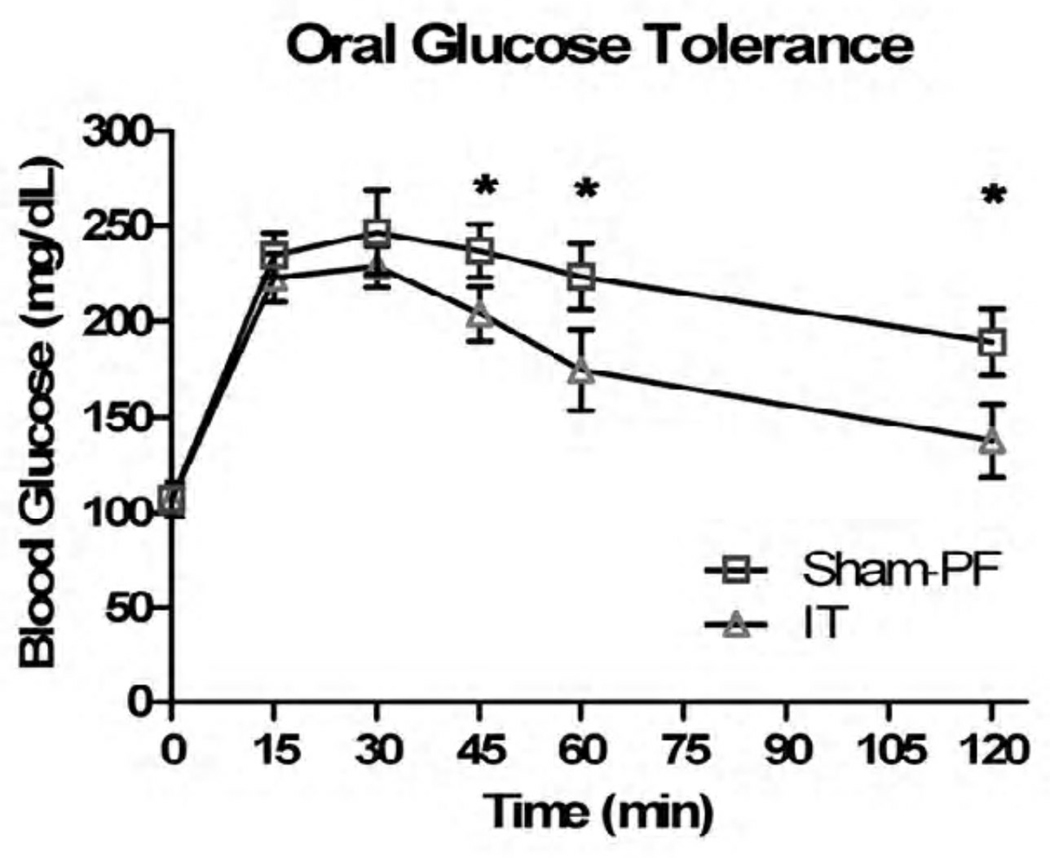
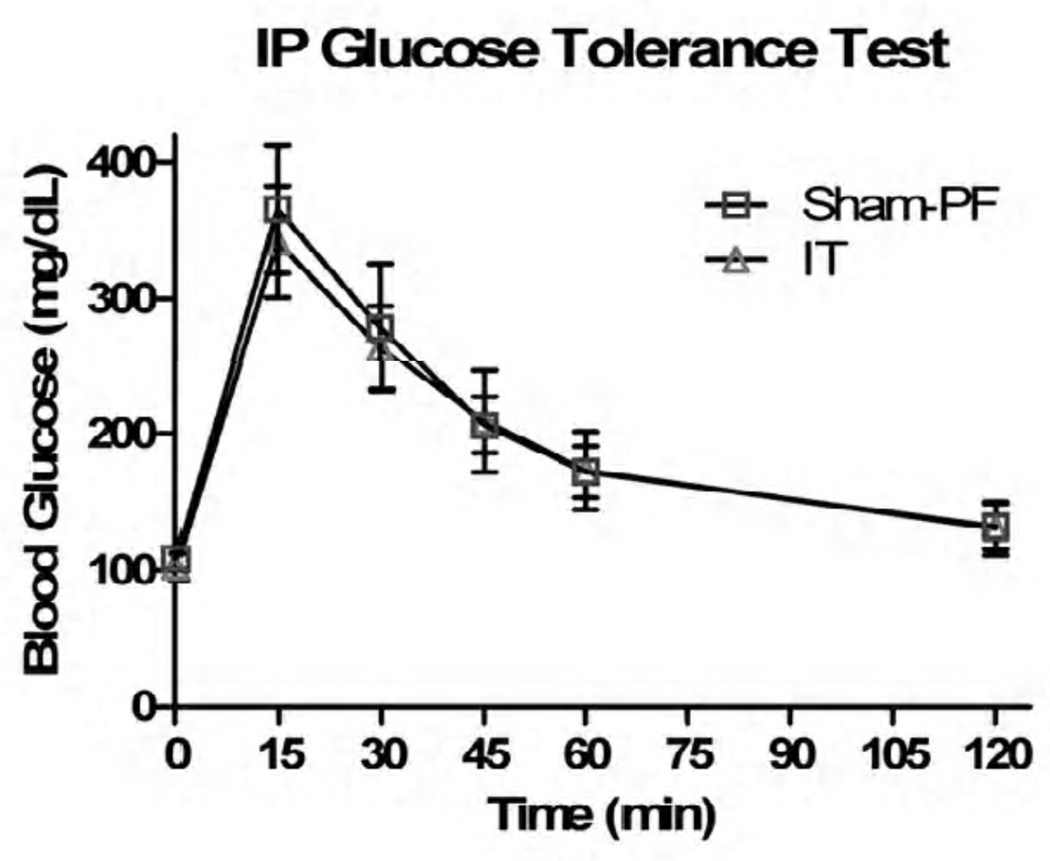
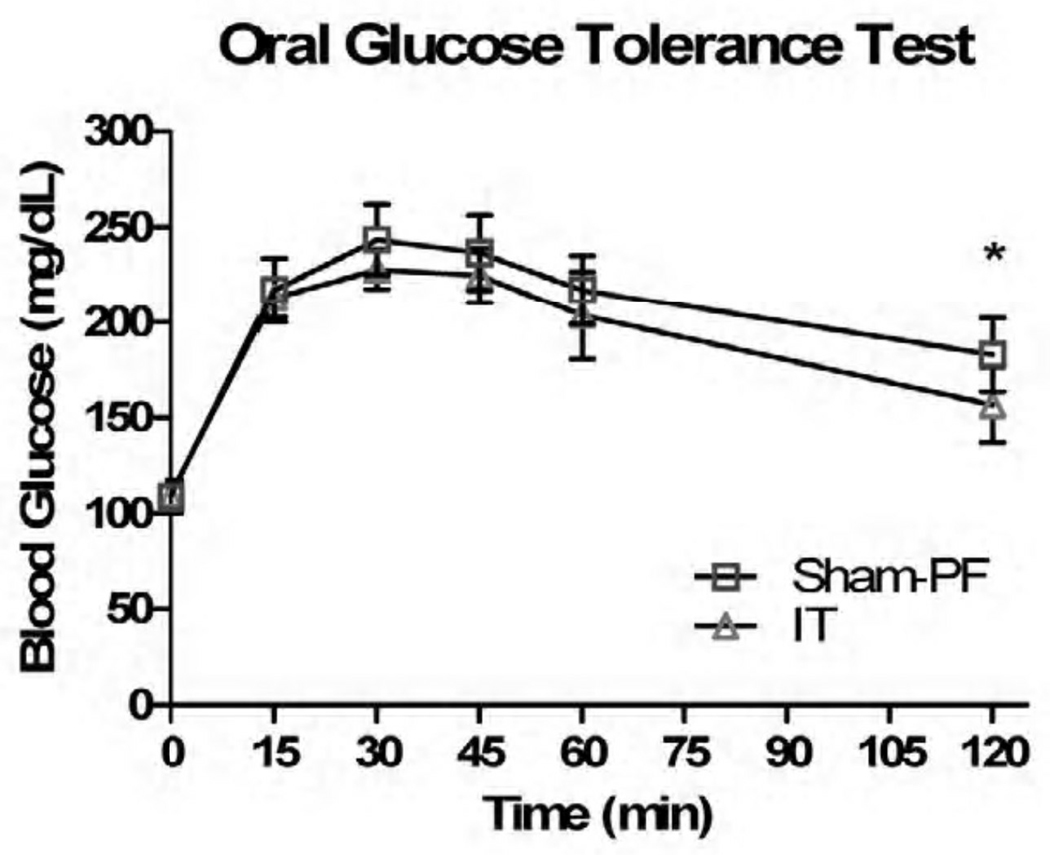
In response to 1gm of oral glucose, the IT animals did have a statistically significant response in their glucose tolerance, namely at the 45, 60, and 120 minute time points (p < 0.05) (Figure V). In regards to 1.5gm/kg of IP glucose, there was no statistically significant difference in glucose tolerance (Figure VI). When the Sham-PF and IT animals had an oral glucose tolerance test repeated in the presence of the known GLP-1R antagonist Exendin 9–39, the improvement in glucose tolerance that was previously seen was negated at all time points except 120 minutes (Figure VII).
