Abstract
Objectives
Alcohol abuse is one of the most common factors associated with acute and chronic pancreatitis. Although it is evident that alcohol abuse can have an important role in the development of pancreatitis, it does not appear that alcohol abuse alone is responsible for this disease. We investigated the involvement of ethanol in impairment of pancreatic repair after induction of pancreatitis.
Methods
A biologically relevant mouse model of alcoholic pancreatitis, combining chronic ethanol consumption and coxsackievirus infection, was used to investigate the effects of ethanol on pancreatic regeneration. Tissues were harvested and analyzed by RT-PCR and immunoblot.
Results
These studies demonstrate that chronic ethanol consumption impairs the structural repair of the exocrine pancreas. This is accompanied by a delay in the restitution of lipase expression. Additionally, impaired expression of the critical pancreatic transcription factors, PDX1 and PTF1, and the mediator of Notch signaling, HES1, were observed.
Conclusions
Chronic ethanol consumption impairs the structural repair and functional restitution of the pancreas after severe injury. These impairments may, in part, be explained by impaired expression of factors important in the development and maintenance of the exocrine pancreas. Impaired pancreatic regeneration may have a role in the pathogenesis of alcoholic pancreatitis.
Keywords: Acute Pancreatitis, Coxsackievirus, Ethanol, Tissue Repair, Alcoholic Pancreatitis, Developmental Factors
Introduction
Pancreatitis is a necroinflammatory disease of the exocrine pancreas that is normally classified as either acute or chronic. In developed countries, alcohol abuse is the most common factor associated with chronic pancreatitis and the second most common factor associated with acute pancreatitis 1, 2. Although the association between alcohol abuse and pancreatitis has been recognized for well over a century 3, the mechanisms by which ethanol abuse leads to pancreatitis are not well understood. Only about 5% of individuals who chronically abuse alcohol develop alcoholic pancreatitis. Therefore, it does not appear that alcohol abuse alone is sufficient to cause acute or chronic alcoholic pancreatitis 4. Because only a small percentage of alcoholics develop pancreatitis, it has been suggested that development of alcoholic pancreatitis requires a cofactor or additional susceptibilities. Commonly suggested cofactors are smoking, genetic predisposition, a high lipid diet, and infectious agents 5.
Although alcohol abuse alone does not appear to be sufficient to cause alcoholic pancreatitis, alcohol does affect the pancreas. It has been suggested that alcohol sensitizes the pancreas to more severe injury from factors that normally would cause minimal tissue damage or disease. The mechanisms by which ethanol sensitizes the pancreas are unknown. Interestingly, the pancreas, like the liver, is able to metabolize ethanol both oxidatively via alcohol dehydrogenase and cytochrome P450 2E1, and nonoxidatively via fatty acid ethyl esterases to fatty acid ethyl esters 6–8. The metabolism of ethanol causes a number of metabolic changes in cells. These metabolic changes have been proposed to predispose the pancreas to injury. Oxidative metabolism of ethanol results in the production of acetaldehyde and reactive oxygen species. Both of these byproducts have been shown to have detrimental effects on the pancreas 9–11. Likewise, the production of fatty acid ethyl esters, which result from the nonoxidative metabolism of ethanol have been shown to be toxic to the pancreas12, 13.
Tissue damage occurs when cellular death exceeds the capacity of the tissue to repair itself. Many studies have investigated the mechanisms by which ethanol damages pancreatic tissue, but few studies have examined the effects of ethanol on pancreatic repair and regeneration after injury. Like the liver, the pancreas has a tremendous capacity to regenerate after injury 14–17. It is generally thought that after acute pancreatitis, the pancreas is structurally and functionally restored in about 2 weeks. Under most circumstances, acinar cells act as facultative progenitors to repair the exocrine pancreas 14, 15. In order to accomplish this, mature acinar cells dedifferentiate and repair of the injured pancreatic tissue generally recapitulates the developmental program of the pancreas. This process requires the expression of factors associated with development and maturation of the exocrine pancreas 16.
We have previously described a model of alcoholic pancreatitis that combines chronic ethanol consumption with coxsackievirus infection in mice 18–20. Using this model, we have investigated the effects of ethanol on repair of the injured pancreas. The results of these studies indicate that chronic ethanol consumption delayed both the structural regeneration and functional restitution of the injured pancreas. Additionally, we found altered expression of transcription factors critical in the maturation of the exocrine pancreas and impaired expression of regulators of cellular development. Altered expression of these factors may, at least in part, be responsible for the delayed pancreatic repair in mice that have chronically consumed ethanol. Impaired recovery of the pancreas may increase the severity and duration of pancreatitis.
