Abstract
Aims
Multiple lines of evidence have implicated β-amyloid (Aβ) in the pathogenesis of Alzheimer’s disease (AD). However, the mechanism(s) whereby Aβ is involved in the disease process remains unclear. The dominant hypothesis in AD has been that Aβ initiates the disease via toxicity from secreted, extracellular Aβ aggregates. More recently, an alternative hypothesis has emerged focusing on a pool of Aβ that accumulates early on within AD vulnerable neurons of the brain. Although the topic of intraneuronal Aβ has been of major interest in the field, technical difficulties in detecting intraneuronal Aβ have also made this topic remarkably controversial. Here we review evidence pointing to the critical role of intraneuronal Aβ in AD and provide insights both into challenges faced in detecting intracellular Aβ and the prion-like properties of Aβ.
Main methods
Immunoprecipitation and Western blot are used for Aβ detection.
Key findings
We highlight that a standard biochemical method can underestimate intraneuronal Aβ and that extracellular Aβ can up-regulate intracellular Aβ. We also show that detergent can remove intraneuronal Aβ.
Significance
There is a growing awareness that intraneuronal Aβ is a key pathogenic pool of Aβ involved in causing synapse dysfunction. Difficulties in detecting intraneuronal Aβ are an insufficient reason for ignoring this critical pool of Aβ.
Keywords: amyloid, Alzheimer’s disease, intraneuronal, prion, detergent
Introduction
The progressive and anatomically selective accumulation of β-amyloid (Aβ) peptides is a hallmark of Alzheimer’s disease (AD) neuropathology, and reduction of Aβ is the leading target of ongoing experimental therapies for AD. Aβ is normally processed from the amyloid precursor protein (APP) by three major cleavages: first by either α- or β-secretase, and then by α-secretase. In non-neuronal cells α-secretase cleavage predominates, whereas in neurons β-cleavage predominates (Gouras et al., 1998). Despite being among the most studied peptides/proteins, the mechanism(s) whereby Aβ/APP is involved in AD has remained obscure. The leading hypothesis, the amyloid cascade hypothesis, has posited that progressive secretion of Aβ into the extracellular space with aging leads to progressive Aβ aggregation first to soluble Aβ oligomers and then into the insoluble fibrils found in plaques (Hardy and Allsop, 1991; Hardy and Selkoe, 2002). These extracellular assemblies of Aβ are then thought to directly damage surrounding synapses and neurons with the development of AD. A modified version of the amyloid cascade hypothesis, based on the aberrant intraneuronal accumulation of Aβ, has had a remarkably difficult time in challenging the traditional amyloid hypothesis (Wirths et al., 2004; Gouras et al., 2010). One major reason has been the difficulty in analyzing the highly insoluble and self-aggregating Aβ peptides accumulating within neurons.
Following the identification of Aβ in plaques, a role for intracellular Aβ was hypothesized to be important (Grundke-Iqbal et al., 1989). Although the fact that the antibodies directed at Aβ also reacted to its normally much more abundant precursor protein APP, precluded immunohistochemical evidence for its presence within neurons of the brain. Intracellular Aβ was first shown in cell lysates by Western blot (Wertkin et al., 1993). The subsequent development of C-terminal end-specific Aβ40 and Aβ42 antibodies allowed for the separation of Aβ from APP immunolabeling. Remarkably, early intraneuronal Aβ accumulation was first seen to occur in AD-vulnerable neurons in human brains with Down syndrome even before plaque deposition (Gouras et al., 2000; Gyure et al., 2001; Busciglio et al., 2002; Mori et al., 2002; Cataldo et al., 2004). Intraneuronal Aβ was also detected in postmortem brain tissue in mild cognitive impairment and AD (Gouras et al., 2000; D'Andrea et al., 2001; Ohyagi et al., 2005), as well as in AD transgenic models (Wirths et al., 2001; Takahashi et al., 2002; Oddo et al., 2003; Sheng et al., 2003; Echeverria et al., 2004; Lord et al., 2006; Knobloch et al., 2007; Levi et al., 2007; Espana et al., 2010; Gandy et al., 2010). This Aβ accumulation in AD vulnerable neurons preceded the presence of hyperphosphorylated tau. Remarkably, it was typically Aβ42/43 that was most pronounced within AD vulnerable neurons. Aβ40 had been viewed as the predominant form of Aβ, because in conditioned media of cells it represented the vast majority of Aβ compared to the more highly aggregation prone Aβ42/43. Curiously, already the early light microscopy studies on intraneuronal Aβ42 noted a subsequent decline of Aβ42 accumulation in soma of neurons (after the initial increases prior to the appearance of plaques) with progressive plaque pathology.
