Abstract
Cadherin superfamily proteins mediate cell-cell adhesion during development. The C. elegans embryo is a powerful system for analyzing how cadherins function in highly stereotyped morphogenetic events. In the embryo, the classical cadherin HMR-1 acts along with the Rac pathway and SAX-7/L1CAM during gastrulation. As adherens junctions mature, PAR complex proteins differentially regulate cadherin complex localization, and SRGP-1/Slit/Robo GAP aids adhesion by promoting membrane bending. Once adherens junctions form, actin is linked to the cell surface via HMP-1/α-catenin, whose actin binding activity is regulated in novel ways. FMI-1/Flamingo and CDH-4/Fat-like regulate axonal morphology of both pioneer and follower neurons. C. elegans thus continues to be useful for uncovering precise functions for cadherin superfamily proteins and their associates in a simple metazoan.
Keywords: cadherin, α-catenin, Flamingo, C. elegans, morphogenesis, actin, gastrulation, axon migration
C. elegans: a simple system for studying cadherins
The classical cadherin system connects cadherins to the actin cytoskeleton via β- and α-catenin to maintain tissue integrity in metazoans [1]. How the cadherin-catenin complex (CCC) is regulated and how it functions together with other adhesion systems in complicated, 3d tissue environments has important implications for understanding morphogenesis and how CCC dysfunction promotes cancer. The nematode C. elegans is a useful model for analyzing CCC function in such complex environments. It possesses a single classical cadherin, HMR-1; the short isoform, HMR-1A, forms a complex with HMP-1/α-catenin and HMP-2/β-catenin [2] in epithelia, where the HMR/HMP complex functions like the vertebrate adherens junction (AJ) to mediate cell-cell adhesion and junctional actin organization. An alternative HMR-1 isoform, HMR-1B, is expressed in neurons [3]. C. elegans also possesses conserved members of the atypical cadherin superfamily [4] (Table 1). Here we review recent work that sheds light on the CCC during gastrulation and epidermal morphogenesis in C. elegans. We also discuss recent studies implicating FMI-1/Flamingo and the Fat-like atypical cadherin, CDH-4, in axon fasciculation in the ventral nerve cord.
Table 1.
Cadherin superfamily proteins in C. elegans
| Class | C. elegans cadherin | Localization | Observed functions |
|---|---|---|---|
| Classical E-cadherin | HMR-1A | Blastomeres | ABar spindle orientation. Blastomere compaction. Ingression of Ea, Ep, and PGCs. Cleft closure. |
| Epithelia | Epidermal sheet sealing. Actomyosin force transmission. Maintenance of epidermal and intestinal tissue integrity. | ||
| Classical N-cadherin | HMR-1B | Neurons | AS, DD, and VS axon guidance. AS fasciculation. |
| Flamingo | FMI-1 | Neurons | PVP, PVQ, and HSN axon guidance and fasciculation. DD and VD axon guidance and synapse formation. |
| Fat-like | CDH-3 | Excretory cell | Excretory cell outgrowth |
| hyp10, hyp11 | Tail morphology | ||
| Anchor, arcade, F, seam, U, and uterine seam cells, VC neurons, and vulval precursors | - | ||
| CDH-4 | Neurons, vulva, spermatheca | ||
| Calsyntenin | CASY-1 | Intestine, gonadal sheath | |
| Neurons | Associative learning | ||
| Daschous? | CDH-1 | Larval viability, growth, locomotion. | |
| Dcad96Cb | CDH-9 | Pharynx | |
| Nematode-specific | CDH-5, 7, 8, 12 | ||
| CDH-10 | mc1, mc2, K, K', and seam cells |
Not alone: the cadherin complex cooperates during gastrulation
C. elegans morphogenesis begins with gastrulation, initiated by ingression of the endodermal precursor cells, Ea and Ep (Fig. 1A). Ingression of Ea and Ep is partly driven by actomyosin-mediated apical constriction, which helps to pull adjacent cells over the apical surface of Ea/Ep and displaces the pair internally [5]•. Cadherin-based adhesion appears to be involved in this process: knockdown of maternal and zygotic hmr-1 message by RNAi reduces the rate of Ea/Ep apical constriction by approximately 50% [6••]. Nevertheless, the eventual ingression of endodermal precursors in hmr-1 loss of function embryos indicates that one or more redundant systems contribute to Ea/Ep ingression. SAX-7/L1CAM has recently been identified as a component of such a system. Knockdown of hmr-1, hmp-1, or hmp-2 in sax-7 mutants results in failure of apical constriction and ingression, which may be partly due to reduced adhesion between blastomeres in these backgrounds, based on blastomere isolation experiments [7••].
