Abstract
Data from 635 very poor men who have sex with men (MSM) were used to identify seroadaptation with 1,102 male partners reported between 2005-2007 in Los Angeles as part of the Sexual Acquisition and Transmission of HIV Cooperative Agreement Program. The mean age of the sample was 41.7 years; 53% had experienced homelessness in the past year. Condoms were reported in 51% of sexual events involving anal intercourse. HIV seroconcordance was reported in 41% of sexual partnerships among HIV-positive participants. HIV-positive men were more likely to have oral-only or unprotected receptive anal intercourse and less likely to have unprotected insertive anal intercourse with HIV-negative or unknown partners compared to HIV-positive partners. Even in the face of poverty, HIV-positive MSM report mitigating risks of HIV-transmission though seroadaptation in the context of modest rates of condom use.
Keywords: serosorting, seropositioning, oral-only sex, poverty
Introduction
Seroadaptation is an umbrella term to describe behaviors that use HIV status to inform sexual decision making (1). Seroadaptive behaviors are quite common among U.S. men who have sex with men (MSM); a population that accounted for 56% of new HIV infections in the United States from 2003-2006 (2). Data from the 2008 National HIV Behavioral Surveillance (NHBS) survey in San Francisco suggest that 41% of HIV-negative and 51% of HIV-positive MSM engaged in some form of seroadaptation over a 6 month period (3). More seroadaptation may be an important “risk management” strategy among Los Angeles area MSM, as 56% reported unprotected anal intercourse (UAI) in the past year as part of the 2008 LA Men’s Survey (4).
Serosorting is a type of assortive mixing in which individuals preferentially select sex partners of the same HIV status (5). A distinction is sometimes made between concepts of “pure serosorting,” in which MSM engage in anal intercourse (AI) only within seroconcordant partnerships and “condom serosorting,” where men use condoms when partners are serodiscordant or unknown and not when the partner is seroconcordant (1). Seropositioning (also called strategic positioning) typically refers to adopting insertive or receptive roles during UAI in such a way that minimizes the risk of transmission in serodiscordant or status unknown partnerships (6-7). For example, an HIV-positive man may choose to adopt the receptive position during UAI with HIV-negative or unknown status partners in an attempt to reduce the risk of spreading the infection (8).
Although evidence of seroadaptation has been observed in several studies of MSM (9-13), its effectiveness as a “risk management” strategy is widely debated (14-22). For example, while the EXPLORE study found a modest decreased risk of seroconversion among HIV-negative MSM who condom serosorted (15), a study by Jin et al. found that in 38% of sexual events involving UAI, HIV infection occurred while the participant was serosorting, seropositioning, or having sex with a partner believed to have an undetectable viral load (23). In order for seroadaptation to be a successful HIV “risk management” strategy, individuals must correctly identify not only their own HIV status but also that of their partner. This is problematic given high rates of unrecognized infection and the fact that contextual factors, such as physical appearance or behavioral attributes are often used to guess partner’s HIV status (24-26). Adding further complexity, studies have shown that selection of sexual partners who are either HIV-negative or unknown status decreases shortly following an HIV diagnosis and then rebounds within 9 months (27). Seroadaptation, which is aimed at reducing the risk of HIV transmission, has also been implicated as a potential mechanism for the spread of bacterial STIs when used as a replacement for consistent condom use (28-29).
Although several studies have examined seroadaptation among MSM as a whole or within particular racial/ethnic communities, little is known about the utilization of these behaviors among low-socioeconomic status (SES) MSM (1, 6, 9, 21, 30). In Los Angeles, at the time of this study, traditional HIV-prevention efforts such as outreach, education, and condom distribution were underway. HIV-primary care for poor HIV-positive MSM was available via the AIDS Drug Assistance Program (ADAP) and Ryan White Care Act (RWCA). Primary care resources for poor HIV-negative MSM were largely absent, especially among minority MSM. Despite these efforts, MSM living in challenging, resource-poor environments comprise a particularly vulnerable population. It is unclear however if and how seroadaptation is being utilized by MSM living in this context. On one hand, resource-poor environments may serve as barriers to seroadaptation by limiting resiliency in the sexual decision making process. On the other hand, decreased access to mainstream care, competing needs, and the struggle of daily life may hinder the ability of poor MSM to engage in traditional HIV-prevention activities, leaving them with few HIV-prevention options other than seroadaptation. Given the clustering of HIV in neighborhoods characterized by poverty, drug use, and larger populations of MSM, and the increased HIV prevalence among minority, incarcerated, and homeless populations, a better understanding of how these seroadaptive behaviors are being used among marginalized MSM is warranted (31-36). In this analysis we examine several types of seroadaptation: serosorting, oral-only sex, condom use, and seropositioning within a population of low-SES MSM in Los Angeles.
Methods
Procedure
Data are from the Los Angeles site of NIDA’s Sexual Acquisition and Transmission of HIV Cooperative Agreement Program (SATH-CAP); details on methodology have been reported elsewhere (37-38). Briefly, Respondent Driven Sampling (RDS) was used to recruit a core population of drug users and/or MSM or men who have sex with men and women (MSMW) and their sexual partners. An initial set of “seeds,” i.e., members of the core population, were passively recruited via fliers and screened for eligibility to start the RDS process. Seeds who met study criteria were compensated $50 for their study visit and were given enrollment vouchers to distribute to individuals they knew to be drug users and/or MSM or MSMW and to their sexual partners (either male or female). To incentivize the referral process, all study participants were compensated $20 for each person who enrolled in the study using one of their coupons. Individuals referred to the study using the coupons were screened for eligibility, and if appropriate, completed a study visit and in turn were given coupons to recruit the next set of participants. This process was repeated until enrollment goals were reached. All study procedures were overseen by the UCLA Human Subjects Protection Committee.
Materials
Demographics, drug use, and overall sexual behaviors were collected using Audio Computer Assisted Self Interviews (ACASI). Additionally, participants provided detailed partnership-specific sexual behaviors (including partner type, drug use, and sexual position at last sex) with up to 3 partners that they had sex with in the past 6 months. Saliva and blood samples were collected for HIV rapid testing (OraQuick Advance) with confirmatory western blot. All HIV-positive samples were assayed for HIV viral load (Los Angeles County Department of Public Health Laboratory) using a cut-off value of ≥400 copies per mL to classify detectable and undetectable cases (39-40). Urine specimens were collected for chlamydia and gonorrhea testing as well as for the detection of marijuana, amphetamine/methamphetamine, cocaine, and heroin/opiate use within the past 72 hours. Participants were informed of their test results and referred to HIV/STI and/or substance abuse treatment as appropriate. Additionally, participants were asked about other hardships they may be experiencing and were referred to local service providers using the Los Angeles and Ventura Counties Rainbow Resource Directory.