The slow acceptance of intraneuronal Aβ in AD pathogenesis has hinged on several factors. Foremost among these appears to be that the extracellular Aβ hypothesis is entrenched; it has been difficult to consider a potential role for an intracellular pool of Aβ in a disease characterized by extracellular plaques. Another factor that contributed is that immunohistochemical detection of intraneuronal Aβ is tricky. For one, common antibodies sold as directed at “β-amyloid” typically also react to the Aβ domain that is present in its normally much more abundant precursor APP. Thus, it is important to use end-specific antibodies that react to Aβ but not to APP such as C-terminal Aβ42 specific antibodies. Alternatively, antibodies directed at the N-terminus of β-amyloid that do not cross-react with full-length APP can be used, although it is possible here that such N-terminal antibodies may cross-react with the same N-terminus found in the APP C-terminal fragment C99 that is generated following initial cleavage by the beta-site APP cleaving enzyme (BACE) 1 (Vassar et al., 1999).
Another difficulty for the acceptance of intraneuronal Aβ is that even when specific Aβ antibodies and appropriate controls are used, immunohistochemistry is not the leading method in AD research given the growing emphasis on molecular and cellular biology over the past few decades, and the biochemical characterization of intraneuronal Aβ has lagged behind. Why has this been the case? As a low molecular weight hydrophobic and aggregation-prone peptide Aβ is notoriously difficult to detect and work with. Since part of the Aβ domain within APP is normally within the membrane, it might be expected to be membrane associated, which could contribute to making it more difficult to detect. Here we provide results emphasizing the importance and also the challenges in detecting this intraneuronal pool of Aβ and provide further insights into the prion-like characteristics of Aβ peptides.
Materials and Methods
Cell culture
Mouse N2a neuroblastoma cells either untransfected (N2a) or stably transfected with the wild-type human APP (WT) or the 670/671 Swedish mutation human APP (SWE) were grown on 10 cm dishes (Thinakaran et al., 1996).
Antibodies
Well-characterized Aβ/APP antibodies: monoclonal 6E10 (human Aβ residues 4–9; IgG1) and 4G8 (Aβ residues 17–24; IgG2B) (Signet Laboratories); Aβ42 C-terminal specific antibody: 12F4 (Covance). Secondary antibodies: anti-mouse horseradish peroxidase (HRP)-conjugated (Pierce), rabbit anti-mouse secondary antibody (Cappell).
Treatments
Trypsin treatment: SWE N2a cells were washed with ice-cold phosphate buffered saline (PBS) and incubated for 20 min with either PBS (control, CTRL) or 10 μg/ml trypsin on ice.
Saponin and triton X treatment: SWE N2a cells were washed with ice-cold PBS and incubated for 5 min with, PBS (control, CTRL), 0,1% saponin or 0,1% triton X-100 on ice.
Cells were then washed, collected in ice-cold PBS, and centrifuged. Pellets were lysed and used for Western blot as described in the next paragraph.
Media treatment: WT and SWE N2a cell media were collected from dishes containing respective confluent cells. Two 65% confluent WT cell dishes had their media removed and replaced with either WT or SWE medium; one 65% confluent N2a dish also had its medium removed and replaced with SWE medium. Cells were incubated for 24 hours. Cells were then washed and collected for Western blot as described in the following paragraph.