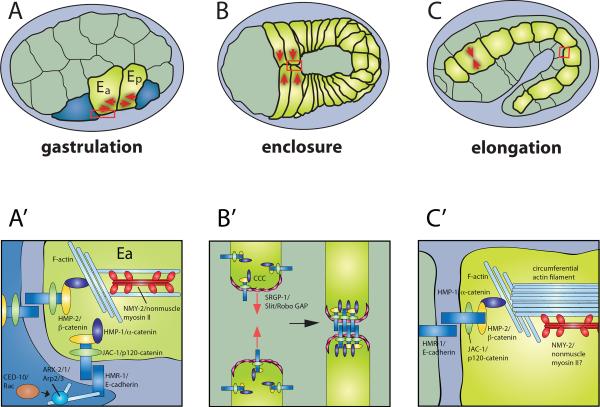
Figure 1. The classical cadherin-catenin complex (CCC) regulates multiple morphogenetic events during C. elegans embryogenesis.
Developmental stages are depicted in A – C, and postulated subcellular events in the boxed regions are depicted in A'– C'. A, A'. Gastrulation involves internalization of the endodermal precursors, Ea and Ep, via apical constriction (A, red arrows), which internalizes Ea and Ep and aids covering of the space they vacate by pulling on neighboring cells (blue). Normal apical constriction requires the CCC, as well as SAX-7/L1CAM, and components of Rac signaling. B, B'. Ventral enclosure involves migration of epithelial cells towards the ventral midline (B, red arrows). The first cells to meet at the midline (“leading cells”) rapidly make cadherin-dependent contacts, which are aided by the SRGP-1/Slit-Robo GAP. C, C'. Elongation of the embryo from a bean to a worm shape requires coordinated, actomyosin-mediated changes in the shape of epidermal cells (C, red arrows). The CCC is required for attachment of actin, including circumferential filament bundles (CFBs) to the cell surface, and hence for transmission of forces to the cell surface. HMP-1/α-catenin is particularly crucial in this process. Anterior is to the left in each panel.
Additionally, HMR-1 and SAX-7 contribute to endodermal precursor ingression by promoting apical enrichment of NMY-2/nonmuscle myosin II in Ea and Ep [7], and HMR-1 may help transmit actomyosin tension to the membrane (Fig. 1A'). In hmr-1(RNAi) endodermal precursors, membrane movements concomitant with myosin translocation are diminished [6••], similar to other systems in which actomyosin contractions are uncoupled from AJs [8,9]. Given the role of the CCC in stabilizing F-actin linkages to the AJ in other tension-driven processes [10,11], it is possible that HMR-1 contributes to stabilizing the cytoskeletal machinery required for apical constriction here as well. Consistent with this possibility, the CCC interacts genetically with the Rac pathway in endodermal precursors. Knockdown of CCC components along with Rac pathway components, including CED-5/Dock180, CED-12/ELMO, and CED-10/Rac, abrogates Ea/Ep apical constriction without loss of apical NMY-2 [6••,9]. Thus Rac may function as part of a molecular clutch, linking actomyosin contractions to the CCC within Ea/Ep. Alternatively, the CCC may synergize with Rac by providing adhesive traction for Arp2/3-dependent lamellipodia, which neighboring mesodermal precursors extend over Ea/Ep [12,13].
While the specific cells in which HMR-1-mediated adhesion is required remains to be experimentally investigated during Ea/Ep ingression, such specificity has been determined for another HMR-1-dependent internalization event, ingression of the primordial germ cells (PGCs). Following Ea/Ep ingression, (PGCs) maintain contact with endodermal daughters and are eventually drawn into the interior by the dorsal movement of the endoderm [14••]. PGC internalization requires surface enrichment of HMR-1 in the PGCs, driven post-transcriptionally via a specific element in the 3'-UTR of the hmr-1 mRNA[14••]. As HMR-1 expression in PGCs alone is both necessary and sufficient for their internalization [14••], it is possible that HMR-1 participates in heterotypic interactions in this context, but this remains to be demonstrated.