Figure V.
Figure VI.
Figure VII.
Discussion
It has long been known that bariatric surgery causes improvement in glucose homeostasis, both acutely, and in long term human studies6,16,17,18. Similar to previous studies, we have shown that IT results in preferential loss of fat mass, compared to both naïve, and pair-fed sham group. When exposed to oral glucose loads, IT animals show improved glucose tolerance. However, that effect is less obvious when glucose is delivered IP and therefore does not engage the GI tract. This outcome points to the importance of GI signaling in the improved glucose regulation. In our hands, independent of food intake and body weight, early exposure of the distal gut to the nutrient-rich chyme of the stomach after an oral glucose load causes a GLP-1 dependent improvement in glucose tolerance. We have demonstrated the novel ability to reverse this improved oral glucose tolerance by inhibiting the GLP-1 receptor with Exendin 9–39. This indicates that the majority, if not all of the glucose regulation improvements seen after IT, is the result of increased GLP-1R activation, a direct result of increased GLP-1 secretion from the ileal L cells. Also, this implies that GLP-1 receptor activation is due to intraluminal factors, and not by circulating hormones.
As seen previously, IT results in increased circulating levels of GLP-110,15. Though many of the breakdown products of ingested energy such as carbohydrates, protein, and fat are known to stimulate GLP-1 secretion, the exact stimulus for its dramatic upregulation after bariatric surgery is as yet unidentified11–13. Despite having identical concentrations and amounts of nutrients ingested in our animals, altering the timing of exposure of these nutrients to the ileal segment induced a physiologically significant difference in activation of the GLP-1 receptor. Given the surgical design of IT, this increased activation must come from signals either created by, or leading to, early activation of the ileal enteroendocrine cells. In performing a gastrojejunostomy to allow for outlet of the newly created gastric pouch in RYGB, the distal gut is similarly “prematurely” activated. Although the aformentioned components of the nutrient rich chyme leaving the stomach all have the potential to be the sole stimulus for the improvements in glucose tolerance, it has been difficult to tease out the respective contributions of each of these factors. Identifying a narrower subset of catalysts for these changes, could help to direct research efforts towards the exact metabolic circuitry that is altered in bariatric surgery, leading to the observed phenotypic changes.
Another component of the nutrient rich chyme entering the small bowel are bile acids. Similar to the known increased GLP-1 levels post surgical intervention, and considerable evidence exists regarding increased bile acids levels post gastric bypass19. Bile acids have long been understood to contribute to the metabolism of plasma lipids, and have been targeted in the treatment of hyperlipidemia20. Carefully examining all of the bariatric surgeries that most effectively treat diabetes, common elements include delivery of the biliopancreatic secretions far more rapidly to the distal gut than in non-altered anatomy. In the case of RYGB, the length of the alimentary limb has been shown to be directly proportional to weight loss noted in super obese individuals32,33. Stated differently, the more distally biliopancreatic secretions are diverted, the greater the phenotypic changes.
Duodenal-Jejunal Bypass (DJB) and Jejunoileal Bypass (JIB) both deliver nutrients to the distal gut sooner as well, but in all surgical approaches there is profound variability in this premature delivery given the lack of standardization of the length of the alimentary limb21. In the case of RYGB, it is primarily surgeon preference which determines alimentary (Roux) limb length, and can vary from 75–200cm. On the other hand, the biliopancreatic limb has a more uniform length (30–40cm), thought variability is seen here is well. When examining this as a percentage of bypassed intestine, gastric nutrients are typically only “short circuited” 30–40cm in a RYGB, where the accepted total length of small intestine is generally considered to be about 6 meters in humans. This represents only a small though notable change in total distance that nutrients must travel. On the other hand, the biliopancreatic secretions including bile acids, bypass on average about 100–150cm, a far larger percentage. Given this, the drastic changes seen in hormonal profiles after bariatric surgery could be due to the change in the bile flow, rather than nutrients.
Evidence of bile acids acting as candidate hormones for the noted ileal hormone changes is centered on recent evidence linking the G-protein coupled bile acid receptor TGR5 to GLP-1 secretion. TGR5 has been known to induce type 2 thyroid deiodinase to increase energy expenditure, and is differentially expressed in various tissues, though most prominently noted in muscle and brown adipose tissue. In vitro, it has been demonstrated that bile acids induce GLP-1 release, through TGR5, likely through a cAMP mediated mechanism. Additionally, alterations in gene expression of TGR5 modulated GLP-1 secretion in a similar manner22. More recently in a series of experiments by Engelstoft et al. using both pharmacological and gain and loss of function models in vivo, bile acids were shown to induce GLP-1 secretion in a dose dependent manner. This increased GLP-1 resulted in improved glucose tolerance as one would expect. This evidence suggests that bile acids also play a role in the hormonal changes seen after bariatric surgery. RYGB, IT, DJB, and JIB all exhibit elements in their surgical design that cause markedly earlier exposure of bile acids to the distal gut. Many also include bypass of some portions of the foregut.