Aβ immunoprecipitation and Western blot
Cells were washed twice, harvested in ice-cold PBS, and centrifuged. Cells were lysed in 6% SDS containing 10 μl/ml of β-mercaptoethanol, sonicated, and then heated at 95°C for 6 min. After centrifugation, supernatants were transferred into new tubes. Protein concentration of supernatants was measured with BCA Protein kit (Pierce); samples were then used for either direct load Western blot or immunoprecipitation (IP) followed by Western blot.
IP: samples were immunoprecipitated overnight at 4°C with 4G8 antibody (12μg/ml), a rabbit anti-mouse secondary antibody together with protein A-sepharose beads (Pharmacia) in IP buffer containing: 190 mM NaCl, 50 mM Tris-HCl pH 8.3, 6 mM EDTA and 2.5% triton X-100. Beads were then washed, boiled for 5 min in Tricine loading buffer and loaded into 10–20% Tricine gels (Invitrogen) for Aβ detection. Samples were subjected to electrophoresis and transferred to polyvinylidine difluoride membranes (Millipore). Membranes were boiled in PBS for 5 min, incubated 30 min in PBS containing 0.1% Tween-20 (PBST) and 5% milk, and immunoblotted overnight at 4°C with 6E10 antibody (2μg/ml) diluted in PBST and 5% milk. Membranes were washed with PBST, and incubated at room temperature with a secondary HRP-conjugated antibody. The immunoreaction was visualized by a chemiluminescence system (Pierce). Bands were quantified by using Scion Imaging (National Institutes of Health).
Metabolic labeling
N2a cells were plated in 6 cm dishes. 80% confluent cultures were used, and after 30 min starvation in cysteine/methionine free medium (Invitrogen), cells were pulsed for 12 hours in fresh cysteine/methionine free medium with 1 mCi [35S] methionine/cysteine (Perkin Elmer) in the presence of 0, 10 or 25 μM human Aβ1–42 (Tocris, prepared as previously described (Tampellini et al., 2009). Cells were collected in ice-cold PBS and lysed. Samples were immunoprecipitated with Aβ antibody 4G8. [35S] signal was visualized using a phosphoimager system (Hewlett-Packard Cyclone).
Statistical analysis
Statistical comparisons were made using unpaired t tests with significance placed at p<0.05. Data were expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 3.0 software (Graph Pad Software).
Results
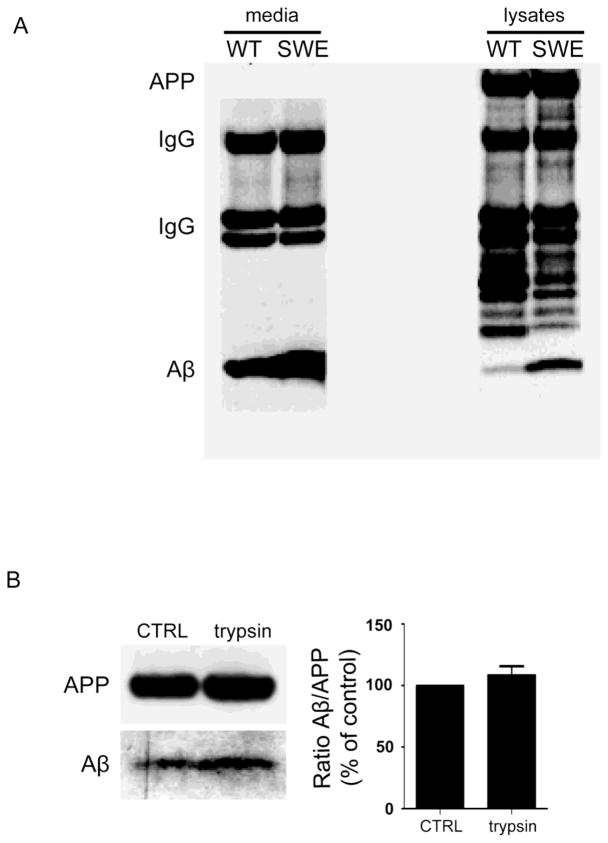
It is well established that familial AD (FAD) mutations in APP or presenilin (PS) can increase the levels of Aβ in the conditioned media of clonal cells (Citron et al., 1992; Borchelt et al., 1996). Less well known is that such FAD mutations also induce an increase of Aβ within cells, which is proportionally even greater than the increase in the medium, as was previously reported using ELISA (Wild-Bode et al., 1997). This proportionally greater elevation of intracellular Aβ with a FAD mutation is also evident on Western blot of cell lysate compared to media from N2a cells harboring the APP Swedish mutation (Fig. 1A). Interestingly, more recent studies have reported that some familial mutations linked to AD, such as the APP E693Delta mutation, do not even increase levels of Aβ peptides in the conditioned media (Shioi et al., 2007; Tomiyama et al., 2008). Interestingly, transgenic mice over-expressing the APP E693Delta mutation, associated with a clinical phenotype identical to AD in patients harboring this mutation, develop only intraneuronal Aβ accumulation, oligomerization and neurodegeneration but no plaques (Tomiyama et al., 2010).
