Abstract
Children with autism spectrum disorder (ASD) who have native exposure to a sign language such as American Sign Language (ASL) have received almost no scientific attention. This paper reports the first studies on a sample of five native-signing children (four deaf children of deaf parents and one hearing child of deaf parents; ages 4;6 to 7;5) diagnosed with ASD. A domain-general deficit in the ability of children with ASD to replicate the gestures of others is hypothesized to be a source of palm orientation reversal errors in sign. In Study 1, naturalistic language samples were collected from three native-signing children with ASD and were analyzed for errors in handshape, location, movement and palm orientation. In Study 2, four native-signing children with ASD were compared to 12 typically-developing deaf children (ages 3;7 to 6;9, all born to deaf parents) on a fingerspelling task. In both studies children with ASD showed a tendency to reverse palm orientation on signs specified for inward/outward orientation. Typically-developing deaf children did not produce any such errors in palm orientation. We conclude that this kind of palm reversal has a perceptual rather than a motoric source, and is further evidence of a “self-other mapping” deficit in ASD.
Keywords: Autism spectrum disorder, sign language
1. Introduction
Autism spectrum disorder (ASD) consists of a set of neurobiological developmental disorders characterized by communicative and social deficits as well as repetitive, stereotyped behaviors. It is estimated that ASD affects approximately 1 in 88 children in the United States (Centers for Disease Control, 2012). The prevalence of congenital deafness is much lower, estimated at 1 to 6 per 1,000 newborns (Cunningham & Cox, 2003; Kemper & Downs, 2000). However, ASD appears to be more frequent in the deaf population than in the general population; Szymanski, Brice, Lam, and Hotto (2012) reported that 1 in 59 deaf or hard of hearing children in the 2009–2010 Annual Survey of Deaf and Hard of Hearing Children and Youth (Gallaudet Research Institute, 2011) carried an ASD diagnosis. At least two other studies have found that a higher-than-expected percentage of deaf children in their samples had ASD (7%: Chess, Fernandez, & Korn, 1978; 4%: Jure, Rapin, & Tuchman, 1991). Conversely, there is evidence that severe hearing loss occurs in the ASD population at a rate of about ten times that in the general population (Rosenhall, Nordin, Sandström, Ahlsén, & Gillberg, 1999).
Despite the fact that language deficits form one of the core symptoms of ASD, very little research has investigated the sign language development of deaf children with ASD. Most prior studies on signing and ASD focused on the teaching of signs to hearing children with severe ASD and little speech (for reviews, see Bonvillian, Nelson, & Rhyne, 1981; Carr, 1979). Some, but not all, nonverbal hearing children with ASD were able to learn and use some manual signs, even when prior speech training had failed (e.g., Fulwiler & Fouts, 1976). However, most of these children did not progress beyond the use of a small number of signs. Moreover, the sign vocabulary sizes claimed in the literature may be considerably overstated, as most signs trained to criterion in such studies were not observed outside training sessions in spontaneous usage (Bonvillian & Blackburn, 1991: 276). Previous work with hearing children has also examined the relationship between motor deficits in ASD and sign acquisition. In a study on 14 nonverbal hearing students with severe ASD (mean age 13;8), Seal and Bonvillian (1997) found that sign formation accuracy was highly correlated with fine motor age and apraxia, suggesting that sign formation errors could in part be a result of underlying motor deficits.
Just as most research on the use of sign language in ASD has focused on hearing children, most research on the acquisition of signed languages has focused on typically-developing deaf children (for summaries, see Emmorey, 2002; Newport & Meier, 1986). The scant literature on the signing of deaf people with ASD includes a brief report of a single 21-year-old deaf signer with autism, whose echolalia was contrasted with the agrammatism of a signer with Broca's aphasia (Poizner, Klima, & Bellugi, 1990). Morgan and colleagues have reported extensively on the acquisition of British Sign Language (BSL) by Christopher, a hearing language savant who lacks an ASD diagnosis but exhibits characteristics typical of autism (Morgan, Smith, Tsimpli, & Woll, 2002, 2007; Smith, Tsimpli, Morgan, & Woll, 2011). Recently, Denmark (2011) investigated the comprehension and production of affective and linguistic facial expression in deaf British signers with ASD.