Dropping the HMR: PAR proteins and cadherin complex localization
Cadherin localization in metazoan epithelia depends on the establishment of apicobasal polarity [15], which crucially depends on PAR proteins [16,17]. While PAR-3/Bazooka and PAR-6 do not appear to affect HMR-1 localization at basolateral cell-cell contacts in the early C. elegans embryo [18], they are critical for establishing apicobasal polarity and CCC localization in epithelia. At the earliest stages of C. elegans intestinal differentiation, CCC and polarity complex proteins colocalize to foci, which then migrate and aggregate at the future apical surface [19••,20]. As apicobasal polarity is established, CCC puncta coalesce into persistent junctional belts that maintain intestinal tissue integrity. Postembryonic knockdown of CCC components results in loss of apical F-actin in the intestine and dilation of the intestinal lumen, consistent with this role [21•]. In the absence of PAR-3, intestinal HMR-1 is initially dispersed and mislocalized, whereas HMP-1 is still recruited into foci [19••]. HMP-1 localization is aberrant, however; it colocalizes with DLG-1/Discs Large [18], normally found basal to the CCC [22]. Basolateral foci of HMP-1 and DLG-1 accumulate despite unperturbed localization of LET-413/Scribble [19••], which normally excludes AJ components from basolateral surfaces [23,24]. Similar localization defects are seen in the mesendodermal epithelium of the pharynx in par-3 mutants [19••].
While PAR-3 is critical for CCC establishment in endodermal and mesodermal epithelia, it is surprisingly dispensable in the epidermis, although localization of HMR-1 and DLG-1 is progressively lost and the epidermis tears [19••]. This difference may be due to an inherent difference in tissue organization: the epidermis is in contact with the developing cuticle. PAR-3-independent apical targeting of cadherin is also seen during cellularization of the first epithelium in Drosophila [25]. In both the C. elegans intestine and the epidermis, PAR-6 is required for junctional maturation, but not targeting of CCC to initial spot junctions [20]. Curiously, apical enrichment of PAR-6 does not occur in par-3 mutants [19••]. How PAR-6 can aid junctional maturation in such mutant backgrounds but not be at its normal location is unclear.
Bending adhesions into shape: membrane curvature and cadherin-based adhesion
Morphogenesis of the C. elegans epidermis can be divided into three major events: dorsal intercalation, ventral enclosure, and elongation [26]. To date, no function for the CCC has been identified during intercalation. However, AJs must be remodeled during intercalation [27] and cadherins and catenins are key proteins in convergent extension events in other systems [28,29], so the possibility remains that functions for HMR-1 will be uncovered during intercalation when examined in an appropriately sensitized backgrounds. In contrast, the CCC is crucial for enclosure and elongation. During ventral enclosure, ventral epidermal cells extend protrusions as they migrate and initiate sealing at the ventral midline via these protrusions (Fig. 1B) [30]. Maternal and zygotic CCC knockdown results in loss of adhesion between opposing cells, failure of epidermal enclosure, and consequently the Hammerhead (Hmr) phenotype [31]. Once enclosed, the embryo elongates via actomyosin-mediated tension, which is transmitted via circumferential F-actin filament bundles (CFBs) in the epidermis [32,33] (Fig. 1C). In zygotic null mutants for hmp-1 and hmp-2, maternal mRNA carries the embryo through ventral enclosure, but mutants fail during elongation, as the embryos retract dorsally and adopt a characteristic Humpback (Hmp) phenotype [2].
Due to the mechanical stress exerted on the epidermis during elongation, its morphogenesis requires robust cell-cell adhesion. However, adhesion must also be dynamically regulated. The hypomorphic catenin alleles hmp-1(fe4) and hmp-2(qm39) display elongation defects and serve as sensitized backgrounds for identifying genetic modulators of CCC function. Such modulators include UNC-34/Ena, ZOO-1/ZO-1, and JAC-1/p120ctn [34–36]. Another is the Slit-Robo GAP homologue, SRGP-1 [37••]. Like its vertebrate counterparts, SRGP-1 contains an N-terminal F-BAR (Bin1, Amphiphysin, RVS167) domain, and a central GTPase activating (GAP) domain, which are thought to allow such proteins to regulate membrane curvature and modulate Rho family GTPase activity, respectively. srgp-1 knockdown enhances catenin hypomorphic phenotypes, and interestingly, expression of only the F-BAR domain and 200 downstream amino acids of SRGP-1 is sufficient to target the transgene to AJs and rescue hmp-2(qm39); srgp-1(RNAi) synthetic phenotypes [37••], independent of its GAP activity. In contrast, in its role during engulfment of cell corpses, SRGP-1's GAP activity is required [38].