Fig. 1. Greater relative increase of Aβ in cell lysate compared to conditioned media of Swedish mutant APP compared to wild-type human APP expressing N2a cells.
A. Representative blot of Aβ (lowest molecular weight bands) showing a greater relative increase in cell lysates (right blot) compared to conditioned media (left blot) in Swedish mutant human APP (“SWE”) compared to wild-type human APP (“WT”) expressing N2a cells using IP and Western blot for Aβ detection. Note the equivalent amounts of full-length APP in cell lysates, which also provides evidence that cell confluence was similar for the conditioned media. 4G8 antibody was used for immunoprecipitation; 6E10 antibody was used for immunoblotting. B. Lack of decrease in Aβ levels using direct load Western blot of lysates from Swedish mutant APP N2a cells after trypsin treatment, which is similar to untreated cells (CTRL), supports the intracellular localization of this Aβ. n=3.
As was previously shown for differentiated neuronal NT2N cells (Turner et al., 1996), pretreatment of cells with trypsin on ice before harvesting confirmed that the intracellular pool of Aβ is not attached to the extracellular cell surface (Fig. 1B). To highlight the discrepancy between the relative high intensity of intracellular Aβ labeling inside cells using immunofluorescence microscopy (Almeida et al., 2006) compared to the difficulty in detecting Aβ with a common biochemical detection method, we spiked cell lysates and conditioned media with known amounts of synthetic human Aβ1–42 and evaluated how efficiently the added amount could be detected. Using immunoprecipitation (IP) followed by Western blotting, a common method used to detect Aβ in cells, we show that only a small fraction of the added synthetic Aβ1–42 was retrieved from cell lysates, media and also IP buffer using these standard methods (Fig. 2A), although this may be a general problem with IP/Western detection. To underscore how a standard method can affect levels of cellular Aβ, we show that a commonly used detergent can alter levels of cell-associated Aβ. Specifically, treatment of cells with a detergent such as triton X, commonly used for solubilization, permeabilization, and cell lysis, induced loss of both Aβ and APP. Therefore, detergents need to be used with care, and the use of milder detergents, such as saponin, may be preferable for some assays (Fig. 2B).
Fig. 2. Biochemical methods can underestimate intracellular levels of Aβ.
A. Aβ immunoprecipitation underestimates Aβ. The Aβ signal coming from the lane with direct load of synthetic Aβ1–42 (lane 1) is much higher than the signal coming from the lanes where synthetic Aβ1–42 was added to lysate (lane 4), media (lane 6) or IP buffer (lane 7) and then immunoprecipitated (MWM, molecular weight markers). 4G8 antibody was used for immunoprecipitation; 6E10 antibody was used for immunoblotting. n=3, **p<0.01 B. Treatment of N2a cells over-expressing the human APP Swedish mutation with triton X induces loss of Aβ and APP, as assessed by direct load Western blot. n=3, *p<0.05.