Data Analysis
This analysis is based on the first two waves of data collection (2005-2006; 2006-2007) from the Los Angeles site for SATH-CAP and is restricted to males who reported at least one male sexual partner in the past 6 months. For partnership-level analysis, sexual encounters with up to 3 male partners within the past 6 months were included. A partnership was considered to be seroconcordant if the HIV status of the participant matched that of their partner. If serodiscordant or partner’s HIV status was unknown then the partnership was classified as non-concordant. It should be noted that while the participant’s HIV status was ascertained by diagnostic testing conducted by research staff, their partner’s HIV status was based on the report of the index participant. Sexual position at last sex was classified as oral-only (either insertive or receptive), protected anal intercourse (PAI, insertive or receptive), unprotected insertive anal intercourse (UIAI), unprotected receptive anal intercourse (URAI), and unprotected versatile (both insertive and receptive anal intercourse; with either being unprotected). In order to make these categories mutually exclusive, participants who reported multiple positions were classified as the highest risk category. For example, if a participant reported engaging in oral sex and any other position, they were classified as the latter.
All analyses were conducted using un-weighted data as our goal was to identify patterns of seroadaptation and not produce population estimates. For descriptive purposes, chi-square and t-tests were used to test independence between HIV status and demographic characteristics and substance use. We stratified the sample by HIV status to assess correlates of seroadaptation among HIV-positive and negative MSM separately. To evaluate serosorting, we first compared HIV status of index participants to the HIV status of their partners. Next, we fit generalized logistic random intercept models with seroconcordance as the outcome and demographics, partnership type (main vs. non-main), exchanging sex for drugs/money, amphetamine/methamphetamine use at last sex, and UAI at last sex as predictors. Models for the strata of HIV-positive men also included regularly taking antiretroviral medication and viral load. For seropositioning analyses, identical models were fit with oral-only sex, PAI, UIAI, URAI, and unprotected versatile as outcomes. As before, categories were mutually exclusive and participants were classified based on their highest risk behavior. In addition to the serosorting predictors, partner HIV status was also included. All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
Sample Characteristics
This final sample size was 635 males and 1,102 MSM partnerships. Participants were mostly middle aged (mean 41.7, SD 9.2), poor (83% earned ≤ $1,000 in past 30 days), highly prevalent for homelessness within the past year (53%), and had been incarcerated at some point in their lives (60%). The sample was 47% non-Hispanic black, 27% Hispanic, 20% non-Hispanic white, and 6% non-Hispanic other race. Drug use was common in the sample with 32%, 28%, and 36% reporting marijuana, amphetamine/methamphetamine, and cocaine use in the past 30 days, respectively. HIV prevalence of the sample was 44%. In univariate analysis, significant differences between HIV-positive and HIV-negative participants were observed with more HIV-positive men being Hispanic, born outside of the U.S., and reporting amphetamine/methamphetamine use. Fewer HIV-positive men reported experiencing homelessness within the past year and having both male and female sexual partners in the past 6 months (Table 1).
Table 1. Demographics, SES, drug use, and sexual behavior by HIV status.
| Covariates | Total (n=635)a %(n) |
HIV+ (n=279)a %(n) |
HIV- (n=356)a %(n) |
T-Value or Chi-Square |
|---|---|---|---|---|
| Age (mean, SD) | 41.7(9.2) | 41.7(7.8) | 41.8(10.1) | 0.1 |
| Race | 40.0*** | |||
| White | 19.8(126) | 18.6(52) | 20.8(74) | |
| Black | 46.6(296) | 36.6(102) | 54.5(194) | |
| Hispanic | 27.4(174) | 39.8(111) | 17.7(63) | |
| Other | 6.2(39) | 5.0(14) | 7.0(25) | |
| Country of birth | 50.9*** | |||
| US | 81.0(514) | 69.2(193) | 90.2(321) | |
| Mexico | 9.1(58) | 17.2(48) | 2.8(10) | |
| Other | 09.9(63) | 13.6(38) | 07.0(25) | |
| Education | 11.1** | |||
| Less than high school | 20.5(130) | 21.6(60) | 19.7(70) | |
| High school | 32.8(208) | 25.9(72) | 38.2(136) | |
| More than high school | 46.7(296) | 52.5(146) | 42.1(150) | |
| Income (past month) | 8.9* | |||
| $0-$500 | 60.0(376) | 53.6(148) | 65.0(228) | |
| $501-$1000 | 23.1(145) | 27.9(77) | 19.3(68) | |
| More than $1000 | 16.9(106) | 18.5(51) | 15.7(55) | |
| Homeless (past year) | 52.6(334) | 44.8(125) | 58.7(209) | 12.1*** |
| Jail/Prison (ever) | 59.6(372) | 56.4(155) | 62.2(217) | 2.2 |
| Drug use (past 30 days) | ||||
| Marijuana | 32.0(203) | 29.8(83) | 33.8(120) | 1.2 |
| Amphetamine/Methamphetamine | 27.6(175) | 33.0(92) | 23.4(83) | 7.2** |
| Cocaine | 36.0(228) | 28.7(80) | 41.7(148) | 11.5*** |
| Heroin | 6.0(38) | 1.4(4) | 9.6(34) | 18.4*** |
| Non-prescription opiates | 8.7(55) | 6.9(19) | 10.2(36) | 2.1 |
| Laboratory drug tests (past 72 hours) | ||||
| Marijuana | 19.1(121) | 26.5(74) | 13.2(47) | 18.0*** |
| Amphetamine/Methamphetamine | 7.6(48) | 13.6(38) | 2.8(10) | 26.2*** |
| Cocaine | 17.5(111) | 12.2(34) | 21.6(77) | 9.7** |
| Heroin/Opiates | 04.3(27) | 04.7(13) | 03.9(14) | 000.2 |
| Gender of sex partners (past 6 months) | ||||
| Male Only (MSM) | 59.7(379) | 86.4(241) | 38.8(138) | 147.4*** |
| Male and Female (MSMW) | 40.3(256) | 13.6(38) | 61.2(218) |
p<0.05
p<0.01
p<0.001
May not sum to column total due to missing values
HIV Disclosure and Treatment
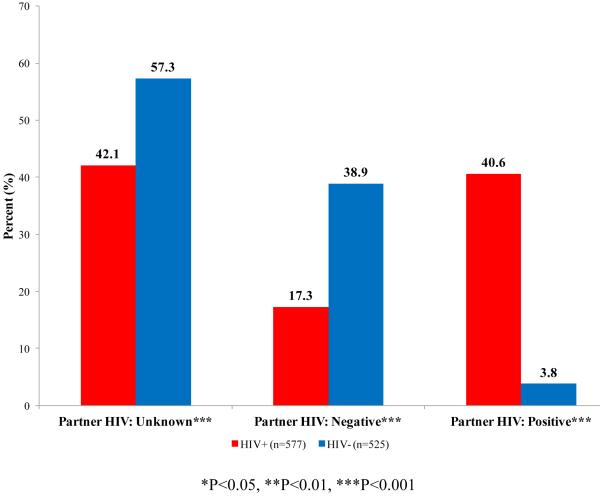
Index participants disclosed their HIV status in 51.3% of partnerships reported by HIV-negative men and 66.3% of the partnerships reported by HIV-positive men. Additionally, participants were unaware of their partner’s HIV status in 57.3% of the partnerships reported by HIV-negative men and 42.1% among HIV-positive men. Among HIV-positive men, 83.9% reported seeing a doctor for HIV on a regular basis and 62.0% reported that they were regularly taking anti-retroviral (ARV) medications; 44.9% had a viral load ≥400 copies/ml.