The F-BAR domains of vertebrate srGAP2 and srGAP3 produce outward protrusions [39,40]. Similarly, overexpression of SRGP-1 induces numerous sizeable excursions of the junctional membrane, which appear to be outward bends, based on mosaic expression. These excursions contain HMR-1 and HMP-1, but not DLG-1 [37••], suggesting that SRGP-1 may contribute to adhesion by increasing the surface area of contact between adjacent cells specifically at the level of the CCC (Fig. 1B'). Consistent with this model, srgp-1(RNAi) embryos display defects in gastrulation cleft closure and a decrease in membrane dynamics at nascent contacts during ventral enclosure. BAR proteins also modulate actin dynamics [41,42•]; it remains to be determined whether SRGP-1 possesses this function as well.
Hidden talent: New insights into α-catenin regulation through HMP-1
How the cadherin-catenin complex mediates dynamic connections to the actin cytoskeleton has been a subject of considerable debate. α-catenin can bind and bundle actin at sites of E-cadherin-mediated adhesion, and β-catenin can act as a molecular bridge between cadherin and α-catenin [1,11]. Bringing α-catenin to the junction via direct fusion with cadherin rescues some adhesive functions of adhesion-defective cells, both in vitro [11] and in Drosophila [43] suggesting that the CCC can function as a quaternary complex. Such a model may not fully capture how vertebrate αE-catenin normally functions, however. αE-catenin can associate with the CCC, but does not bind actin simultaneously in vitro; to do so it forms homodimers with high affinity for F-actin [44]. Recent experiments also indicate that the vertebrate CCC can undergo mechanosensitive adhesive strengthening, which involves the actin-binding protein vinculin [45]; αE-catenin may undergo conformational changes as part of this process that allow it to bind and recruit vinculin [46].
HMP-1 provides some advantages that simplify analysis of α-catenin function in vivo. Vinculin is not present in epithelia in C. elegans, and deletion of the region homologous to the αE-catenin sequence that binds vinculin seems to have little effect on HMP-1 function (S. Maiden and J. Hardin, unpublished). Moreover, the tension-bearing mechanical requirements on HMP-1 due to the stresses of elongation are stringent (Fig. 1C, C'). Recent experiments provide unambiguous evidence that both linkage to the CCC via HMP-2 and F-actin binding are essential for HMP-1 function. Mutations that truncate HMP-1 prior to the C-terminal F-actin binding domain result in complete failure of elongation. Deletion of the N-terminal β-catenin binding domain similarly results in morphogenetic failure, with loss of recruitment of HMP-1 to junctions [47••]. Interestingly, HMP-1 appears conformationally autoinhibited in vitro: full-length HMP-1 only binds F-actin weakly in vitro, whereas C-terminal fragments bind actin avidly [47••]. Whether HMP-1 is regulated in vivo by application of tension to relieve this autoinhibition remains to be determined, but a common theme seems to be emerging that α-catenin functions as a switch through which CCC dynamics can be modulated. This pivotal function of α-catenin may reflect its evolutionary origins. Recent work in Dictyostelium suggests that an α-catenin-like molecule predates cadherin as a polarized epithelial regulator of morphogenesis [48].
Follow the leader: FMI-1/Flamingo and CDH-4/Fat-like during neuronal morphogenesis
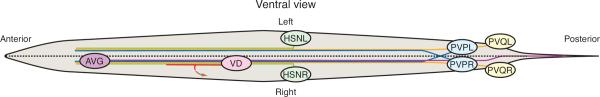
The N-cadherin homolog HMR-1B and the Fat-like atypical cadherin CDH-4 have previously been demonstrated to promote axon guidance and fasciculation of neurons in the ventral nerve cord (VNC) [3,49]. Recent experiments show that FMI-1/Flamingo is also required for these processes, perhaps through a trans interaction with CDH-4. During embryogenesis, the left VNC tract is pioneered by the right PVP neuron (PVPR), which extends an axon across the ventral midline and proceeds anteriorly to the nerve ring (Fig. 2). Subsequently, neurons such as PVQL and HSN extend axons that follow the established tract by fasciculating with the pioneer. FMI-1 contributes to both of these processes, being required for left VNC pioneering and for pathfinding and fasciculation of the PVQ and HSN axons that follow [50••].