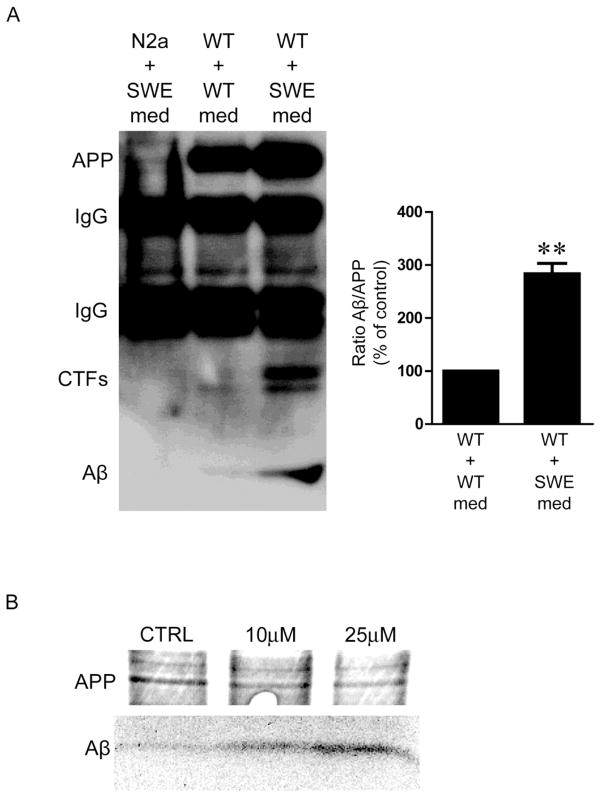
Increasing evidence supports an intriguing common theme among neurodegenerative diseases of aging, where disease-linked proteins can spread by seemingly prion-like mechanism in brain, the biology of which remains poorly defined. Studies have shown that addition of brain extracts containing Aβ can propagate Aβ pathology in susceptible host brains such as of AD transgenic mice (Kane et al., 2000). Although rarely discussed in the growing literature on Aβ spread in the brain, previous cellular work had shown that addition of extracellular Aβ1–42 leads to marked up-regulation of newly generated Aβ in human embryonic kidney 293 (HEK293) cells by metabolic labeling (Yang et al., 1999) and in primary neurons by immunofluorescence microscopy (Tampellini et al., 2009). In fact, we previously reported that the synapse-altering effect of extracellular Aβ1–42 was prevented in APP knockout neurons or in wild-type neurons concomitantly treated with an inhibitor of Aβ generation (Tampellini et al., 2009). Here we show additional evidence of extracellular Aβ1–42 induced up-regulation of Aβ in cells by IP and Western blot (Fig. 3A) and by metabolic labeling (Fig. 3B). Addition of conditioned medium from N2a cells harboring the Swedish APP mutation (with elevated levels of Aβ) for 24 hours enhanced generation of intracellular Aβ in wild-type cells (Fig. 3A, right lane) compared to treatment with wild-type N2a medium (Fig. 3A, center lane). The majority of the detected Aβ is not likely to be internalized from the media, or an Aβ band would have been expected in the Western blot of the untransfected N2a cell lysate (Fig. 3A, left lane). The higher levels of β-CTFs seen in Figure 3A fit with the increase of intracellular Aβ observed upon addition of conditioned medium to wild-type N2a cells. The mechanism(s) for this APP CTF increase are unclear, although it could be from reduced degradation and/or augmented amyloidogenic APP processing from, for example, increased APP endocytosis and/or elevated BACE1 activity.
Fig. 3. Addition of Aβ in the cell medium induces generation of Aβ inside cells.
A. Addition of conditioned medium from Swedish mutant APP N2a cells (SWE, right lane) induces an increase of intracellular Aβ in wild-type (WT) cells compared to medium from wild-type cells (center lane). The detected Aβ is not likely to primarily come from the medium via internalization, since N2a cells treated with the SWE medium do not show a band (left lane). 4G8 antibody was used for immunoprecipitation; 6E10 antibody was used for immunoblotting. n=3, **p<0.01. B. Metabolic labeling demonstrates an increase in newly generated Aβ in Swedish mutant APP N2a cells in proportion to the concentration of synthetic human Aβ1–42 (10 or 25μM) added to the medium.