Materials and Methods
Virus
Coxsackievirus, group B, type 3, strain CO (CVB3/CO) 21 (a kind gift from Dr. Nora Chapman, University of Nebraska Medical Center) was used throughout these studies. CVB3/CO was chosen for these studies because it is a human pathogen that causes a similar disease in both mice and human beings, resulting in pancreatitis. The virus has not been mouse adapted, and was propagated using HeLa cells.
The viral titers of infected mice were determined by performing plaque assays with spleen homogenates. The spleens were homogenized in DMEM containing 2% fetal bovine serum, diluted, and incubated with HeLa cell monolayers for 1 hr. The viral inoculum was removed and the monolayers were overlaid with DMEM containing 1% agarose and incubated for 48 hr. After incubation the cells were fixed with 20% formalin, the agarose was removed, and the cells were stained with 1% crystal violet to visualize the plaques. The plaques were counted and the viral titers of each sample calculated.
Animal Subjects
The Guidelines for the Use and Care of Laboratory Animals, published by the National Institutes of Health, were followed in all animal studies. The Institutional Animal Care and Use Committee of the VA Nebraska-Western Iowa Health Care System approved all animal studies and procedures. In all experiments, female C57Bl/6 mice were used.
Ethanol Exposure and Viral Infection
Mice were divided into two groups, ethanol and control. Mice receiving ethanol were provided 20% ethanol in their drinking water, as described by Song et al. 22. The group that was provided the ethanol was gradually acclimated to the ethanol by providing increasing concentrations of ethanol in their water, until the ethanol concentration reached 20%. The other group of mice was provided water and served as controls. After 6–8 weeks of ethanol consumption, mice were infected with CVB3/CO. Ethanol has been shown to affect the severity of a number of viral infections 23–25. Therefore, it was necessary to determine the concentration of coxsackievirus that would result in similar pancreatitis in control mice and mice that had been chronically provided ethanol. To accomplish this control mice were infected with 5 × 105 tissue culture infective dose50 (TCID50) of coxsackievirus CVB3/CO and mice provided ethanol were infected with either 5 × 105, 2.5 × 105, or 1 × 105 TCID50. Having determined that infection of control mice with 5 × 105 TCID50 and mice provided ethanol with 1 × 105, resulted pancreatitis in of similar severity these concentrations of virus were used for the remainder of the studies. These doses of virus are sublethal, but cause severe pancreatitis.
Histologic Examination and BrdU Staining
Pancreata were harvested, fixed in 10% buffered formalin, paraffin embedded, and sectioned. Histologic changes were determined by staining sections with hematoxylin and eosin (H&E). The replication of acinar cells, in the regenerating pancreas, was determined by monitoring the incorporation of the DNA analogue 5-bromo-2-deoxyuridine (BrdU) into newly synthesized DNA. Mice were inoculated intraperitoneally with .2 ml of 10 mg/ml BrdU 2 hours prior to euthanasia. Tissue sections were deparaffinized and rehydrated. Endogenous peroxidases were quenched with 3% hydrogen peroxide. DNA was denatured with 1M HCL, followed by neutralization with 0.1M sodium borate. Sections were blocked for 1 hour using mouse on mouse (M.O.M.) IgG blocking reagent (Vector Labs, Burlingame, CA). The sections were incubated at room temperature for 30 minutes with the primary antisera at 1:100 dilution (BD Biosciences, Franklin Lakes, NJ), followed by biotinylated anti-mouse IgG for 10 minutes, and Vectastain ABC reagent for 10 minutes. Subsequently, the sections were incubated with Vector DAB until a light brown color developed, counterstained with hematoxylin, dehydrated, mounted, and examined microscopically.
Serum Enzyme Assays
As an indicator of pancreatic damage, serum amylase and lipase levels were monitored. Serum amylase and lipase levels were determined using Liquid Select amylase reagent and Lipase Color kit respectively (Sekisui Diagnostics Framingham, MA), according to the manufacturers instructions.
Immunoblot
Pancreata were homogenized in RIPA buffer (50 mM Tris pH 7.4, 1% NP-40, 0.25% Na deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaVO4, 1 mM NaF). Complete mini protease cocktail was added to the buffer as recommended by the manufacturer (Roche, Indianapolis, IN). The protein concentrations of the homogenates were determined using the Bio-Rad protein reagent (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as a standard. Sixty micrograms of protein from each sample was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were electroblotted onto nitrocellulose membranes in 25 mM Tris, 192 mM glycine, and 20% methanol at 100 volts for 60 min at 4 °C. The membranes were blocked in TBST (20 mM Tris pH 7.6, 136 mM NaCl, 0.1% Tween-20) containing 5% nonfat dry milk for 1 hour at room temperature, and incubated at 4°C overnight with the primary antibody diluted in TBST/milk solution. The membranes were washed in TBST and incubated for 1 hour with a peroxidase-conjugated secondary antibody (Jackson Labs, Bar Harbor, ME) diluted in TBST/milk, washed, and the proteins visualized by chemiluminescence using the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). Antibodies used were anti-lipase, anti-amylase (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-tubulin (Sigma, St Louis, MO).