Serosorting
The HIV status of SATH-CAP index participants by reported HIV status of their sexual partners is presented in Figure 1. Although the percentage of seroconcordant partnerships was similar between HIV-positive (41%) and HIV-negative (39%) men, compared to HIV-positive men, HIV-negative men reported more partners of unknown status (57% vs. 42%, p<0.001). Among HIV-positive men, an increased odds of seroconcordance was observed in partnerships involving main partners compared to non-main partners (AOR:2.23, 95%CI:1.21-4.14; p=0.011) and in partnerships that had UAI at last sex compared to partnerships with no UAI (AOR:2.19, 95%CI:1.23-3.88; p=0.008). Among HIV-negative men, having a less than high school education vs. more than high school (AOR:0.26, 95%CI:0.11-0.64; p=0.004) and experiencing homelessness within the past year compared to not being homeless (AOR:0.51, 95%CI:0.27-0.96; p=0.038) were negatively associated with seroconcordance. Main partners compared to non-main were positively associated with seroconcordance (AOR:1.93, 95%CI:1.05-3.54; p=0.034) (Table 2).
Figure 1.
HIV status of partner by HIV status of index participant
Table 2. Generalized logistic random intercept models predicting seroconcordant partnerships among HIV-positive and HIV-negative participants.
| Covariate | HIV-Positive Participants |
HIV-Negative Participants |
||
|---|---|---|---|---|
| OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | |
| Age | 1.02 [0.99-1.05] | 1.02 [0.99-1.06] | 1.01 [0.99-1.03] | 1.02 [0.99-1.04] |
| Race (ref = white) | ||||
| Black | 0.75 [0.40-1.39] | 0.84 [0.34-2.12] | 1.16 [0.66-2.04] | 1.67 [0.73-3.86] |
| Hispanic | 0.54 [0.29-0.99] * | 0.60 [0.25-1.42] | 0.91 [0.46-1.83] | 0.84 [0.32-2.24] |
| Other | 2.40 [0.79-7.23] | 1.83 [0.46-7.26] | 2.89 [1.11-7.54] * | 5.32 [1.44-19.62] * |
| Monthly income (ref = >$1,000) | ||||
| ≤$500 | 0.90 [0.48-1.68] | 1.26 [0.51-3.14] | 0.60 [0.33-1.09] | 0.95 [0.41-2.19] |
| $501-$1,000 | 0.91 [0.46-1.81] | 0.75 [0.30-1.90] | 0.69 [0.34-1.43] | 1.04 [0.42-2.59] |
| Education (ref = more than high school) | ||||
| Less than high school | 0.61 [0.34-1.08] | 1.29 [0.56-2.98] | 0.43 [0.23-0.82] * | 0.26 [0.11-0.64] ** |
| High school | 0.61 [0.35-1.06] | 0.82 [0.40-1.70] | 0.90 [0.56-1.46] | 0.89 [0.47-1.65] |
| Gender of sex partners: MSM (ref = MSMW)a | 0.99 [0.49-2.01] | 0.73 [0.25-2.10] | 0.97 [0.63-1.51] | 1.05 [0.57-1.94] |
| Homeless (past year) | 1.15 [0.73-1.81] | 1.52 [0.79-2.95] | 0.72 [0.46-1.12] | 0.51 [0.27-0.96] * |
| Main partner | 1.96 [1.20-3.21] ** | 2.23 [1.21-4.14] * | 1.66 [1.02-2.69] * | 1.93 [1.05-3.54] * |
| Received money/drugs for sex (last sex) | 0.68 [0.38-1.22] | 0.80 [0.38-1.67] | 0.71 [0.44-1.15] | 0.95 [0.52-1.74] |
| Used amphetamine/methamphetamine (last sex) | 1.94 [1.11-3.38] * | 1.72 [0.82-3.58] | 0.45 [0.21-0.99] * | 0.42 [0.17-1.04] |
| Unprotected anal intercourse (last sex) | 2.79 [1.81-4.31] *** | 2.19 [1.23-3.88] ** | 1.06 [0.68-1.66] | 1.21 [0.69-2.11] |
| Regularly taking anti-retroviral medications (ref = no) | ||||
| Yes | 1.13 [0.67-1.89] | 1.48 [0.68-3.22] | ---- | ---- |
| No, new diagnosis | 0.29 [0.12-0.69] ** | 0.20 [0.06-0.67] ** | ---- | ---- |
| Viral load ≥ 400 copies/ml | 1.08 [0.69-1.71] | 1.19 [0.59-2.38] | ---- | ---- |
<0.05
<0.01
<0.001
MSM: Men who have sex with men, MSMW: Men who have sex with men and women
Seropositioning
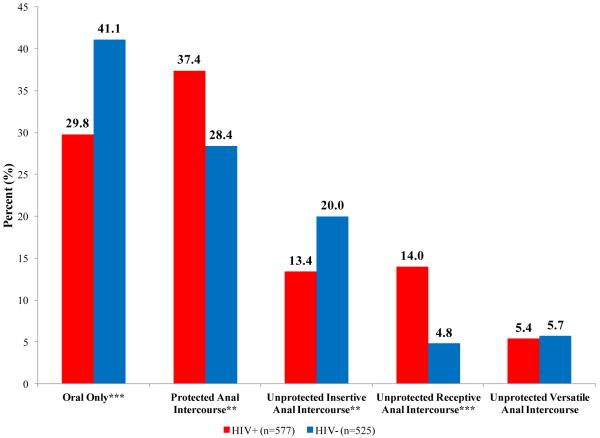
A wide range of sexual positions were reported in the sample; 35%, 33%, 17%, 10%, and 6% reported oral-only, PAI, UIAI, URAI, and unprotected versatile sex respectively. Compared to HIV-negative participants, more HIV-positive men reported engaging in PAI (37% to 28%; p=0.008) and URAI (14% to 5%; p<0.001) and fewer reported oral-only (30% to 41%; p<0.001) and UIAI (13% to 20%; p=0.009) with their partners at last sex. No differences in unprotected versatile between HIV-positive and negative men were observed (5% to 6%; p=0.838) (Figure 2). To assess what type of PAI was occurring in the sample, we split PAI into protected insertive anal intercourse (PIAI) and protected receptive anal intercourse (PRAI). No differences in PIAI were found between HIV-positive and negative participants (15% vs. 18%; p=0.313); however, 22% of HIV-positive participants reported PRAI compared to 11% of HIV-negative participants (p<0.001).