To further verify that the intracellular Aβ detected is not the one added to the cells, we performed metabolic labeling. Fig. 3B is a representative Western blot highlighting that the increase in newly generated Aβ in N2a cells expressing the APP Swedish mutation is proportional to the concentration of synthetic human Aβ1–42 that was added to the medium. Overall, these biological insights of how extracellular Aβ can up-regulate endogenous Aβ production by cells have implications for the cellular mechanism of the prion-like transmission of Aβ, which has been mainly studied using in vivo models (Jucker and Walker, 2011).
Discussion
Aβ is one among numerous factors that is likely to play an important role in AD. However, the mechanism(s) whereby Aβ is involved in the disease process remains a leading unanswered question in AD research. Aβ has attained a central role in AD, because converging evidence has pointed to its involvement. These include genetic linkage in families afflicted by rare autosomal dominant forms of AD, and its presence in the characteristic neuropathological lesions of AD. Extensive resources have been allocated to studying Aβ over the past few decades and have underscored its relevance in AD, such as the recent finding showing that declining Aβ42 levels in the cerebrospinal fluid (CSF) is the best biomarker for predicting the development of AD that can precede clinical symptoms by a decade (Buchhave et al., 2012). Therefore, it is imperative for the field to continue to work on the molecular underpinnings of AD, which includes elucidating the more precise pathogenic role of Aβ.
The established amyloid hypothesis of AD needs to be further modified to consider the importance of Aβ and its aggregated forms not only in the extracellular space, but also within neurons (Takahashi et al., 2004; Capetillo-Zarate et al., 2011). Intraneuronal Aβ could have critical implications for Aβ based therapy. Given recent failures in clinical trials targeting Aβ, a turn to alternative targets may neglect this important upstream target that may have been approached incorrectly. Amyloid plaques were long known to be a poor correlate of cognitive decline in AD, but only recent work has shown that they may even be misleading indicators of disease progression. The brains of patients who took part in an aborted active vaccine clinical trial and reached autopsy showed disappearance of plaques, while they continued to decline cognitively (Holmes et al., 2008). In vivo studies in AD transgenic mice showed that in the setting of chronic inhibition of synaptic activity, synapses were damaged and cognitive function further impaired, despite a decrease in plaques but with a concomitant increase in intraneuronal Aβ (Tampellini et al., 2010). Thus, in this work AD-like synapse damage tracked with intraneuronal Aβ and not at all with plaques. Remarkably, more recent data showed that neurons derived from APP mutant transgenic mice, but not wild type mice, showed declines in Aβ secretion with time in culture, concomitant with a build-up of intraneuronal Aβ (Tampellini et al., 2011). The declining levels of extracellular Aβ in brains of APP mutant mice with aging assayed by in vivo microdialysis (Hong et al., 2011) could support such declining secretion. It is important to note that the studies from the 1990s highlighting elevations of secreted Aβ with FAD mutations using clonal cell lines looked only at one time point, and thereby failed to consider the importance of aging. One should also keep in mind that patients harboring FAD mutations are fine when they are young (and presumably secreting elevated amounts of Aβ). It is only with aging that they develop a phenotype. Thus, FAD mutations, such as the Swedish mutation, could initially raise both secreted and intracellular Aβ, but then, with time/aging, secretion could decline while intraneuronal Aβ rises, leading to the disease phenotype.