RT-PCR
Pancreata were isolated and immediately homogenized in 1 ml of RNA STAT-60 (Tel-Test Inc. Friendswood, TX). Two hundred microliters of chloroform was added to the homogenates and the samples were centrifuged at 15,000 × g for 15 min. The aqueous phase was collected and RNA was isolated using the RNeasy Mini Kit, essentially as described by the manufacturer (Qiagen, Valencia, CA). cDNA was produced using a High Capacity cDNA Kit (Applied Biosystems, Foster City, CA). RT-PCR was performed with the Taqman system on an Applied Biosystems 7500 RT-PCR themocycler, using primers specific for the transcript of interest. Primers specific for GAPDH of murine origin were used as internal controls for each reaction. Differences between control mice and mice provided ethanol are expressed as fold changes of the target transcripts, as determined by the 2−ΔΔCt method 26.
Statistics
Data are expressed as the mean values ± SEM. Statistical significance between two groups was determined by Student’s t-test. A probability (p ≤ 0.05) was considered statistically significant.
Results
Viral Infection and Pancreatitis: Because alcohol itself is not sufficient to cause overt pancreatitis in mice, we combined administration of ethanol with pancreatitis induced by infection with coxsackievirus. Ethanol has been shown to affect the severity of a number of viral infections 23–25. Therefore, it was necessary to first determine the concentration of coxsackievirus that would result in similar pancreatitis in control mice and mice that had been chronically provided ethanol. Control mice were infected with 5 × 105 TCID50 of coxsackievirus CVB3/CO and mice provided ethanol were infected with either 5 × 105, 2.5 × 105, or 1 × 105 TCID50. The results, shown in Figure 1, reveal similar viral titers were obtained from the spleens of control mice infected with 5 × 105 TCID50 and mice provided ethanol infected with 1 × 105 TCID50.
Figure 1.
Viral Titers of CVB3/CO Infected Mice. Control mice were infected with 5 × 105 TCID50 and mice provided ethanol for 6–8 weeks were infected with 1 × 105 TCID50 of CVB3/CO. Mice were euthanized on day 2, 4, 6, 8, 10, and 12 after infection and viral titers were determined by plaque assays using spleen homogenates. Data represent the mean + SEM. * indicates samples significantly different from control (p ≤ .05) (n=6).
To determine if infection of control mice with 5 × 105 TCID50 and mice provided ethanol with 1 × 105 TCID50 yielded similar pancreatic damage, we first examined pancreata histologically. Histologic examination of the pancreata had similar exocrine pathology in both control and mice administered ethanol (Figure 2). Two days after infection the pancreata from both groups revealed the presence of inflammatory cells and some degranulated necrotic acinar cells. Four days after infection, severe destruction of acinar cells was observed in both control and mice provided ethanol. Compared to the two day samples, the inflammation was much more severe and the majority of the acinar cells were degranulated and necrotic. No differences between the pancreata from control or mice provided ethanol were observed at these time points.
Figure 2.
Micrographs of Pancreatic Damage. Representative micrographs of pancreata from control mice infected with 5 × 105 TCID50 and mice provided ethanol for 6–8 weeks infected with 1 × 105 TCID50 of CVB3/CO. Mice were euthanized on day 2 and 4 after infection. Pancreata were isolated, fixed, sectioned, and stained with hematoxalin and eosin (H&E).
Next, we determined the severity of pancreatitis by monitoring the serum amylase and serum lipase levels (Figure 3 and 4 respectively). The results of these studies revealed that both the serum amylase and serum lipase levels were significantly different 4 days after infection, but similar in both the control mice and mice administered ethanol throughout the remainder of the study. These results indicated that similar pancreatitis occurred in both groups of animals throughout the majority of the course of the virally induced pancreatitis.
Figure 3.
Serum Amylase Levels. Control mice or mice provided ethanol for 6–8 weeks were infected with CVB3/CO. Mice were euthanized on day 0, 2, 4, 6, 8, 10, 12 and 14, and serum amylase activity was determined. Data represent the mean + SEM. * indicates samples significantly different from control (p ≤ .05) (n=6).
Figure 4.
Serum Lipase Levels. Control mice or mice provided ethanol for 6–8 weeks were infected with CVB3. Mice were euthanized on day 0, 2, 4, 6, 8, 10, 12 and 14, and serum lipase activity was determined. Data represent the mean + SEM. * indicates samples significantly different from control (p ≤ .05) (n=6).