Figure 2. The atypical cadherins FMI-1/Flamingo and CDH-4/Fat-like are required for axon guidance and synapse development.
PVP neurons reside in the pre-anal ganglion, where they embryonically extend axons that cross the ventral midline and proceed anteriorly to the nerve ring. Pioneering of the left ventral nerve cord (VNC) by PVPR requires FMI-1 and CDH-4. The right VNC is pioneered by posterior axon extension of AVG, which is unaffected by loss of either cadherin. PVQ axons originating in the lumbar ganglia extend along the ipsilateral nerve cord immediately following PVP processes. PVQL navigation requires both FMI-1 and CDH-4, while PVQR displays only a moderate requirement for FMI-1. After the L1 stage, 13 VD and 6 DD GABAergic motor neurons lie in the right VNC (one pictured), where they extend axons anteriorly and through commissures to the dorsal cord. VD and DD synapse with ventral and dorsal body wall muscles, respectively, and their axon guidance and synapse formation are dependent on both FMI-1 and CDH-4. During L2 and L3 larval stages, HSN neurons extend axons ventrally, innervating the vulva and fasciculating with the ipsilateral VNC to extend anteriorly. Both HSN neurons require FMI-1 for correct axon morphology.
The cadherin domains target FMI-1 to axons and contribute to PVQ and HSN guidance [50••], implicating FMI-1-mediated adhesion in fasciculation of followers in the VNC. Evidence from VD motor neurons in the VNC suggests that FMI-1 may interact with CDH-4 [51•], which participates in heterophilic binding in other systems [52]. Mutations in cdh-4 and fmi-1 also produce similar synaptic defects that are not enhanced in double mutants [51•], suggesting that heterophilic binding between Flamingo and Fat-like cadherins may also promote proper synapse formation at neuromuscular junctions.
Structure-function analysis also sheds light on the intracellular events downstream of FMI-1. The intracellular domain of FMI-1 is necessary for PVP pathfinding but dispensable for PVQ and HSN fasciculation [50••], suggesting that FMI-1 may have signaling activity necessary for pioneer axon guidance. Drosophila Flamingo can mediate neuronal morphogenesis through both Planar Cell Polarity (PCP) pathway-dependent and PCP-independent mechanisms [53–55]. However, there is currently no clear evidence for a full PCP pathway in C. elegans, and FMI-1 does not appear to function in a PCP-like pathway [50••,56]. This raises the possibility that further study of Flamingo cadherins in the VNC of C. elegans may shed additional light on PCP-independent mechanisms of atypical cadherin function in neuronal morphogenesis.
Conclusions
C. elegans is a powerful system for studying both classical and atypical cadherins. The ability to identify highly specific cellular behaviors that are regulated by cadherin family members and their associates should continue to make C. elegans useful for clarifying how these important cell adhesion proteins function during morphogenesis in multicellular organisms.
Highlights
-
*
HMR-1/cadherin cooperates with SAX-7/L1CAM and the Rac pathway to drive gastrulation.
-
*
PAR-3/Bazooka and PAR-6 function differently between germ layers to localize HMR-1.
-
*
SRGP-1/Slit-RoboGAP interacts with the CCC to promote epidermal morphogenesis.
-
*
HMP-1/α-catenin experiments reveal domains important for α-catenin function.
-
*
FMI-1/Flamingo promotes neural morphogenesis in conjuction with CDH-4/Fat-like.
Acknowledgements
This work was supported by NIH grant R01GM58038 awarded to JH. TJ was supported by Molecular Biosciences predoctoral training grant T32GM007215. We thank Jeremy Nance for sharing data prior to publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated References
- 1.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev. 2011;91(2):691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the caenorhabditis elegans embryo. J Cell Biol. 1998;141(1):297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broadbent ID, Pettitt J. The c. Elegans hmr-1 gene can encode a neuronal classic cadherin involved in the regulation of axon fasciculation. Curr Biol. 2002;12(1):59–63. doi: 10.1016/s0960-9822(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 4.Pettitt J. The cadherin superfamily. WormBook. 2005:1–9. doi: 10.1895/wormbook.1.50.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 5.Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: A cell shape change that can drive morphogenesis. Dev Biol. 2010;341(1):5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Performs genome-wide RNAi screening using an end-3 and ced-5/DOCK180 sensitized backgrounds to identify molecular components that act redundantly during gastrulation, including Wnt and Rac pathway components, as well as cell adhesion proteins such as HMR-1/cadherin.