Figure 2.
Sexual position with male partners at last sex by HIV status
In multivariate models, HIV-positive participants were more likely to engage in oral-only and URAI and less likely to engage in UIAI with HIV-negative and unknown partners compared to HIV-positive partners (Table 3.1). Additionally, amphetamine/methamphetamine use at last sex compared to no use at last sex was negatively associated with PAI (AOR:0.42, 95%CI:0.21-0.82; p=0.012) and positively associated with UIAI (AOR:3.38, 95%CI:1.35-8.49; p=0.009). Regularly taking anti-retroviral medications and viral load were not significantly associated with any sexual position. The Pearson Correlation Coefficient for regularly taking ARV medication and viral load was −0.52. In multivariate models that excluded regularly taking ARV medication, viral load was not significantly associated with any sexual position.
Table 3.1. Generalized logistic random intercept models predicting sexual position at last sex among HIV-positive participants.
| Covariates | Model 1: Oral-Only |
Model 2: Protected AI |
Model 3: Unprotected Insertive AI |
Model 4: Unprotected receptive AI |
Model : Unprotected Versatile AI |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI]a | OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | |
| Partner HIV status (ref = HIV+) | ||||||||||
| HIV- | 2.22 [1.23-4.00] ** | 2.09 [0.99-4.41] | 1.38 [0.77-2.45] | 1.20 [0.58-2.47] | 0.30 [0.12-0.75] ** | 0.27 [0.08-0.88] * | 1.13 [0.53-2.39] | 2.76 [1.00-7.62] * | ||
| Unknown | 1.82 [1.13-2.91] * | 1.34 [0.73-2.45] | 1.52 [0.97-2.37] | 1.49 [0.84-2.64] | 0.34 [0.18-0.65] ** | 0.40 [0.17-0.92] * | 1.03 [0.57-1.86] | 2.56 [1.15-5.71] * | ---- | ---- |
| Main partner | 0.36 [0.19-0.65] *** | 0.28 [0.13-0.58] *** | ---- | 1.34 [0.74-2.43] | 1.74 [0.94-3.24] | 1.90 [0.88-4.10] | 1.24 [0.65-2.34] | 1.66 [0.73-3.79] | 2.14 [0.92-4.98] | ---- |
| Received money/drugs for sex (last sex) | 1.41 [0.80-2.47] | 1.17 [0.59-2.35] | ---- | 1.37 [0.69-2.72] | 0.61 [0.26-1.43] | 0.60 [0.22-1.70] | 0.90 [0.42-1.92] | 0.66 [0.26-1.71] | 1.12 [0.38-3.27] | ---- |
| Used amphetamine/methamphetamine (last sex) | 0.62 [0.35-1.10] | 0.55 [0.26-1.14] | 0.42 [0.24-0.75] ** | 0.42 [0.21-0.82] * | 2.12 [1.08-4.18] * | 3.38 [1.35-8.49] ** | 2.34 [1.21-4.53] * | 2.01 [0.86-4.69] | 2.25 [0.92-5.50] | ---- |
| Homeless (past year) | 1.32 [0.84-2.09] | 0.80 [0.42-1.53] | ---- | 0.74 [0.40-1.36] | 0.83 [0.46-1.51] | 0.43 [0.19-0.99] * | 2.42 [1.36-4.30] ** | 4.24 [1.82-9.84] *** | 0.92 [0.40-2.09] | ---- |
| Regularly taking anti-retroviral medications (ref = no) | ||||||||||
| Yes | 1.01 [0.59-1.73] | 1.15 [0.53-2.50] | 1.21 [0.72-2.04] | 1.07 [0.51-2.21] | 1.18 [0.57-2.43] | 0.83 [0.30-2.27] | 0.67 [0.36-1.26] | 0.95 [0.37-2.43] | 0.86 [0.33-2.21] | ---- |
| No, new diagnosis | 1.25 [0.57-2.75] | 1.45 [0.52-4.05] | 0.84 [0.38-1.85] | 0.60 [0.22-1.64] | 2.24 [0.85-5.91] | 3.16 [0.88-11.25] | 0.36 [0.12-1.13] | 0.31 [0.07-1.41] | 1.10 [0.28-4.33] | ---- |
| Viral load > 400 copies/ml | 0.92 [0.58-1.46] | 0.93 [0.48-1.80] | ---- | 0.79 [0.42-1.49] | 0.95 [0.52-1.73] | 0.92 [0.38-2.22] | 2.02 [1.14-3.57] * | 1.84 [0.77-4.40] | 1.88 [0.82-4.28] | ---- |
<0.05
<0.01
<0.001
Adjusted models also controlled for: age, race/ethnicity, income, education and MSM vs. MSMW
---- Model did not converge
In multivariate models among HIV-negative participants, no statistically significant results were observed when comparing sexual positions with partners reported to be HIV-positive or unknown to partners reported to be HIV-negative. Receiving money/drugs for sex was positively associated with oral-only sex (AOR:2.52, 95%CI:1.48-4.30; p<0.001) and negatively associated with PAI (AOR:0.39, 95%CI:0.20-0.77, p=0.007). Amphetamine/methamphetamine use at last sex was strongly associated with engaging in unprotected versatile sex (AOR:13.40, 95%CI:3.41-52.65, p<0.001) (Table 3.2).