Pancreatic Regeneration
Having established that similar pancreatic damage occurred in both the control mice and mice that were provided ethanol, we investigated the effects of ethanol on the repair of the pancreas. Initially, we monitored the repair of the pancreas microscopically. As shown in Figure 5, by day six after infection the damaged pancreas had begun to show signs of repair. Acinar cells organized into acini were observed in the pancreata from both control mice and mice provided ethanol, although greater numbers of both acinar cells and acini were detected in the pancreata of control mice. Eight days after infection, greater numbers of acini were detected in the pancreata of mice from both groups and the gross architecture of the pancreas was apparent. Again, it was evident that fewer acini were present in the pancreata of mice provided ethanol. This pattern of pancreatic repair continued throughout the period of observation. By twelve days after infection, nearly complete repair of the pancreas and restitution of pancreatic architecture was observed in the control mice. Although the pancreata of mice provided ethanol showed signs of repair, at each time point the repair of the pancreas was retarded in the mice provided ethanol. Because fewer acinar cells and acini were observed in the pancreata of mice provided ethanol, it appeared that the regeneration of the pancreatic parenchyma was impaired in these animals.
Figure 5.
Micrographs from Regenerating Pancreata. Representative micrographs of pancreata from control mice infected with 5 × 105 TCID50 and mice provided ethanol for 6–8 weeks infected with 1 × 105 TCID50 of CVB3/CO. Mice were euthanized on day 6, 8,10, and 12 after infection. Pancreata were isolated, fixed, sectioned, and stained with hematoxylin and eosin (H&E). (I) indicates an islet of Langerhans, arrows indicate acini, and arrowheads indicate acinar cells. The scale bar represents 100 μm.
To quantify the regeneration of the pancreas, incorporation of BrdU into newly synthesized DNA was determined at various days following coxsackievirus-induced pancreatitis. The results, presented in Table 1, showed that significantly more cells were replicating in the pancreata of control mice at days 4 and 6 after infection. At day 8 after infection, nearly equal numbers of replicating cells were observed in the pancreata of mice from both groups. Thereafter, at days 10, 12 and 14, more replicating cells were observed in the pancreata of mice provided ethanol. These results demonstrate that chronic ethanol consumption resulted in delayed replacement of acinar cells and an impairment of pancreatic repair.
Table 1.
Number of Cells Actively Replicating at Various Days During Pancreatic Regeneration
| Day | 4D | 6D | 8D | 10D | 12D | 14D |
|---|---|---|---|---|---|---|
| Control | 20.3 ± 3.3* | 60.1 ± 27.4* | 30.0 ± 5.4 | 48.9 ± 13.7 | 75.9 ± 51.6 | 70.6 ± 6 |
| Ethanol | 14.2 ±2.9 | 47.6 ± 16.1 | 29.5 ± 8.5 | 95.9 ± 21.6* | 91.9 ± 27.1 | 131.7 ± 31.8* |
Number of cells stained positively for incorporation of BrdU/435 μm × 326 μm field.
signifies significantly different from the other treatment group (p ≤ 0.05).
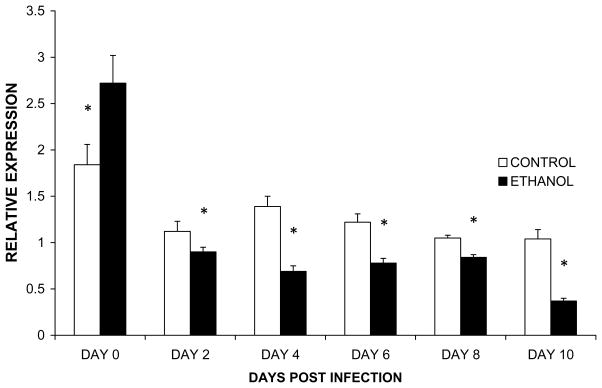
To investigate if the impaired replacement of acinar cells corresponded with a functional impairment, we first investigated if the transcription of pancreas specific genes was impaired. To accomplish this, we isolated mRNA from the pancreata of infected animals at time points after induction of pancreatitis and determined the expression of lipase mRNA. The results of these studies (Figure 6) showed that although the expression of lipase was initially higher in the pancreata of mice provided ethanol, the expression of this transcript was reduced in the pancreata of mice provided ethanol during the regenerative phase of the pancreatitis. Analysis of amylase expression revealed a similar pattern of impairment (data not shown).
Figure 6.
Expression of Lipase Specific mRNA in the Regenerating Pancreas. Control or mice or mice provided ethanol for 6–8 weeks were infected with CVB3/CO. Mice were euthanized on day 0, 2, 4, 6, 8, and 10 after infection. Lipase mRNA expression was determined in the pancreata by RT-PCR. Data represent the mean + SEM. * indicates samples that are significantly different from control (p ≤ .05) (n=6).