- •• 6.Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, Gao L, Betzig E, Kiehart DP, Goldstein B. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335(6073):1232–1235. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that actomyosin-mediated contractions appear to occur continuously at the apex of endodermal precursors prior to their ingression, and that cell-cell adhesion, along with components of the Rac pathway, are required to engage the “clutch” that transmits forces to the plasma membrane during gastrulation.
- •• 7.Grana TM, Cox EA, Lynch AM, Hardin J. Sax-7/l1cam and hmr-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during caenorhabditis elegans gastrulation. Dev Biol. 2010;344(2):731–744. doi: 10.1016/j.ydbio.2010.05.507. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that general blastomere adhesion, as well as nonmuscle myosin II localization and apical constriction of endodermal precursors, involves the functionally redundant activity of HMR-1A/cadherin and SAX-7/L1CAM in the early embryo.
- 8.Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The drosophila afadin homologue canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186(1):57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawyer JK, Choi W, Jung KC, He L, Harris NJ, Peifer M. A contractile actomyosin network linked to adherens junctions by canoe/afadin helps drive convergent extension. Mol Biol Cell. 2011;22(14):2491–2508. doi: 10.1091/mbc.E11-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez GA, McLachlan RW, Yap AS. Productive tension: Force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21(9):499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Maiden SL, Hardin J. The secret life of alpha-catenin: Moonlighting in morphogenesis. J Cell Biol. 2011;195(4):543–552. doi: 10.1083/jcb.201103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nance J, Priess JR. Cell polarity and gastrulation in c. Elegans. Development. 2002;129(2):387–397. doi: 10.1242/dev.129.2.387. [DOI] [PubMed] [Google Scholar]
- 13.Roh-Johnson M, Goldstein B. In vivo roles for arp2/3 in cortical actin organization during c. Elegans gastrulation. J Cell Sci. 2009;122(Pt 21):3983–3993. doi: 10.1242/jcs.057562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 14.Chihara D, Nance J. An e-cadherin-mediated hitchhiking mechanism for c. Elegans germ cell internalization during gastrulation. Development. 2012;139 doi: 10.1242/dev.079863. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that primordial germ cell (PGC) ingression is driven by HMR-1/cadherin-mediated adhesion between PGCs and endodermal precursors. Demonstrates the necessity and sufficiency of HMR-1 expression in the PGCs for their internalization, and that this expression is regulated by the 3' UTR in the hmr-1 mRNA.
- 15.Lynch AM, Hardin J. The assembly and maintenance of epithelial junctions in c. Elegans. Front Biosci. 2009;14:1414–1432. doi: 10.2741/3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: Parallels and diversity. Cell. 2010;141(5):757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Nance J, Zallen JA. Elaborating polarity: Par proteins and the cytoskeleton. Development. 2011;138(5):799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 18.Nance J, Munro EM, Priess JR. C. Elegans par-3 and par-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130(22):5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]; Uses an elegant genetic approach to zygotically deplete PAR proteins in the embryo. PAR-3 is required for proper polarization and junctional assembly in the intestinal epithelium, but surprisingly, there is a less important role for PAR-3 in the epidermis. There is a separable requirement for PAR-6 in both epithelia.
- 19.Achilleos A, Wehman AM, Nance J. Par-3 mediates the initial clustering and apical localization of junction and polarity proteins during c. Elegans intestinal epithelial cell polarization. Development. 2010;137(11):1833–1842. doi: 10.1242/dev.047647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Totong R, Achilleos A, Nance J. Par-6 is required for junction formation but not apicobasal polarization in c. Elegans embryonic epithelial cells. Development. 2007;134(7):1259–1268. doi: 10.1242/dev.02833. [DOI] [PubMed] [Google Scholar]
- • 21.Bernadskaya YY, Patel FB, Hsu HT, Soto MC. Arp2/3 promotes junction formation and maintenance in the caenorhabditis elegans intestine by regulating membrane association of apical proteins. Mol Biol Cell. 2011;22(16):2886–2899. doi: 10.1091/mbc.E10-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates a role for the Arp2/3 complex in normal apical localization of junctional components in the developing intestine.