The sign language of children with ASD is of theoretical interest because sign language acquisition depends crucially on social and cognitive skills known to be impaired in ASD. For example, children with ASD are impaired in their comprehension of facial expressions (Grossman & Tager-Flusberg, 2008; Lacroix, Guidetti, Rogé, & Reilly, 2009; Rump, Giovannelli, Minshew, & Strauss, 2009; Volker, Lopata, Smith, & Thomeer, 2009), yet facial expressions signal questions, conditionals, and other aspects of sign language grammar (Liddell, 1980). The imitation of signs in three-dimensional space could also pose problems for children with ASD, whose deficits in theory of mind and/or perspective-taking (e.g., Baron-Cohen, Leslie, & Frith, 1985; Hamilton, Brindley, & Frith, 2009) could impede the recognition and reproduction of signs viewed from varying angles in everyday life.
Previous work (Williams, Whiten, & Singh, 2004 for a review) has found that hearing children with ASD are impaired in their ability to imitate the body movements of others; this impairment might have particular bearing on the acquisition of sign by children with ASD. A compelling finding from a number of studies is that hearing children with ASD tend to reverse the direction of observed palm orientations when imitating the gestures of others. Ohta (1987) was the first to report such errors (which he called “partial imitations”): children with ASD often imitated a wave-like gesture (in which the experimenter's open palm was oriented toward the child) with their palms facing inward toward themselves. In another study, Smith and Bryson (1998) found that, in the imitation of 8 ASL handshapes and 8 bimanual gestures, hearing children with ASD made significantly more 180° reversal errors (e.g., palm toward the viewer rather than away from him) than age-matched language-impaired and typically-developing children. These reversed palm orientations suggest that children with ASD imitate gestures as they appear from their own perspective. Williams et al. conclude that such errors are evidence of an autism-specific deficit in “self–other mapping,” that is, a deficit in the process(es) by which children and adults observe the movements of others and map them onto their own bodies (Rogers & Pennington, 1991).
The acquisition of signs could likewise be affected by a deficit in self-other mapping, inasmuch as the learning of signs entails the imitation of the bodily movements of others. Specifically, signs in ASL that are specified for an inward or outward palm orientation, for example tuesday1 and bathroom (Figure 1), could exhibit reversal errors. The sign tuesday is produced with the palm facing inward toward the signer's body; a child facing the signer sees the back of the signer's hand. If that child reproduced what he saw from his own perspective, the child would articulate the sign with his palm facing away from his body; as a result, the child would see the back of his own hand. Interestingly, such a reversal could lead to lexical confusion in ASL, since the signs tuesday and bathroom are near-minimal pairs that differ primarily in the direction the palm faces. Not all signs pose this problem: we would not predict palm orientation errors on signs with the palm facing up or down, for example, since an upward facing palm appears upward no matter the viewer's angle on it. Thus, the imitation of upward or downward palm orientation (and the learning of signs specified for such palm orientations) is not complicated by the varying configurations of signers and learners in a three-dimensional world.
Figure 1.
The ASL signs TUESDAY (left) and BATHROOM (right).
In a preliminary pilot study, we observed one five-year-old deaf child (of hearing parents) who had been identified as having ASD by administrators of the residential school for the Deaf he attended. As we had no access to his educational records, we could not verify this diagnosis. However, in two videotaped naturalistic observation sessions (a 27-minute session at age 5;3 and a 24-minute session at age 5;5), this child produced 46 palm orientation errors in a corpus of 171 sign tokens. Twenty-eight of the 46 errors involved an inward/outward palm reversal. Most of these tokens were signs for days of the week, on which his palm faced outward rather than inward, and signs for numbers, on which his palm faced downward toward the table rather than inward. This evidence suggested that it would be worth exploring the production of signs by signing children with ASD.
In this paper, we ask whether the reversal errors reported in the gesture imitation of hearing children with ASD will also be produced by children with ASD who have been exposed from birth to ASL by their deaf parents. The vast majority of deaf children are born to hearing parents (90–95%; Mitchell & Karchmer, 2004) and may exhibit linguistic and cognitive delays due to impoverished or delayed language exposure (Schick, de Villiers, de Villiers, & Hoffmeister, 2007) that could mask the effects of ASD. Crucially, children born to deaf parents have access to excellent sign language models from birth.