To determine if impairment in the expression of lipase mRNA corresponded to impaired expression of the protein, pancreata were isolated at 8 and 10 days post infection. Protein samples were prepared and immunoblots performed. The results of these studies revealed striking differences in the protein expression of lipase (Figure 7). Lipase was readily detected in samples isolated from control mice, but difficult to detect in samples isolated from mice chronically provided ethanol. As with the mRNA analysis, amylase expression revealed a similar pattern of impairment (data not shown). The results of these studies show that the structural and functional repair of the injured pancreas is delayed in mice chronically provided ethanol.
Figure 7.
Immunoblot of Pancreatic Lipase 8 and 10 Days After Infection. Control mice or mice provided ethanol for 6–8 weeks were infected with CVB3/CO and homogenates were prepared 8 and 10 days after infection. Proteins were separated, blotted, and incubated with antisera specific for lipase.
Pancreas Differentiation and Development
Recent studies have shown that after pancreatic damage the remaining acinar cells normally accomplish repair and regeneration of the exocrine pancreas. The surviving acinar cells act as facultative progenitor cells, acquiring traits of progenitor cells and express genes, such as pdx1, p48/ptf1a, and hes-1, normally associated with the developing pancreas.
Because repair of the damaged pancreas was delayed in mice provide ethanol we investigated if ethanol mediated this effect by altering the expression of these important developmental factors. We found that the expression of PDX1 mRNA was reduced in the pancreata of mice provided ethanol as early as 4 days after infection, and remained reduced at least 10 days after infection (Figure 8).
Figure 8.
Expression of PDX1 Specific mRNA in the Regenerating Pancreas. Control mice and mice provided ethanol for 6–8 weeks were infected with CVB3/CO. Mice were euthanized on day 0, 2, 4, 6, 8, and 10, and PDX1 mRNA expression was determined in the pancreata by RT-PCR. Data represent the mean + SEM. * indicates samples that are significantly different from control (p ≤ .05) (n=6).
As a marker for the expression of PTF1 in acinar cells, we monitored the expression of P48, one of the components of this heterotrimeric complex. The results of these studies revealed that P48 mRNA expression in the pancreata of mice provided ethanol was reduced early during the course of the disease, at 4 and 6 days after infection (Figure 9). This indicates that ethanol alters the normal expression of P48 in the regenerating pancreas, and provides, at least in part, an explanation for the altered expression of pancreas specific proteins that we have observed.
Figure 9.
Expression of P48 Specific mRNA in the Regenerating Pancreas. Control mice or mice provided ethanol for 6–8 weeks were infected with CVB3/CO. Mice were euthanized on day 0, 2, 4, 6, 8, and 10, and P48 mRNA expression was determined in the pancreata by RT-PCR. Data represent the mean + SEM. * indicates samples that are significantly different from control (p ≤ .05) (n=6).
Hes-1 is an important component of the Notch signaling pathway. The Notch signaling pathway promotes pancreatic progenitor self-renewal and/or exocrine lineage commitment. Analysis of the expression of HES-1 mRNA expression, revealed it was reduced at all time points analyzed in mice provided ethanol (Figure 10). These results reveal that chronic ethanol consumption impairs the expression of factors important in the regeneration of the pancreas. Impairment of these factors could, in part, explain the delay observed in the structural repair and functional restitution of the pancreas in mice chronically consuming ethanol.
Figure 10.
Expression of HES-1 Specific mRNA in the Regenerating Pancreas. Control mice or mice provided ethanol for 6–8 weeks were infected with CVB3/CO. Mice were euthanized on day 0, 2, 4, 6, 8, and 10, and HES1 mRNA expression was determined in the pancreata by RT-PCR. Data represent the mean + SEM. * indicates samples that are significantly different from control (p ≤ .05) (n=6).
Discussion
The association between alcohol abuse and pancreatitis has been recognized for well over a century 3. Despite this long established association, the critical mechanism(s) by which ethanol abuse leads to alcoholic pancreatitis is not well defined. Interestingly, although it is evident that alcohol abuse can have an important role in the development of pancreatitis, it does not appear that alcohol abuse alone is responsible for the development of alcoholic pancreatitis. Rather, it appears that ethanol alters the normal physiologic responses of pancreatic cells to injury. Because of this, it has been proposed that, alcoholic pancreatitis results from exposure to agents that would not normally cause overt disease or from agents that would normally cause mild disease. In this way, alcohol in combination with cofactors leads to alcoholic pancreatitis 4, 5. A number of cofactors for alcoholic pancreatitis have been proposed, including exposure to viruses that infect the pancreas 5, 19, 20, 27. Coxsackieviruses are known to infect the pancreas and cause pancreatitis in both human beings and animals 19,21,28,29. Using coxsackievirus as a cofactor, we have developed a biologically relevant mouse model of alcoholic pancreatitis that combines coxsackievirus-mediated pancreatitis with chronic ethanol consumption 19, 20, 30.