Table 3.2. Generalized logistic random intercept models predicting sexual position at last sex among HIV-negative participants.
| Covariates | Model 1: Oral-Only |
Model 2: Protected AI |
Model 3: Unprotected Insertive AI |
Model 4: Unprotected receptive AI |
Model : Unprotected Versatile |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | OR [95%CI] | AOR [95%CI] | |
| Partner HIV status (ref = HIV-) | ||||||||||
| HIV+ | 1.03 [0.37-2.90] | 1.63 [0.48-5.58] | 1.57 [0.55-4.51] | 1.97 [0.55-7.04] | 0.52 [0.11-2.56] | 0.41 [0.07-2.30] | 0.93 [0.10-8.37] | 0.78 [0.06-9.74] | 0.69 [0.08-6.27] | 1.17 [0.01-2.69] |
| Unknown | 1.27 [0.85-1.92] | 1.17 [0.70-1.98] | 0.76 [0.49-1.20] | 0.98 [0.54-1.75] | 1.34 [0.81-2.22] | 1.43 [0.77-2.64] | 0.64 [0.26-1.55] | 0.47 [0.14-1.56] | 0.61 [0.27-1.40] | 0.31 [0.08-1.11] |
| Main partner | 0.83 [0.52-1.32] | 1.29 [0.73-2.30] | 0.85 [0.50-1.42] | 0.58 [0.30-1.15] | 0.89 [0.50-1.57] | 0.96 [0.49-1.90] | 1.41 [0.54-3.69] | 0.52 [0.12-2.24] | 3.35 [1.45-7.76] ** | 3.59 [1.14-11.26] * |
| Received money/drugs for sex (last sex) | 1.84 [1.19-2.85] ** | 2.52 [1.48-4.30] *** | 0.39 [0.23-0.67] *** | 0.39 [0.20-0.77] ** | 0.78 [0.45-1.36] | 0.61 [0.32-1.17] | 1.95 [0.80-4.72] | 1.95 [0.56-6.72] | 1.42 [0.61-3.34] | 1.16 [0.33-4.06] |
| Used amphetamine/methamphetamine (last sex) | 0.54 [0.26-1.10] | 0.59 [0.27-1.30] | 0.63 [0.29-1.35] | 0.48 [0.20-1.15] | 1.15 [0.53-2.47] | 1.32 [0.55-3.16] | 1.67 [0.47-5.96] | 1.84 [0.41-8.25] | 7.43 [2.76-20.04] *** | 13.40 [3.41-52.65] *** |
| Homeless (past year) | 1.16 [0.77-1.75] | 0.82 [0.47-1.43] | 0.77 [0.49-1.20] | 1.01 [0.52-1.94] | 0.83 [0.51-1.36] | 0.87 [0.45-1.69] | 2.47 [0.92-6.65] | 2.81 [0.64-12.27] | 1.25 [0.53-2.94] | 2.40 [0.61-9.44] |
<0.05
<0.01
<0.001
Adjusted models also controlled for: age, race/ethnicity, income, education and MSM vs. MSMW
---- Model did not converge
Discussion
The study captured a large sample of very poor MSM in Los Angeles, many of whom had experienced homelessness within the past year and had a history of incarceration. The estimated prevalence of HIV among Los Angeles MSM from behavioral surveillance is 19% (41). The fact that 41% of the sexual episodes reported by HIV-positive participants were with partners who they believed to be HIV positive implies that as a group, HIV-positive MSM in our sample are engaging in seroconcordant partnerships more than would be expected by chance. Within these partnerships an increased odds of UAI was observed. Taken together, this suggests that many HIV-positive MSM in our sample are engaging in UAI within concordant partnerships. While this is indicative of serosorting as an intentional “risk management” strategy, several other possible explanations exist. For example, HIV-positive men may select HIV-positive partners for the sake of support and shared life experiences. Previous studies have shown that increased intimacy is associated with decreased condom use and by extension, increased UAI among MSM (42-43). Our finding that seroconcordance was more common with main partnerships would seem to support the latter explanation. Regardless of the reason, the serosorting behaviors observed among HIV-positive MSM in our sample should theoretically reduce the risk of HIV transmission to uninfected partners. However, inferred partner HIV status is often incorrect (24-25). If serosorting partnerships among HIV-positive men commonly involve both the highest frequency of sex (within main partnerships) and higher transmission risk behaviors, then risk of transmission would increase, even if unrecognized, in circumstances where HIV status of the partner is incorrectly identified. It is possible that increased risks of HIV transmission within main partnerships reported by other studies of MSM may in part be attributed to “failed” attempts at serosorting (44-45).
Although only 4% of partnerships reported by HIV-negative participants were with HIV-positive partners, it is not clear that this avoidance of positive partners translates into serosorting among the HIV-negative MSM in our sample. Over half of the partners among HIV-negative participants were of an unknown HIV status, posing a substantial risk of infection. Additionally, increased odds of UAI with seroconcordant partners were not observed in univariate or multivariate models. As only 20 partnerships among HIV-negative men were with a HIV-positive partner, it is also possible that small cell sizes limited our ability to detect significant associations. For these reasons, evidence of serosorting among HIV-negative participants in our sample is not supported.
Substantial differences in sexual position by HIV status were noted within the sample. Interestingly, the percentage of participants reporting partnerships with UAI at last sex were similar between HIV-positive and negative men; 33% and 31% respectively. The type of UAI however, was different as more HIV-negative men reported UIAI and more HIV-positive men reported URAI, which is consistent with seropositioning as a risk management strategy. Differences in non-AI behaviors were observed as well and may indicate different strategies for HIV-positive and negative MSM who wish to avoid UAI.