If ASD entails a deficit in self-other mapping, and self-other mapping is a general cognitive mechanism that affects linguistic and non-linguistic domains alike, then we would predict the appearance of palm reversal errors in the signing of children with ASD. Alternatively, native exposure to a sign language might lead to enhanced visuo-spatial abilities (cf. Bosworth & Dobkins, 2002), which could attenuate the effects of such a deficit. In this case, palm reversals would not be evident in the signing of such children.
We report two studies of a sample of five signing children with ASD (four deaf children of deaf parents and one hearing child of deaf parents) and compare their performance to a sample of 12 typically-developing deaf children of deaf parents.
2. Study 1: Naturalistic Observation of Signing Children with ASD
All experiments described hereafter were approved by the Institutional Review Board at the University of Texas at Austin, and informed consent was obtained from parents of all participants prior to data collection.
2.1. Method
2.1.1. Assessments
Language exposure
Parents were asked to fill out a screening form reporting the hearing status of the parents themselves or other caregivers and the language or languages used at home. At least one parent had to report being deaf and using ASL as a primary means of communication at home in order for a child to be included in the study.
ASD
Educational and medical records of each child were thoroughly examined. A report in the child's records of a prior diagnosis, made by a qualified clinician (psychiatrist or clinical psychologist), of autistic disorder, ASD, or pervasive developmental disorder-not otherwise specified (PDD-NOS), was considered a criterion for inclusion in the study.
No diagnostic or screening instruments for ASD have been designed for use with deaf populations. Gold standard instruments such as the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989) and the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) have not been adapted for deaf children, and the ADOS explicitly warns against use with deaf children. The adaptation of such instruments for use with deaf children is likely to be complex, as some items are inappropriate for this population (e.g., the ADOS scores the child's response to his/her name being called by the examiner). Szymanski (2010) found that only 50% of 52 deaf children with a reported diagnosis of an ASD had scores in the clinically significant range on three common screeners for ASD, the Gilliam Autism Rating Scale - Second Edition (GARS-2; Gilliam, 2006), the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), and the Social Responsiveness Scale (SRS; Constantino, 2002). It is likely that the presence of items on these instruments that are inappropriate for deaf children (e.g., SCQ item 38 asks if the child looks up and pays attention when the adult rater comes into a room and talks to him or her without calling his or her name) prevents some of those children from reaching standard cut-off scores for ASD, since such items cannot be scored. Thus, existing instruments may be insufficiently sensitive to detect ASD in deaf children.
The lack of ASL-adapted gold-standard instruments makes it difficult to be certain of an ASD diagnosis in deaf children. However, Quinto-Pozos, Forber-Pratt, and Singleton (2011) recently argued that deaf and hearing professionals working in deaf schools are often best-suited to notice sign language disorders in native-signing children, since they are the most familiar with individual children's language level and signing style. We thus followed a three-step protocol for verifying the ASD diagnosis of children included in this study:
indication by school psychologist or teacher that the child was on the autism spectrum;
documentation of autistic behaviors in medical and educational records, and documentation of prior diagnosis of ASD according to DSM-IV criteria by a qualified clinician;
direct observation of autistic behaviors (sign echolalia, aversion to eye contact, armflapping, spinning, lack of appropriate facial expression, etc.) by the investigators.
Language level
The Language Proficiency Profile-2 (LPP-2: Bebko & McKinnon, 1993) was collected as a measure of language development and skill. The LPP-2 is a multiple-choice rating scale that can be completed by an adult familiar with the child's language skills (usually a teacher or parent). It examines five domains of language development: content (what the child refers to in his communication), form (structure of language), use (functions of language), cohesion (how much the child's language is related to what precedes and follows it), and reference (ability to refer to non-present information). Questions are structured so as to be independent of language modality and it has good concurrent validity with other language measures used with hearing and deaf children (Bebko, Calderon & Treder, 2003). Each item on the LPP-2 is scored a 2 if the child has already acquired the skill in question, a 1 if the child is in the process of acquiring the skill, or a zero if the child does not possess the skill. The maximum score is 112, with hearing children reaching ceiling levels (80% of the maximum score; i.e. ≥ 90) by about age 4, while deaf children reach ceiling by about age 8.2 Information is not yet available for children with ASD, though J. Bebko (personal communication, May 17, 2012) is at the time of this writing collecting LPP-2 scores for children with ASD.