- 22.McMahon L, Legouis R, Vonesch JL, Labouesse M. Assembly of c. Elegans apical junctions involves positioning and compaction by let-413 and protein aggregation by the maguk protein dlg-1. J Cell Sci. 2001;114(Pt 12):2265–2277. doi: 10.1242/jcs.114.12.2265. [DOI] [PubMed] [Google Scholar]
- 23.Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of ajm-1 controls junctional integrity in caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3(11):983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 24.Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M. Let-413 is a basolateral protein required for the assembly of adherens junctions in caenorhabditis elegans. Nat Cell Biol. 2000;2(7):415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- 25.McGill MA, McKinley RF, Harris TJ. Independent cadherin-catenin and bazooka clusters interact to assemble adherens junctions. J Cell Biol. 2009;185(5):787–796. doi: 10.1083/jcb.200812146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chisholm AD, Hardin J. Epidermal morphogenesis. WormBook. 2005:1–22. doi: 10.1895/wormbook.1.35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams-Masson EM, Heid PJ, Lavin CA, Hardin J. The cellular mechanism of epithelial rearrangement during morphogenesis of the caenorhabditis elegans dorsal hypodermis. Dev Biol. 1998;204(1):263–276. doi: 10.1006/dbio.1998.9048. [DOI] [PubMed] [Google Scholar]
- 28.Schepis A, Sepich D, Nelson WJ. Alphae-catenin regulates cell-cell adhesion and membrane blebbing during zebrafish epiboly. Development. 2012;139(3):537–546. doi: 10.1242/dev.073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brieher WM, Gumbiner BM. Regulation of c-cadherin function during activin induced morphogenesis of xenopus animal caps. J Cell Biol. 1994;126(2):519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the c. Elegans hypodermis. Development. 1997;124(15):2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 31.Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9(20):1139–1146. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 32.Wissmann A, Ingles J, McGhee JD, Mains PE. Caenorhabditis elegans let-502 is related to rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev. 1997;11(4):409–422. doi: 10.1101/gad.11.4.409. [DOI] [PubMed] [Google Scholar]
- 33.Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: The role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117(1):156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- 34.Lockwood C, Zaidel-Bar R, Hardin J. The c. Elegans zonula occludens ortholog cooperates with the cadherin complex to recruit actin during morphogenesis. Curr Biol. 2008;18(17):1333–1337. doi: 10.1016/j.cub.2008.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The caenorhabditis elegans p120 catenin homologue, jac-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162(1):15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheffield M, Loveless T, Hardin J, Pettitt J. C. Elegans enabled exhibits novel interactions with n-wasp, abl, and cell-cell junctions. Curr Biol. 2007;17(20):1791–1796. doi: 10.1016/j.cub.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 37.Zaidel-Bar R, Joyce MJ, Lynch AM, Witte K, Audhya A, Hardin J. The f-bar domain of srgp-1 facilitates cell-cell adhesion during c. Elegans morphogenesis. J Cell Biol. 2010;191(4):761–769. doi: 10.1083/jcb.201005082. [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterizes a role for the Slit/Robo GAP, SRGP-1, during morphogenesis. SRGP-1 modulates membrane curvature at the leading edge during ventral enclosure, and aids cadherin-dependent nascent junction formation. Surprisingly, although its F-BAR domain is required, the GAP domain of SRGP-1 does not appear to be as important during these processes.
- 38.Neukomm LJ, Frei AP, Cabello J, Kinchen JM, Zaidel-Bar R, Ma Z, Haney LB, Hardin J, Ravichandran KS, Moreno S, Hengartner MO. Loss of the rhogap srgp-1 promotes the clearance of dead and injured cells in caenorhabditis elegans. Nat Cell Biol. 2011;13(1):79–86. doi: 10.1038/ncb2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A, Polleux F. The f-bar domain of srgap2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell. 2009;138(5):990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson BR, Lloyd KE, Kruszewski A, Kim IH, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC, Soderling SH. Wrp/srgap3 facilitates the initiation of spine development by an inverse f-bar domain, and its loss impairs long-term memory. J Neurosci. 2011;31(7):2447–2460. doi: 10.1523/JNEUROSCI.4433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aspenstrom P. Roles of f-bar/pch proteins in the regulation of membrane dynamics and actin reorganization. Int Rev Cell Mol Biol. 2009;272:1–31. doi: 10.1016/S1937-6448(08)01601-8. [DOI] [PubMed] [Google Scholar]
- • 42.Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG, Disanza A, Stradal TB, Cassata G, Confalonieri S, Hardin JD, et al. Requirements for f-bar proteins toca-1 and toca-2 in actin dynamics and membrane trafficking during caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet. 2009;5(10):e1000675. doi: 10.1371/journal.pgen.1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elucidates roles for the BAR proteins TOCA-1 and -2 in multiple processes in C. elegans, including in maintenance of junctional proteins in epidermal cells during morphogenesis.