Prior hypotheses have suggested that the primary stimulus for improved glucose regulation is avoidance of nutrient flow through the proximal small intestine, namely the duodenum23. Dubbed the foregut hypothesis, it has been suggested that bypassing key portions of the proximal gut avoids the secretion of an anti-diabetogenic signal, leading to improved insulin sensitivity and secretion24. Such changes in foregut secretion cannot explain the improvement in glucose tolerance seen in IT since it results in no change in foregut nutrient exposure.
Previous experiments with IT have shown increased levels of the hormones secreted from the distal gut, but these hormones are not exclusive to the distal ileum. GLP-1 is known to be secreted from not only the ileal L-cells, but colonic enteroendocrine cells, neural cells in the caudal regions of the nucleus of the solitary tract (NTS) and from pancreatic islet cells11. GLP-1 receptors are found throughout the body, with effector organs including the pancreatic islets, gastric acid secreting cells, cardiac myocytes, the hepatic portal region, and several brain regions12. Though the major source of secreting GLP-1 is known to occur after direct intraluminal stimulation of ieal L cells, secretion appears to occur prior to nutrient arrival to these portions of the distal gut. Additionally, there is a subset of GLP-1 receptors that are activated independent of GLP-1, likely through a vagally mediated response13. The complex interaction of neural and alimentary signaling has yet to be clearly elucidated.
Our surgical model begins to demonstrate that early activation of the hindgut induces a series of signals that ultimately result in increased GLP-1 receptor activation. Though this may be as simple as increased secretion from the ileal L-cells resulting in in direct activation of GLP-1 receptors, however, as yet there is no direct evidence to suggest this is the sole mechanism. Careful examination of our own data shows that at the 120min time point after administration of Exendin 9–39 and an oral glucose load, there still remains a statistically significant difference in glucose levels. Perhaps downstream early activation of the distal gut induces an as yet unidentified signaling cascade that has late effects on baseline glucose levels. Though a simple explanation of the residual difference in glucose levels could be explained by the reported 30 minute half-life of Exendin 9–39, this difference could be an indication of non-GLP-1 related effects of IT.35 Altering the exposure of nutrients in small bowel may change the composition of nutrients reaching the colonic enteroendocrine cells, resulting in the key signaling changes. Additionally, much attention has been given to the composition of the intestinal flora, and its contributions to glucose regulation and susceptibility to diet induced obesity25,26. Any alimentary surgery alters this microbial environment, but the contributions that these alterations have in metabolic regulation or enhanced GLP-1 secretion is unclear.
The notable exception is Sleeve Gastrectomy (SG). SG has gained increasing popularity amongst surgeons and researchers alike, and some studies have shown comparable results to RYGB when examining endpoints such as body weight and glucose tolerance27,28. If the mechanism behind these changes is due clearly hindgut activation, the process by which SG causes this is unclear. No intestine is bypassed or resected and the pylorus is left intact so presumably gastric transit is likely largely unaffected. There is no obligate vagotomy, so direct sympathetic/parasympathetic signaling affecting intestinal motility should also be unchanged.