2.1.2. Participants
The children observed in Study 1 were the following:
Child 1, a right-handed male age 7;5 at the time of observation, was diagnosed with PDD-NOS at age 4;1 at the Mayo Clinic. The diagnosis was confirmed by a second evaluation at the a major university medical center in the Midwest. His profound bilateral sensorineural hearing loss is hereditary, as his parents, maternal grandparents, and younger brother are also deaf. He does not use hearing aids and his primary means of receptive and expressive communication is ASL, with some augmentative use of the Picture Exchange Communication System (PECS; Frost & Bondy, 1994). In previous testing he obtained an autism index of 93 on the GARS-2, indicating a likely probability of autism, and a 97 on the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997), indicating average intelligence. His score on the LPP-2 was 52, indicating an approximate language age of 4, in comparison to non-autistic deaf children. Autistic behaviors observed by the investigators included limited eye contact, a disinterest in social interaction, and a lack of affective facial expression.
Child 2, a right-handed male age 4;6 at the time of observation, was diagnosed with autistic disorder at a major children's hospital in the Northeast at age 2;3. The diagnosis was confirmed at age 3;4 by a pediatric psychologist at a hospital clinic for deaf and hard-of-hearing children. His severe to profound bilateral sensorineural hearing loss is hereditary, as both his parents and his maternal grandparents are deaf. He does not use hearing aids and his primary means of receptive and expressive communication is ASL. His first sign, MOM, appeared at six months and vocabulary increased until 19 months, when expressive signing suddenly stopped. At age 2;8 he was tested on the Stanford-Binet Intelligence Scales, Fifth Edition (Roid, 2003); the results revealed strengths in nonverbal visual spatial processing and visual motor skills, whereas communication, especially social communication, was a weakness. Autistic behaviors included obsessive interests, stereotyped behaviors, spinning, limited eye contact and rare sharing of joint attention.
Child 3, a left-handed hearing male age 6;6 at the time of observation, was diagnosed with PDD-NOS after evaluation by a licensed clinical psychologist. He scored 32½ (threshold for autism = 30) on the Childhood Autism Rating Scale (CARS; Schopler, Reichler, DeVellis, & Daly, 1980) and his LPP-2 score was 26, indicating a language age well below his chronological age. Both parents are deaf and communicate primarily through ASL; however both Child 3 and his younger brother are hearing. He communicates with both parents through signing, though both his and his parents' signing is often accompanied by vocalization. Autistic behaviors observed by the investigators included signed and spoken echolalia, limited eye contact and joint attention, and arm-flapping. In addition, parental report confirmed that he has hypotonia affecting his fine motor skills, for which he had previously received occupational therapy. A formal assessment of his motor skills was not performed as part of this study.
2.1.3. Protocol and coding
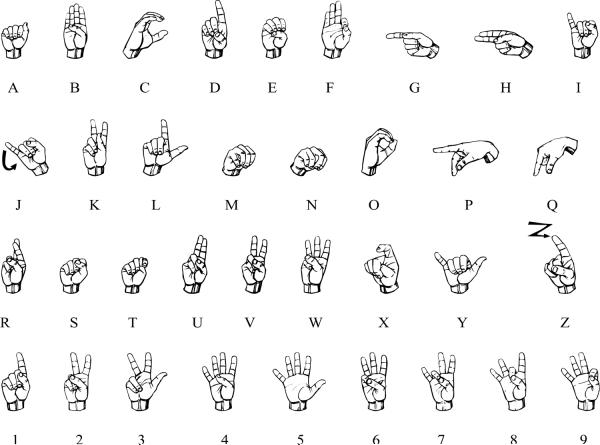
We videotaped the signing children with ASD in naturalistic interaction with teachers and other children at school (Children 1 and 2), or with parents and siblings at home (Child 3). Observation sessions lasted between 20 and 60 minutes; 20-minute sections of the data were then chosen for transcription. Signs were transcribed using FileMaker Pro® software; the parameters of location, movement, palm orientation, and handshape (Stokoe, 1960) were noted and each parameter value was coded as either correct or incorrect. For readers unfamiliar with ASL, a chart of sign handshapes is included in the Appendix.
2.2. Results
The naturalistic data collected from each ASD participant are described below. For each child, we report the number of signs produced as well as the number and types of errors.
2.2.1. Child 1 (age 7;5)
Child 1 produced 94 sign tokens; 18 were lexical signs and the remaining 76 were fingerspelled letters. Fingerspelling is a system whereby each letter of the written alphabet is represented by a different hand configuration. Of the 94 tokens, 68 (72.3%) contained one or more formational errors. Child 1 produced 61 palm orientation errors, 6 movement errors, 1 handshape error, and 0 location errors. A complete list of the signs produced by Child 1 can be seen in Table 1.