- 43.Sarpal R, Pellikka M, Patel RR, Hui FY, Godt D, Tepass U. Mutational analysis supports a core role for drosophila alpha-catenin in adherens junction function. J Cell Sci. 2012;125(Pt 1):233–245. doi: 10.1242/jcs.096644. [DOI] [PubMed] [Google Scholar]
- 44.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281(47):35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates e-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin ii-dependent manner. J Cell Biol. 2010;189(7):1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. Alpha-catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12(6):533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- •• 47.Kwiatkowski AV, Maiden SL, Pokutta S, Choi HJ, Benjamin JM, Lynch AM, Nelson WJ, Weis WI, Hardin J. In vitro and in vivo reconstitution of the cadherin-catenin actin complex from caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107(33):14591–14596. doi: 10.1073/pnas.1007349107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first analysis in vivo analysis of anα-catenin loss of function mutant during dynamic morphogenetic processes in vivo. Shows that the N-terminal (β-catenin binding) and C-terminal (F-actin binding) domains are essential. In vitro experiments suggest that HMP-1 undergoes intramolecular inhibition that may regulate its ability to bind F-actin, even when associated with HMR-1A/cadherin and HMP-2/β-catenin.
- 48.Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science. 2011;331(6022):1336–1339. doi: 10.1126/science.1199633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz C, Wacker I, Hutter H. The fat-like cadherin cdh-4 controls axon fasciculation, cell migration and hypodermis and pharynx development in caenorhabditis elegans. Dev Biol. 2008;316(2):249–259. doi: 10.1016/j.ydbio.2008.01.024. [DOI] [PubMed] [Google Scholar]
- •• 50.Steimel A, Wong L, Najarro EH, Ackley BD, Garriga G, Hutter H. The flamingo ortholog fmi-1 controls pioneer-dependent navigation of follower axons in c. Elegans. Development. 2010;137(21):3663–3673. doi: 10.1242/dev.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identifies roles for FMI-1/Flamingo during neuronal migration and fasciculation in C. elegans. FMI-1 is required for follower axons to utilize pioneer axons during migration. Structure-function analysis indicates that follower axon fasciculation depends on the extracellular but not on the intracellular domain.
- • 51.Najarro EH, Wong L, Zhen M, Carpio EP, Goncharov A, Garriga G, Lundquist EA, Jin Y, Ackley BD. Caenorhabditis elegans flamingo cadherin fmi-1 regulates gabaergic neuronal development. J Neurosci. 2012;32(12):4196–4211. doi: 10.1523/JNEUROSCI.3094-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that fmi-1 mutants exhibit defective axon pathfinding and synapses. FMI-1 is required for correct morphology of ventral D-type (VD) and dorsal D-type (DD) GABAergic motorneurons, which do not express FMI-1, and instead express CDH-4. Mutations in cdh-4 causes similar defects, suggesting that both cadherin superfamily proteins are involved.
- 52.Ishiuchi T, Misaki K, Yonemura S, Takeichi M, Tanoue T. Mammalian fat and dachsous cadherins regulate apical membrane organization in the embryonic cerebral cortex. J Cell Biol. 2009;185(6):959–967. doi: 10.1083/jcb.200811030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann K, Wang M, Luu SH, Ohler S, Hakeda-Suzuki S, Suzuki T. A putative tyrosine phosphorylation site of the cell surface receptor golden goal is involved in synaptic layer selection in the visual system. Development. 2012;139(4):760–771. doi: 10.1242/dev.074104. [DOI] [PubMed] [Google Scholar]
- 54.Matsubara D, Horiuchi SY, Shimono K, Usui T, Uemura T. The seven-pass transmembrane cadherin flamingo controls dendritic self-avoidance via its binding to a lim domain protein, espinas, in drosophila sensory neurons. Genes Dev. 2011;25(18):1982–1996. doi: 10.1101/gad.16531611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mrkusich EM, Flanagan DJ, Whitington PM. The core planar cell polarity gene prickle interacts with flamingo to promote sensory axon advance in the drosophila embryo. Dev Biol. 2011;358(1):224–230. doi: 10.1016/j.ydbio.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 56.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in c. Elegans. Cell. 2007;130(4):704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]