When examining associations of partner HIV status on sexual position among a sub-sample of HIV-positive participants, a clear pattern of seroadaptation was observed. HIV-positive men had lower odds of UIAI and higher odds of URAI with partners of negative or unknown status compared to positive partners, indicating seropositioning. To our knowledge, there are no official recommendations that promote seropositioning as a risk management strategy for HIV-positive or HIV-negative men. Seroadaptation is often thought of as a “folk belief” based heavily on a 1999 Vittinghoff et al. paper among HIV-negative MSM, which estimated a lower per-contact risk of HIV seroincidence for engaging in UIAI with an HIV-positive or unkown partner compared to engaging in URAI with an HIV-positive or unkown partner (8). It is unclear to what extent seroadaptation has been incorporated into mainstream HIV counseling and prevention approaches. Anecdotally, in many Los Angeles area HIV service organizations, seroadaptation is described to clients in terms of a “heirarchy of risk,” in which URAI is explained to pose a greater risk of acquiring HIV than UIAI. Similar to serosorting, no clear pattern of seropositioning was observed among the HIV-negative participants. Future studies should assess how participants are becoming informed about seroadaptation, delve into how these messages influence participants’ cognitions related to seroadaptation, and determine the level of awareness of newer studies showing mixed evidence for seroadaptaton as a “risk management” strategy.
The finding that 30% of HIV-positive men had oral-only sex and the fact that the odds of oral-only sex was higher with serodiscordant partners, although not statistically significant, suggests that oral sex may be a commonly used seroadaptive behavior among poor, HIV-positive MSM. Surprisingly, no association between discordant partners and condom use was observed. A recent qualitative study of HIV-positive minority MSM in Los Angeles noted a lack of availability of condoms in homeless shelters and incarcerated settings (46). One-third (34%) of our sample reported their current housing situation as “a shelter, boarding house, or halfway house” or “a squat, abandoned building, on the street” and 53% said they had been homeless at some point in the past year. Structural barriers may prevent access to condoms and leave many individuals with few options other than seroadaptation to protect themselves from HIV infection, which provides some evidence of resilience in sexual decision making. Increased condom distribution, particularly in jails and areas with large populations of poor MSM, may be necessary to ensure adequate access among this population.
Studies of HIV risks in low-SES and homeless populations, especially youth, generally focus on non-main partners with whom sex is exchanged for food, money, shelter, or drugs (47-49). While important, our findings suggest that among older marginalized MSM more attention needs to be paid to the role of main partnerships. In this study, only 4% of partnerships were identified as trade partners; 30% were considered main or regular partners (data not shown). The potential for “failed” serosorting in main partnerships, coupled with our findings of a negative association between main partnerships and oral-only sex among HIV-positive men and a positive association between main partners and unprotected versatile sex among HIV-negative men, underscores the need to better understand the role of main partnerships in HIV transmission of very low-SES MSM populations. This is especially so given the high HIV prevalence among MSM in Los Angeles and even higher prevalence in this sample, which is comprised of men who purportedly had sexual, social, and/or drug using ties between them.
These findings should be viewed in light of some limitations. As noted in previous studies using RDS, recruitment may not have reached the entire universe of the target population and therefore caution should be taken when generalizing results to younger or higher SES MSM (50). Partner HIV status was based on the report of the index participant and a large proportion of partners’ HIV status was unknown. Moreover, the survey did not ask about seroadaptation directly; therefore, we do not know if the observed patterns are merely correlated or represent intentional “risk management” approaches. Caution should be taken when comparing the results between HIV-positive and HIV-negative men in this sample. The two groups differed substantially in terms of demographics, homelessness, substance use, and gender of sex partners (men-only vs. men and women). It is therefore possible that the differences in seroadaptive behaviors between HIV-positive and HIV-negative men in our sample can be attributed to factors other than their HIV status. For example, more HIV-negative men reported experiencing homelessness within the past year compared to HIV-positive men. If homelessness limits resiliency in sexual decision making, as we might expected given studies documenting relatively high rates of survival sex among the homeless (47, 51), then the lack of seroadaptation observed among HIV-negative men in the sample may be in part attributed to their increased exposure to homelessness.
This study provides evidence of seroadaptation among poor HIV-positive MSM in Los Angeles. Even in the face of abject poverty, HIV-positive MSM in our sample are attempting to mitigate the risk of transmission to others though a combination of seroadaptive behaviors, namely serosorting, oral-only sex, and seropositioning. This reflects an altruism that is especially noteworthy in a population with substantial un-met needs and several structural, social, and behavioral barriers to provision of their own health care (52-54). Future studies should examine individuals’ cognitions related to seroadaptation, address the influence of partnerships, and determine the effect that structural factors play in encouraging seroadaptation as well as consistent condom use.
Acknowledgements
This study was funded by the National Institute on Drug Abuse Award Number U01DA17394. The project described was supported in part by Award Number T32AA007240, Graduate Research Training on Alcohol Problems, from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health.
References
- 1.Snowden JM, Raymond HF, McFarland W. Prevalence of seroadaptive behaviours of men who have sex with men, San Francisco, 2004. Sex Transm Infect. 2009 Oct;85(6):469–76. doi: 10.1136/sti.2009.036269. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008 Aug 6;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snowden JM, Raymond HF, McFarland W. Seroadaptive behaviours among men who have sex with men in San Francisco: the situation in 2008. Sex Transm Infect. 2011 Mar;87(2):162–4. doi: 10.1136/sti.2010.042986. [DOI] [PubMed] [Google Scholar]
- 4.HIV Epidemiology Program, Los Angeles County Department of Public Health [Accessed March 22, 2012];An Epidemiologic Profile of HIV and AIDS in Los Angeles County. 2009 http://publichealth.lacounty.gov/wwwfiles/ph/hae/hiv/2009-epi.pdf.