Table 1.
Sign tokens and errors produced by Child 1 in Study 1
| Time | Gloss | Error, if any | Time | Gloss | Error, if any |
|---|---|---|---|---|---|
| 0:00 | A | palm inward rather than outward | 2:15 | L | palm inward rather than outward |
| 0:01 | G | 2:17 | K | palm inward rather than outward | |
| 0:01 | E | palm inward rather than outward | 2:18 | L | palm inward rather than outward |
| 0:04 | G | 2:18 | M | palm inward rather than outward | |
| 0:05 | H | 2:20 | N | palm inward rather than outward | |
| 0:07 | I | palm inward rather than outward | 2:22 | O | palm inward rather than outward |
| 0:08 | J | palm inward rather than outward | 2:23 | P | |
| 0:10 | K | palm inward rather than outward | 2:24 | 0 | |
| 0:13 | L | palm inward rather than outward | 2:26 | R | palm to the side rather than outward |
| 0:13 | M | palm inward rather than outward | 2:28 | S | palm to the side rather than outward |
| 0:15 | N | palm inward rather than outward | 2:36 | W | palm inward rather than outward |
| 0:17 | O | 2:37 | V | palm inward rather than outward | |
| 0:19 | R | palm inward rather than outward | 2:38 | W | palm inward rather than outward |
| 0:20 | Q | 2:40 | X | palm inward rather than outward | |
| 0:22 | R | palm inward rather than outward | 2:41 | Y | palm inward rather than outward |
| 0:24 | S | palm inward rather than outward | 2:41 | Z | palm inward rather than outward; movement reduced |
| 0:25 | R | palm inward rather than outward | 3:18 | FIND | baby-O-handshape instead of F |
| 0:26 | T | palm inward rather than outward | 3:19 | BOOK | movement towards midline rather than away |
| 0:27 | U | palm inward rather than outward | 3:20 | PEN | |
| 0:28 | V | palm inward rather than outward | 4:44 | DAY | |
| 0:29 | W | palm inward rather than outward | 5:39 | FRIDAY | palm downward rather than inward |
| 0:30 | X | palm inward rather than outward | 5:41 | WEDNESDAY | palm to the side rather than inward; arm on table rather than up in neutral space |
| 0:31 | Y | palm inward rather than outward | 10:50 | SUNDAY | |
| 0:32 | Z | palm inward rather than outward | 10:51 | MONDAY | |
| 1:27 | B | palm inward rather than outward | 10:52 | TUESDAY | |
| 1:28 | C | 10:53 | WEDNESDAY | ||
| 1:31 | D | palm inward rather than outward | 10:54 | THURSDAY | |
| 1:32 | G | 10:55 | FRIDAY | ||
| 1:33 | H | 10:57 | MONDAY | ||
| 1:35 | I | palm inward rather than outward | 11:02 | TODAY | reduced movement; assimilation of handshape to non-dominant hand |
| 1:36 | J | palm inward rather than outward | 11:04 | TODAY | reduced movement |
| 1:36 | K | palm inward rather than outward | 11:05 | IS | |
| 1:36 | K | 11:07 | WEDNESDAY | ||
| 1:54 | K | 16:02 | K | palm inward rather than outward | |
| 1:59 | L | palm inward rather than outward | 16:04 | T | palm inward rather than outward |
| 2:00 | A | palm inward rather than outward | 16:06 | E | palm inward rather than outward |
| 2:02 | B | palm inward rather than outward | 16:14 | KITE | |
| 2:02 | C | 16:34 | T | palm inward rather than outward | |
| 2:03 | D | 16:35 | E | palm inward rather than outward | |
| 2:04 | G | palm outward rather than inward | 16:41 | K | palm inward rather than outward |
| 2:07 | F | palm to the side rather than outward | 16:41 | K | palm to the side rather than inward |
| 2:08 | G | palm to the side rather than outward | 16:42 | I | palm to the side rather than inward |
| 2:09 | H | palm to the side rather than outward | 16:43 | K | palm to the side rather than inward |
| 2:10 | T | palm inward rather than outward | 16:46 | I | palm to the side rather than inward |
| 2:11 | I | palm to the side rather than outward | 16:47 | T | palm inward rather than outward |
| 2:11 | J | palm inward rather than outward; movement reduced | 16:48 | E | palm inward rather than outward |
| 2:14 | K | palm inward rather than outward | 16:48 | E | palm inward rather than outward |
Handshape
Child 1 substituted a baby-O-handshape (with the tip of the thumb and index finger touching and all other fingers retracted) for an F-handshape on the sign find (thus failing to extend the middle, ring and pinky fingers).