The pancreas and the liver are closely related developmentally, and in a broad sense have many similarities 31. The pancreas, like the liver, is able to metabolize ethanol and it is thought that this is the reason these organs are targets for the toxic effects of ethanol 6–8. Additionally, the exocrine pancreas has a remarkable regenerative capacity 14, 16, 17, 32. There is extensive evidence that ethanol metabolism delays or impairs the regeneration of the liver, as well as the replication of hepatocytes and cultured hepatic cells 30, 33–35. It is thought that in some instances this impairment is intimately associated with disease progression 36, 37. Thus, when investigating the pathogenesis of alcohol-induced tissue injury, one must not only consider tissue damage and cell death, one must also consider the ability of tissues to respond appropriately to injury and replace damaged cells.
Remarkably, little is known about the mechanisms by which ethanol affects the regeneration of the pancreas, or how these effects contribute to the initiation and progression of alcoholic pancreatitis. Using our model of virus-induced alcoholic pancreatitis, we investigated the effects of chronic ethanol consumption on the regeneration of the pancreas. Initially, we monitored the damage and regeneration of the pancreas histologically. These studies demonstrated that chronic ethanol consumption prolonged the damage and delayed the recovery of the pancreas after injury. The delay in the regeneration of the pancreas was also evident by the fact that more replicating cells were observed in the pancreata of mice provided ethanol 10, 12, and 14 days after injury. Additionally, pancreatic weight was lower in mice provided ethanol than in control mice, 10 and 12 days after induction of pancreatitis (data not shown). These structural deficiencies were associated with functional impairments. We found that chronic ethanol consumption delayed the expression of lipase in the pancreas after injury. Thus, it appears that chronic ethanol consumption not only impairs the structural repair of the pancreas, but impairs its functional restitution as well.
Impaired recovery from pancreatitis after would prolong the course of the disease. Additionally, it is possible that a mild, subclinical episode of pancreatitis would go unnoticed under conditions of normal pancreatic repair. In the absence of normal repair, damage could accumulate resulting in clinical pancreatitis. Thus, delay in the repair of the injured pancreas could exasperate the severity of pancreatitis, as well as prolong the disease.
Recent studies have shown that, similar to the role of hepatocytes in liver regeneration, differentiated acinar cells possess the ability to act as facultative progenitors during pancreatic regeneration 14, 15, 38. These facultative progenitor cells are mature cell types that, in response to injury, acquire the traits of progenitor cells 15, 38. Jensen et al. demonstrated that after caerulein induced pancreatic injury, the surviving acinar cells repress the terminally differentiated exocrine gene expression program, and express transcripts normally associated with the developing pancreas 16. Because of this, it has been suggested that mature acinar cells accomplish the repopulation of the pancreas by dedifferentiating, replicating, and then redifferentiating 16, 32, 39. Genes normally associated with development of the exocrine pancreas, such as hedgehog, notch, pdx1, and hes-1, are also expressed in the regenerating exocrine pancreas.
Because normal regeneration of the exocrine pancreas requires the appropriate expression of developmental factors, we investigated if chronic ethanol consumption altered the expression of factors important in the development of the exocrine pancreas. We found that the expression of transcription factors important for the identity of acinar cells, PDX1 and P48, were delayed in the pancreata of mice provided ethanol. These transcription factors are required for the appropriate development and differentiation of pancreatic cells; thus, disruption of their expression would alter the maturation of replicating cells in the regenerating pancreas 16, 40–42. Altered expression of these transcription factors may, in part, explain the delay in the functional restitution that was observed.
Likewise, we found that expression of Hes-1, an important mediator of Notch signaling, was impaired. The Notch signaling pathway is important in the regulation of cellular proliferation and cellular differentiation 43. Notch signaling is known to have an important role in the development and regeneration of the exocrine pancreas 16, 17, 44. Impairment of Notch signaling could delay the structural regeneration and functional restitution of the pancreas.
These results lead us to suggest that one mechanism by which ethanol impairs the repair and regeneration of the pancreas is by altering the expression of factors that orchestrate the orderly transitions that occur during normal pancreatic regeneration. Alterations in the expression of these factors would lead to inefficient or inappropriate response to injury. This inappropriate response would exaerbate tissue injury and adversely affect recovery from injury.