To clearly elucidate these mechanisms and the contributions of the hindgut, nutrients, and bile acids to glucose regulation after bariatric surgery, more research is needed. With the obesity afflicting nearly 1/3 of the adult population in the U.S. it is clear that the current surgical solutions can never be the basis for helping more than a small percentage of those afflicted. However, understanding the key mechanisms of these surgeries has enormous benefits. Insight into how these surgeries produce such potent effects can allow for less invasive and more scaleable therapies that range from simpler procedures that could be performed endoscopically to effective medications that allow us to help a much larger segment of the obese population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haslam DW, James WP. Obestiy. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Pi-Sunyer FX. Comorbidities of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999 Nov;31(11 Suppl):S602–S608. doi: 10.1097/00005768-199911001-00019. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28(7):1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 4.McNeely MJ, Boyko EJ. Diabetes-related comorbidities in Asian Americans: results of a national health survey. J Diabetes Complications. 2005;19(2):101–106. doi: 10.1016/j.jdiacomp.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Maddigan SL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with diabetes and comorbidities in a Canadian National Population Survey. Qual Life Res. 2005;14(5):1311–1320. doi: 10.1007/s11136-004-6640-4. [DOI] [PubMed] [Google Scholar]
- 6.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makary MA, Clarke JM, Shore AD, Magnuson TH, Richards T, Bass EB, Dominici F, Weiner JP, Wu AW, Segal JB. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg. 2010;145(8):726–731. doi: 10.1001/archsurg.2010.150. [DOI] [PubMed] [Google Scholar]
- 8.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770. doi: 10.1097/00000658-199006000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Allesio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and sythesis in rats. Am J Physiol Endocrinol Metab. 2005;288(2):E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. Glucagon-like peptide 1 (GLP-1): An intestinal hormone, signalling nutritional abundance, with an unusual therapeutic potential. Trends in Endocrinology and Metabolism. 1999;10(6):229–235. doi: 10.1016/s1043-2760(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 12.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 13.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo M, Ulusoy NB. Glucagon-like peptide-1 inhibitics gastric emptying via vagal afferent-mediated central mechansims. Am J Physiol. 1997;237(4 Pt 1):G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 14.Koopmans HS, Sclafani A. Control of body weight by lower gut signals. Int J Obes. 1981;5:491–495. [PubMed] [Google Scholar]
- 15.Mason EE. Ileal [correction of ilial] transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg. 1999;9:223–228. doi: 10.1381/096089299765553070. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Brawnwald E, et al. Bariatric surgery: a systematic review and meta-anaylsis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 17.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugarman JH, Wolfe LG, Sica DA, et al. Diabetes and hypertension in sever obesity and effects of gastric bypass induced weight loss. Ann Surg. 2003;237:571–576. doi: 10.1097/01.SLA.0000071560.76194.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17(9):1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 21.Kral JG. Malabsorptive procedures in surgical treatment of morbid obesity. Gastroenterol Clin North Am. 1987;2:293–305. [PubMed] [Google Scholar]
- 22.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acids sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubino F, Forgione A, Cummings DE, et al. The Mechanism of Diabetes Control After Gastrointestinal Bypass Surgery Reveals a Role of the Proximal Small Intestine in the Pathophysiology of Type 2 Diabetes. Ann Surg. 2006;244(5):741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knop FK. Resolution of type 2 diabetes following gasric bypass surgery: involvement of gut-derived glucagon and glucagonotropic signalling? Diabetologia. 2009;52:2270–2276. doi: 10.1007/s00125-009-1511-8. [DOI] [PubMed] [Google Scholar]
- 25.Corrodi P, Wideman PA, Sutter VL, et al. Bacterial Flora of the Small Bowel before and after Bypass Procedure for Morbid Obesity. J of Infectious Diseases. 1978;137:1–6. doi: 10.1093/infdis/137.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat feeding. Pathol Biol. 2008;56(5):305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Leyba JL, Aulestia SN, Llopis SN. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy for the Treatment of Morbid Obesity: A Prospective Study of 117 Patients. Obes Surg. 2010 doi: 10.1007/s11695-010-0279-8. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Shi X, Karmali S, Sharma AM, Birch DW. A review of laproscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20(8):1171–1177. doi: 10.1007/s11695-010-0145-8. [DOI] [PubMed] [Google Scholar]
- 29.Nightengale ML, Sarr MG, Kelly KA, Jensen MD, Zinsmeister AR, Palumbo PJ. Prospective evaluation of vertical banded gastroplasty as the primary operation for morbid obesity. Mayo Clin Proc. 1991;67:304–305. doi: 10.1016/s0025-6196(12)61194-x. [DOI] [PubMed] [Google Scholar]
- 30.Howard L, Malone M, Michalek A, Carter J, Alger S, Van Woert J. Gastric bypass and vertical banded gastroplasty – a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995;5:55–60. doi: 10.1381/096089295765558169. [DOI] [PubMed] [Google Scholar]
- 31.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92(4):704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Sahagian KG, Schriver JP. Relationship between varying Roux limb lengths and weight loss in gastric bypass. Curr Surg. 2006;63(4):259–263. doi: 10.1016/j.cursur.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Gleysteen JJ. Five-year outcome with gastric bypass: Roux limb length makes a diference. Surg Obes Relat Dis. 2009;5(2):242–247. doi: 10.1016/j.soard.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal transposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–G660. doi: 10.1152/ajpgi.00221.2010. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards CM, Todd IF, Mahrnoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-3 9. Diabetes. 1999 Jan;48(1):86–93. doi: 10.2337/diabetes.48.1.86. [DOI] [PubMed] [Google Scholar]