A series of important recent studies on intraneuronal Aβ in human brains provided much needed biochemical support for the importance of this pool of Aβ42 in AD (Aoki et al., 2008; Hashimoto et al., 2010). Elucidating the biological mechanism(s) whereby intraneuronal Aβ is involved in pathogenesis is therefore of key importance (Gouras et al., 2010). Importantly, intraneuronal Aβ has been correlated with neuron cell death in transgenic mouse models (Bayer and Wirths, 2010). In the 5×AD transgenic mouse thioflavin S positive amyloid could also be detected inside degenerating neuron cell bodies (Oakley et al., 2006). Thus, a subset of plaques can arise directly from cell bodies, consistent with observations in prior studies on human AD brains (Gouras et al., 2000; D'Andrea et al., 2001). On the other hand, Aβ42 immuno-electron microscopy has emphasized the importance of distal neurites and synaptic compartments in the intraneuronal build-up and oligomerization of Aβ (Takahashi et al., 2002; Takahashi et al., 2004; Takahashi et al., 2010). A recent high-resolution immuno-fluorescent study revealed Aβ fibrils within vulnerable neurites and even isolated spines in brains of AD transgenic mice, supporting that plaques can also develop from neurites (Capetillo-Zarate et al., 2011). That the most prominent Aβ increases are in neurites and synapses rather than in neuron soma, as well as loss of Aβ antibody reactivity with Aβ aggregation, likely contribute to why initial observations noted declining intraneuronal Aβ42 labeling in AD vulnerable neuron cell bodies; for example, standard C-terminal end specific Aβ42 antibodies react mainly to monomers (Takahashi et al., 2004). An additional issue is that dystrophic neurites in AD also show considerable APP accumulation.
Here we highlighted technical challenges in detecting, intracellular Aβ using standard biochemical assays. Although we have focused on intraneuronal Aβ, there is considerable experimental evidence that extracellular Aβ plays a role in the disease. Plaques do appear to act as a reservoir for soluble extracellular Aβ (Hong et al., 2011), internalization of Aβ by neurons occurs (Saavedra et al., 2007), and extracellular Aβ1–42 can up-regulate intraneuronal Aβ42 (Yang et al., 1999; Tampellini et al., 2009).
The effects of extracellular on intracellular Aβ likely plays a critical role in the prion-like progression of AD pathology in the brain, although it has not received attention in the many studies defining Aβ propagation in brains of AD transgenic mice. The precise molecular mechanism whereby extracellular Aβ could impact intracellular Aβ remains to be determined. Evidence supports that at least some extracellular Aβ is taken up by neurons (Saavedra et al., 2007). One could speculate that more aggregated oligomeric Aβ might then act as a seed for aggregation of the Aβ already present in the host neuron, which in turn somehow can lead to up-regulation of de novo Aβ production and further local intraneuronal accumulation and aggregation. The mechanism whereby then such a neuron with elevated Aβ oligomers propagates aggregated Aβ to another neuron might be via transport down neurites of AD vulnerable anatomical circuits, followed by release by, for example, exosomes (Rajendran and Annaert, 2012) near synapses for internalization by the next neuron.
Conclusions
Here we provided insights both on the complexity of assaying intracellular Aβ and the prion-like transmission of extracellular to intracellular Aβ, while stressing the importance of intraneuronal Aβ in the pathogenesis of AD.
Acknowledgments
This study was supported by MultiPark and the Swedish Research Council. We thank Drs. Gopal Thinakaran and Sangram Sisodia at the University of Chicago for sharing their stably transfected human APP N2a cells.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida CG, Takahashi RH, Gouras GK. J Neurosci. 2006;26(16):4277–88. doi: 10.1523/JNEUROSCI.5078-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M, Volkmann I, Tjernberg LO, Winblad B, Bogdanovic N. Neuroreport. 2008;19(11):1085–9. doi: 10.1097/WNR.0b013e328302c858. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Frontiers in Aging Neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Neuron. 1996;17(5):1005–13. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Arch Gen Psychiatry. 2012;69(1):98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Neuron. 2002;33(5):677–88. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- Capetillo-Zarate E, Gracia L, Yu F, Banfelder JR, Lin MT, Tampellini D, Gouras GK. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Neurobiol Aging. 2004;25(10):1263–72. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Nature. 1992;360(6405):672–4. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Histopathology. 2001;38(2):120–34. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Echeverria V, Ducatenzeiler A, Dowd E, Janne J, Grant SM, Szyf M, Wandosell F, Avila J, Grimm H, Dunnett SB, Hartmann T, Alhonen L, Cuello AC. Neuroscience. 2004;129(3):583–92. doi: 10.1016/j.neuroscience.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Espana J, Gimenez-Llort L, Valero J, Minano A, Rabano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. Biol Psychiatry. 2010;67(6):513–21. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Gandy S, Simon AJ, Steele JW, Lublin AL, Lah JJ, Walker LC, Levey AI, Krafft GA, Levy E, Checler F, Glabe C, Bilker WB, Abel T, Schmeidler J, Ehrlich ME. Ann Neurol. 2010;68(2):220–30. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Acta Neuropathol. 2010;119(5):523–41. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Am J Pathol. 2000;156(1):15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Jovanovic JN, Buxbaum JD, Wang R, Greengard P, Relkin NR, Gandy S. J Neurochem. 1998;71(5):1920–5. doi: 10.1046/j.1471-4159.1998.71051920.x. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, George L, Tung YC, Kim KS, Wisniewski HM. Proc Natl Acad Sci U S A. 1989;86(8):2853–7. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Arch Pathol Lab Med. 2001;125(4):489–92. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Trends Pharmacol Sci. 1991;12(10):383–8. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. Science. 2002;297(5580):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Bogdanovic N, Volkmann I, Aoki M, Winblad B, Tjernberg LO. Acta Neuropathol. 2010;119(5):543–54. doi: 10.1007/s00401-010-0661-6. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Lancet. 2008;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. J Neurosci. 2011;31(44):15861–9. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Ann Neurol. 2011;70(4):532–40. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. J Neurosci. 2000;20(10):3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Neurobiol Aging. 2007;28(9):1297–306. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Levi O, Dolev I, Belinson H, Michaelson DM. J Neurochem. 2007;103(3):1031–40. doi: 10.1111/j.1471-4159.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. Neurobiol Aging. 2006;27(1):67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Amyloid. 2002;9(2):88–102. [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. J Neurosci. 2006;26(40):10129–40. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Neuron. 2003;39(3):409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. FASEB J. 2005;19(2):255–7. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Annaert W. Traffic. 2012;13(6):759–70. doi: 10.1111/j.1600-0854.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP. J Biol Chem. 2007;282(49):35722–32. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Neurobiol Dis. 2003;14(1):133–45. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, Baki L, Robakis NK. J Neurochem. 2007;101(3):674–81. doi: 10.1111/j.1471-4159.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. J Neurosci. 2004;24(14):3592–9. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Capetillo-Zarate E, Lin MT, Milner TA, Gouras GK. Neurobiol Aging. 2010;31(7):1145–52. doi: 10.1016/j.neurobiolaging.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Am J Pathol. 2002;161(5):1869–79. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK. J Neurosci. 2010;30(43):14299–304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, Lin MT, Gouras GK. J Neurosci. 2009;29(31):9704–13. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Lin MT, Capetillo-Zarate E, Gouras GK. J Neurosci. 2011;31(43):15384–90. doi: 10.1523/JNEUROSCI.2986-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. J Biol Chem. 1996;271(16):9390–7. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T, Nishitsuji K, Ito K, Shimada H, Lambert MP, Klein WL, Mori H. J Neurosci. 2010;30(14):4845–56. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H. Ann Neurol. 2008;63(3):377–87. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Turner RS, Suzuki N, Chyung AS, Younkin SG, Lee VM. J Biol Chem. 1996;271(15):8966–70. doi: 10.1074/jbc.271.15.8966. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Wertkin AM, Turner RS, Pleasure SJ, Golde TE, Younkin SG, Trojanowski JQ, Lee VM. Proc Natl Acad Sci U S A. 1993;90(20):9513–7. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C. J Biol Chem. 1997;272(26):16085–8. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Bayer TA. J Neurochem. 2004;91(3):513–20. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Neurosci Lett. 2001;306(1–2):116–20. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Yang AJ, Chandswangbhuvana D, Shu T, Henschen A, Glabe CG. J Biol Chem. 1999;274(29):20650–6. doi: 10.1074/jbc.274.29.20650. [DOI] [PubMed] [Google Scholar]