Although we investigated the effects of ethanol in an acute model of pancreatic injury and repair, our findings that ethanol alters the expression of factors important in regeneration may have broader implications. Worldwide, ethanol abuse is the number one factor associated with development of chronic pancreatitis, being associated with approximately 70% of reported cases 1. Chronic pancreatitis is characterized by necrosis, fibrotic scarring, loss of parenchymal tissue, and eventual loss of pancreatic function 1. Because fibrotic scarring is an aberrant repair and regenerative response, the fact that ethanol alters the repair response in the pancreas raises some interesting questions.
Development of chronic pancreatitis dramatically increases one’s risk for pancreatic cancer. Individuals who suffer from chronic pancreatitis have a 20-fold greater probability of developing pancreatic cancer than those unaffected by this disease 45. Interestingly, it has been shown that pancreatic cancer cells inappropriately express factors involved in pancreatic regeneration, including Notch and Hes-1 46. We have shown that ethanol impairs normal regeneration of the pancreas and affects the Notch signaling pathway. Thus, it is tempting to speculate that ethanol-mediated alterations in normal pancreatic repair may, in some circumstances, have a role in the development of pancreatic cancer.
In summary, using a biologically relevant model of alcoholic pancreatitis, we have shown that chronic ethanol consumption impairs the normal regenerative response of the pancreas after severe injury. This ethanol-mediated impairment is associated with the altered expression of factors that are important in pancreatic development. Impaired regeneration of the pancreas would not only accentuate tissue damage, it would also exacerbate the severity and duration of the disease. Additionally, because of the close associations between ethanol abuse, aberrant tissue repair, chronic pancreatitis, and development of pancreatic cancer, the ethanol-mediated impairment of pancreatic regeneration and repair warrants further investigation.
Acknowledgments
Support: This work was supported by grants to DLC from the Veterans Administration and the National Institutes of Health (AA016310)
Footnotes
The Authors declare no conflict of interest.
References
- 1.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996;111(1):224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- 2.Wang GJ, Gao CF, Wei D, et al. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15(12):1427–1430. doi: 10.3748/wjg.15.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friederich N. In Cyclopaedia of the practice of medicine. 18-18. New York: William Wood; 1878. Disease of the Pancreas; pp. 254–312. [Google Scholar]
- 4.Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol. 2010;25(12):1816–1826. doi: 10.1111/j.1440-1746.2010.06445.x. [DOI] [PubMed] [Google Scholar]
- 5.Oruc N, Whitcomb DC. Theories, mechanisms, and models of alcoholic chronic pancreatitis. Gastroenterol Clin North Am. 2004;33(4):733–750. doi: 10.1016/j.gtc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Haber PS, Apte MV, Applegate TL, et al. Metabolism of ethanol by rat pancreatic acinar cells. J Lab Clin Med. 1998;132(4):294–302. doi: 10.1016/s0022-2143(98)90042-7. [DOI] [PubMed] [Google Scholar]
- 7.Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231(4737):497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 8.Norton ID, Apte MV, Haber PS, et al. Cytochrome P4502E1 is present in rat pancreas and is induced by chronic ethanol administration. Gut. 1998;42(3):426–430. doi: 10.1136/gut.42.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology. 2002;122(1):106–118. doi: 10.1053/gast.2002.30302. [DOI] [PubMed] [Google Scholar]
- 10.Masamune A, Kikuta K, Satoh M, et al. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302(1):36–42. doi: 10.1124/jpet.302.1.36. [DOI] [PubMed] [Google Scholar]
- 11.Masamune A, Satoh A, Watanabe T, et al. Effects of ethanol and its metabolites on human pancreatic stellate cells. Dig Dis Sci. 2010;55(1):204–211. doi: 10.1007/s10620-008-0695-y. [DOI] [PubMed] [Google Scholar]
- 12.Hamamoto T, Yamada S, Hirayama C. Nonoxidative metabolism of ethanol in the pancreas; implication in alcoholic pancreatic damage. Biochem Pharmacol. 1990;39(2):241–245. doi: 10.1016/0006-2952(90)90022-d. [DOI] [PubMed] [Google Scholar]
- 13.Werner J, Laposata M, Fernandez-del Castillo C, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113(1):286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 14.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117(4):971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fendrich V, Esni F, Garay MV, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135(2):621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen JN, Cameron E, Garay MV, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128(3):728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Siveke JT, Lubeseder-Martellato C, Lee M, et al. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134(2):544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Clemens DL, Jerrells TR. Ethanol consumption potentiates viral pancreatitis and may inhibit pancreas regeneration: preliminary findings. Alcohol. 2004;33(3):183–189. doi: 10.1016/j.alcohol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Jerrells TR, Chapman N, Clemens DL. Animal model of alcoholic pancreatitis: role of viral infections. Pancreas. 2003;27(4):301–304. doi: 10.1097/00006676-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Jerrells TR, Vidlak D, Strachota JM. Alcoholic pancreatitis: mechanisms of viral infections as cofactors in the development of acute and chronic pancreatitis and fibrosis. J Leukoc Biol. 2007;81(2):430–439. doi: 10.1189/jlb.1004622. [DOI] [PubMed] [Google Scholar]