- 5.Siconolfi DE, Moeller RW. Serosorting. BETA. 2007 Winter;19(2):45–9. [PubMed] [Google Scholar]
- 6.Van de Ven P, Kippax S, Crawford J, Rawstorne P, Prestage G, Grulich A, et al. In a minority of gay men, sexual risk practice indicates strategic positioning for perceived risk reduction rather than unbridled sex. AIDS Care. 2002 Aug;14(4):471–80. doi: 10.1080/09540120208629666. [DOI] [PubMed] [Google Scholar]
- 7.Kippax S, Race K. Sustaining safe practice: twenty years on. Soc Sci Med. 2003 Jul;57(1):1–12. doi: 10.1016/s0277-9536(02)00303-9. [DOI] [PubMed] [Google Scholar]
- 8.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999 Aug 1;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JT, Schrimshaw EW, Wolitski RJ, Halkitis PN, Purcell DW, Hoff CC, et al. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS. 2005 Apr;19(Suppl 1):S13–25. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 10.van den Boom W, Stolte I, Sandfort T, Davidovich U. Serosorting and sexual risk behaviour according to different casual partnership types among MSM: the study of one-night stands and sex buddies. AIDS Care. 2011 Aug 23; doi: 10.1080/09540121.2011.603285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarland W, Chen YH, Nguyen B, Grasso M, Levine D, Stall R, et al. Behavior, Intention or Chance? A Longitudinal Study of HIV Seroadaptive Behaviors, Abstinence and Condom Use. AIDS Behav. 2011 Jun 5; doi: 10.1007/s10461-011-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland W, Chen YH, Raymond HF, Nguyen B, Colfax G, Mehrtens J, et al. HIV seroadaptation among individuals, within sexual dyads, and by sexual episodes, men who have sex with men, San Francisco, 2008. AIDS Care. 2011 Mar;23(3):261–8. doi: 10.1080/09540121.2010.507748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C, Raymond HF, Guadamuz TE, Stall R, Colfax GN, Snowden JM, et al. Racial/Ethnic differences in seroadaptive and serodisclosure behaviors among men who have sex with men. AIDS Behav. 2011 Jan;15(1):22–9. doi: 10.1007/s10461-010-9683-2. [DOI] [PubMed] [Google Scholar]
- 14.Cassels S, Menza TW, Goodreau SM, Golden MR. HIV serosorting as a harm reduction strategy: evidence from Seattle, Washington. AIDS. 2009 Nov 27;23(18):2497–506. doi: 10.1097/QAD.0b013e328330ed8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip SS, Yu X, Donnell D, Vittinghoff E, Buchbinder S. Serosorting is associated with a decreased risk of HIV seroconversion in the EXPLORE Study Cohort. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heymer KJ, Wilson D. Available evidence does not support serosorting as an HIV risk reduction strategy. AIDS. 2010 Mar;24(6):935–6. doi: 10.1097/QAD.0b013e328337b029. [DOI] [PubMed] [Google Scholar]
- 17.Eaton LA, Kalichman SC, O’Connell DA, Karchner WD. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care. 2009 Oct;21(10):1279–88. doi: 10.1080/09540120902803208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008 Oct 1;49(2):212–8. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 19.Pinkerton SD. Acute HIV infection increases the dangers of serosorting. Am J Prev Med. 2008 Aug;35(2):184. doi: 10.1016/j.amepre.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler DM, Smith DM. Serosorting can potentially increase HIV transmissions. AIDS. 2007 May 31;21(9):1218–20. doi: 10.1097/QAD.0b013e32814db7bf. [DOI] [PubMed] [Google Scholar]
- 21.Marks G, Millett GA, Bingham T, Lauby J, Murrill CS, Stueve A. Prevalence and protective value of serosorting and strategic positioning among Black and Latino men who have sex with men. Sex Transm Dis. 2010 May;37(5):325–7. doi: 10.1097/OLQ.0b013e3181c95dac. [DOI] [PubMed] [Google Scholar]
- 22.McFarland W, Chen YH, Nguyen B, Grasso M, Levine D, Stall R, et al. Behavior, intention or chance? A longitudinal study of HIV seroadaptive behaviors, abstinence and condom use. AIDS Behav. 2012 Jan;16(1):121–31. doi: 10.1007/s10461-011-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin F, Prestage GP, Ellard J, Kippax SC, Kaldor JM, Grulich AE. How homosexual men believe they became infected with HIV: the role of risk-reduction behaviors. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):245–7. doi: 10.1097/QAI.0b013e3181565db5. [DOI] [PubMed] [Google Scholar]
- 24.Parsons JT, Severino J, Nanin J, Punzalan JC, von Sternberg K, Missildine W, et al. Positive, negative, unknown: assumptions of HIV status among HIV-positive men who have sex with men. AIDS Educ Prev. 2006 Apr;18(2):139–49. doi: 10.1521/aeap.2006.18.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zablotska IB, Imrie J, Prestage G, Crawford J, Rawstorne P, Grulich A, et al. Gay men’s current practice of HIV seroconcordant unprotected anal intercourse: serosorting or seroguessing? AIDS Care. 2009 Apr;21(4):501–10. doi: 10.1080/09540120802270292. [DOI] [PubMed] [Google Scholar]
- 26.German D, Sifakis F, Maulsby C, Towe VL, Flynn CP, Latkin CA, et al. Persistently High Prevalence and Unrecognized HIV Infection Among Men Who Have Sex With Men in Baltimore: The BESURE Study. Jaids-J Acq Imm Def. 2011 May 1;57(1):77–87. doi: 10.1097/QAI.0b013e318211b41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbach PM, Weiss RE, Jeffries R, Javanbakht M, Drumright LN, Daar ES, et al. Behaviors of recently HIV-infected men who have sex with men in the year postdiagnosis: effects of drug use and partner types. J Acquir Immune Defic Syndr. 2011 Feb 1;56(2):176–82. doi: 10.1097/QAI.0b013e3181ff9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus U, Schmidt AJ, Hamouda O. HIV serosorting among HIV-positive men who have sex with men is associated with increased self-reported incidence of bacterial sexually transmissible infections. Sex Health. 2011 Jun;8(2):184–93. doi: 10.1071/SH10053. [DOI] [PubMed] [Google Scholar]
- 29.Truong HM, Kellogg T, Klausner JD, Katz MH, Dilley J, Knapper K, et al. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect. 2006 Dec;82(6):461–6. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tieu HV, Xu G, Bonner S, Spikes P, Egan JE, Goodman K, et al. Sexual Partner Characteristics, Serodiscordant/Serostatus Unknown Unprotected Anal Intercourse and Disclosure Among Human Immunodeficiency Virus-Infected and Uninfected Black Men Who Have Sex With Men in New York City. Sex Transm Dis. 2011 Jan 6; doi: 10.1097/OLQ.0b013e318203e2d7. [DOI] [PubMed] [Google Scholar]
- 31.Xia Q, Osmond DH, Tholandi M, Pollack LM, Zhou W, Ruiz JD, et al. HIV prevalence and sexual risk behaviors among men who have sex with men: results from a statewide population-based survey in California. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):238–45. doi: 10.1097/01.qai.0000185574.98472.36. [DOI] [PubMed] [Google Scholar]
- 32.Lieb S, Prejean J, Thompson DR, Fallon SJ, Cooper H, Gates GJ, et al. HIV Prevalence Rates Among Men Who Have Sex with Men in the Southern United States: Population-Based Estimates by Race/Ethnicity. AIDS Behav. 2010 Sep 25; doi: 10.1007/s10461-010-9820-y. [DOI] [PubMed] [Google Scholar]
- 33.Robertson MJ, Clark RA, Charlebois ED, Tulsky J, Long HL, Bangsberg DR, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. American Journal of Public Health. 2004 Jul;94(7):1207–17. doi: 10.2105/ajph.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javanbakht M, Murphy R, Harawa NT, Smith LV, Hayes M, Chien M, et al. Sexually transmitted infections and HIV prevalence among incarcerated men who have sex with men, 2000-2005. Sex Transm Dis. 2009 Feb;36(2 Suppl):S17–21. doi: 10.1097/OLQ.0b013e31815e4152. [DOI] [PubMed] [Google Scholar]
- 35.Arriola KR, Braithwaite RL, Kennedy S, Hammett T, Tinsley M, Wood P, et al. A collaborative effort to enhance HIV/STI screening in five county jails. Public Health Rep. 2001 Nov-Dec;116(6):520–9. doi: 10.1016/S0033-3549(04)50084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hixson BA, Omer SB, del Rio C, Frew PM. Spatial clustering of HIV prevalence in Atlanta, Georgia and population characteristics associated with case concentrations. J Urban Health. 2011 Feb;88(1):129–41. doi: 10.1007/s11524-010-9510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iguchi MY, Ober AJ, Berry SH, Fain T, Heckathorn DD, Gorbach PM, et al. Simultaneous recruitment of drug users and men who have sex with men in the United States and Russia using respondent-driven sampling: sampling methods and implications. J Urban Health. 2009 Jul;86(Suppl 1):5–31. doi: 10.1007/s11524-009-9365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorbach PM, Murphy R, Weiss RE, Hucks-Ortiz C, Shoptaw S. Bridging sexual boundaries: men who have sex with men and women in a street-based sample in Los Angeles. J Urban Health. 2009 Jul;86(Suppl 1):63–76. doi: 10.1007/s11524-009-9370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006 Jul 15;43(2):234–42. doi: 10.1086/505212. [DOI] [PubMed] [Google Scholar]
- 40.Moss AR, Hahn JA, Perry S, Charlebois ED, Guzman D, Clark RA, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004 Oct 15;39(8):1190–8. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 41.Los Angeles County Department of Public Health . HIV Epidemiology Program. Los Angeles County Department of Public Health; 2009. An Epidemiologic Profile of HIV and AIDS in Los Angeles County. [Google Scholar]
- 42.Golub SA, Starks TJ, Payton G, Parsons JT. The Critical Role of Intimacy in the Sexual Risk Behaviors of Gay and Bisexual Men. AIDS Behav. 2011 Jun 1; doi: 10.1007/s10461-011-9972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theodore PS, Duran RE, Antoni MH, Fernandez MI. Intimacy and sexual behavior among HIV-positive men-who-have-sex-with-men in primary relationships. AIDS Behav. 2004 Sep;8(3):321–31. doi: 10.1023/B:AIBE.0000044079.37158.a9. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009 Jun 1;23(9):1153–62. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 45.Davidovich U, de Wit J, Albrecht N, Geskus R, Stroebe W, Coutinho R. Increase in the share of steady partners as a source of HIV infection: a 17-year study of seroconversion among gay men. AIDS. 2001 Jul 6;15(10):1303–8. doi: 10.1097/00002030-200107060-00013. [DOI] [PubMed] [Google Scholar]
- 46.Harawa NT, Williams JK, Ramamurthi HC, Bingham TA. Perceptions towards condom use, sexual activity, and HIV disclosure among HIV-positive African American men who have sex with men: implications for heterosexual transmission. J Urban Health. 2006 Jul;83(4):682–94. doi: 10.1007/s11524-006-9067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walls NE, Bell S. Correlates of engaging in survival sex among homeless youth and young adults. J Sex Res. 2011 Sep;48(5):423–36. doi: 10.1080/00224499.2010.501916. [DOI] [PubMed] [Google Scholar]
- 48.Marshall BD, Shannon K, Kerr T, Zhang R, Wood E. Survival sex work and increased HIV risk among sexual minority street-involved youth. J Acquir Immune Defic Syndr. 2010 Apr;53(5):661–4. doi: 10.1097/QAI.0b013e3181c300d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haley N, Roy E, Leclerc P, Boudreau JF, Boivin JF. HIV risk profile of male street youth involved in survival sex. Sex Transm Infect. 2004 Dec;80(6):526–30. doi: 10.1136/sti.2004.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burt RD, Hagan H, Sabin K, Thiede H. Evaluating respondent-driven sampling in a major metropolitan area: Comparing injection drug users in the 2005 Seattle area national HIV behavioral surveillance system survey with participants in the RAVEN and Kiwi studies. Ann Epidemiol. 2010 Feb;20(2):159–67. doi: 10.1016/j.annepidem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reback CJ, Kamien JB, Amass L. Characteristics and HIV risk behaviors of homeless, substance-using men who have sex with men. Addict Behav. 2007 Mar;32(3):647–54. doi: 10.1016/j.addbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Robbins JL, Wenger L, Lorvick J, Shiboski C, Kral AH. Health and oral health care needs and health care-seeking behavior among homeless injection drug users in san francisco. J Urban Health. 2010 Dec;87(6):920–30. doi: 10.1007/s11524-010-9498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palepu A, Milloy MJ, Kerr T, Zhang R, Wood E. Homelessness and adherence to antiretroviral therapy among a cohort of HIV-infected injection drug users. J Urban Health. 2011 Jun;88(3):545–55. doi: 10.1007/s11524-011-9562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson KM, Thiede H, Hawes SE, Golden MR, Hutcheson R, Carey JW, et al. Why the wait? Delayed HIV diagnosis among men who have sex with men. J Urban Health. 2010 Jul;87(4):642–55. doi: 10.1007/s11524-010-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]