- 21.Tracy S, Hofling K, Pirruccello S, et al. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol. 2000;62(1):70–81. doi: 10.1002/1096-9071(200009)62:1<70::aid-jmv11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Song K, Coleman RA, Zhu X, et al. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72(6):1109–1116. [PubMed] [Google Scholar]
- 23.Jamal MM, Morgan TR. Liver disease in alcohol and hepatitis C. Best Pract Res Clin Gastroenterol. 2003;17(4):649–662. doi: 10.1016/s1521-6918(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 24.Jerrells TR, Pavlik JA, DeVasure J, et al. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41(5):357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepulveda RT, Jiang S, Besselsen DG, et al. Alcohol consumption during murine acquired immunodeficiency syndrome accentuates heart pathology due to coxsackievirus. Alcohol Alcohol. 2002;37(2):157–163. doi: 10.1093/alcalc/37.2.157. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Imrie CW, Ferguson JC, Sommerville RG. Coxsackie and mumpsvirus infection in a prospective study of acute pancreatitis. Gut. 1977;18(1):53–56. doi: 10.1136/gut.18.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrysos G, Kokkoris S, Protopsaltis J, et al. Coxsackievirus infection associated with acute pancreatitis. JOP. 2004;5(5):384–387. [PubMed] [Google Scholar]
- 29.Parenti DM, Steinberg W, Kang P. Infectious causes of acute pancreatitis. Pancreas. 1996;13(4):356–371. doi: 10.1097/00006676-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Clemens DL, Calisto LE, Sorrell MF, et al. Ethanol metabolism results in a G2/M cell-cycle arrest in recombinant Hep G2 cells. Hepatology. 2003;38(2):385–393. doi: 10.1053/jhep.2003.50332. [DOI] [PubMed] [Google Scholar]
- 31.Slack JM. Developmental biology of the pancreas. Development. 1995;121(6):1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 32.Gomez G, Englander EW, Wang G, et al. Increased expression of hypoxia-inducible factor-1alpha, p48, and the Notch signaling cascade during acute pancreatitis in mice. Pancreas. 2004;28(1):58–64. doi: 10.1097/00006676-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Clemens DL, Forman A, Jerrells TR, et al. Relationship between acetaldehyde levels and cell survival in ethanol-metabolizing hepatoma cells. Hepatology. 2002;35(5):1196–1204. doi: 10.1053/jhep.2002.32668. [DOI] [PubMed] [Google Scholar]
- 34.Koteish A, Yang S, Lin H, et al. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2002;26(11):1710–1718. doi: 10.1097/01.ALC.0000036923.77613.59. [DOI] [PubMed] [Google Scholar]
- 35.Yang SQ, Lin HZ, Yin M, et al. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol. 1998;275(4 Pt 1):G696–704. doi: 10.1152/ajpgi.1998.275.4.G696. [DOI] [PubMed] [Google Scholar]
- 36.Jung Y, Brown KD, Witek RP, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134(5):1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53(1):106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cano DA, Hebrok M. Hedgehog spikes pancreas regeneration. Gastroenterology. 2008;135(2):347–351. doi: 10.1053/j.gastro.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 39.Reid LE, Walker NI. Acinar cell apoptosis and the origin of tubular complexes in caerulein-induced pancreatitis. Int J Exp Pathol. 1999;80(4):205–215. doi: 10.1046/j.1365-2613.1999.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujikura J, Hosoda K, Kawaguchi Y, et al. Rbp-j regulates expansion of pancreatic epithelial cells and their differentiation into exocrine cells during mouse development. Dev Dyn. 2007;236(10):2779–2791. doi: 10.1002/dvdy.21310. [DOI] [PubMed] [Google Scholar]
- 41.Masui T, Long Q, Beres TM, et al. Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev. 2007;21(20):2629–2643. doi: 10.1101/gad.1575207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taguchi M, Yamaguchi T, Otsuki M. Induction of PDX-1-positive cells in the main duct during regeneration after acute necrotizing pancreatitis in rats. J Pathol. 2002;197(5):638–646. doi: 10.1002/path.1134. [DOI] [PubMed] [Google Scholar]
- 43.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580(12):2860–2868. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Apelqvist A, Li H, Sommer L, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400(6747):877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 45.Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Dig Dis. 2010;28(2):355–358. doi: 10.1159/000319414. [DOI] [PubMed] [Google Scholar]
- 46.De La OJ, Murtaugh LC. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8(12):1860–1864. doi: 10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]