Movement
Of the six movement errors that Child 1 produced, two involved reduced movement on the sign today (which in ASL is a compound consisting of the signs now and day); Child 1 did not produce the first member of the compound (now) and the path movement of the second member (day) was reduced in its trajectory. He executed the path movement of the sign book by moving his hands toward the midline rather than away from the midline. He also had trouble executing the movement of the fingerspelled letters j and z (which, unlike the other fingerspelled letters, have characteristic movements).
Palm orientation
The vast majority of the formational errors Child 1 produced pertained to palm orientation. Of the 61 palm orientation errors found, 50 involved the substitution of an inward palm orientation for an outward orientation; all these errors occurred on fingerspelled letters. Thus, Child 1 held his palm facing inward toward himself while producing fingerspelled letters, which normally face outward toward the signer's interlocutor (except for the letters g and h, which are produced with a palm orientation toward the midline). An additional 11 tokens were produced with an orientation to the side (towards the midline) instead of an inward or outward orientation. So, Child 1 produced the sign wednesday with orientation toward the midline, rather than the required inward orientation.
2.2.2. Child 2 (age 4;6)
Child 2 produced 41 sign tokens, including 14 (34.1%) that contained one or more formational errors. He produced 7 palm orientation errors, 7 movement errors, 2 handshape errors, and 0 location errors. Table 2 is a complete list of the signs produced by Child 2.
Table 2.
Sign tokens and errors produced by Child 2 in Study 1
| Time | Gloss | Error, if any | Time | Gloss | Error, if any |
|---|---|---|---|---|---|
| 19:48 | FINE | 23:25 | WRONG | ||
| 21:00 | ONE | 23:32 | STORY | movement reduced | |
| 21:08 | EAT | 23:35 | LIBRARY | ||
| 21:09 | FISH | movement inward rather than outward | 23:35 | SCHOOL | |
| 21:14 | ONE | 23:42 | CENTERS | ||
| 21:18 | FIVE | 23:48 | OUTSIDE | ||
| 21:19 | SIX | V-handshape instead of W; palm faces inward instead of outward | 23:54 | ART | |
| 21:20 | SEVEN | palm inward instead of outward | 24:02 | SLEEP | |
| 21:21 | EIGHT | palm to the side instead of outward | 24:03 | REST | |
| 21:26 | FISH | movement inward and back rather than outward | 24:04 | LIBRARY | |
| 21:30 | MINE | 24:11 | LIBRARY | ||
| 21:59 | SNACK | 24:21 | LEAVE | ||
| 22:11 | HELP | 25:13 | YES | 5-handshape instead of S | |
| 22:12 | AGAIN | palm of non-dominant hand faces down instead of up | 31:30 | LION | |
| 22:15 | REST | 31:32 | GIRAFFE | movement omitted | |
| 22:16 | LIBRARY | 32:37 | ELEPHANT | ||
| 22:27 | SCIENCE | 33:37 | LION | movement reduced; palm outward rather than down | |
| 22:29 | GO-HOME | movement omitted | 36:39 | SLEEP | movement omitted |
| 23:04 | STORY | 37:09 | FINISH | primary movement error with palm orientation error. does not move wrist and reverse palm. | |
| 23:08 | SNACK | palm toward midline instead of inward | 37:21 | DRAW | |
| 23:13 | ART |
Handshape
The two handshape errors were a V-handshape instead of a W-handshape for the sign six and a 5-handshape instead of an S-handshape for the sign yes.
Movement
Child 2 produced 7 movement errors. The sign fish was produced with a reversed movement; rather than an outward path movement, he executed a path movement that started with his arm extended, retracting his arm and pulling it to the right. He omitted path movements from the signs giraffe, lion and home, and omitted hand-internal movement on the sign sleep, an error type which has been found in typical development in much younger signers (Cheek et al, 2001; Morgan, Barrett-Jones, & Stoneham, 2007). He reduced the repeated movement of the sign story to a single movement cycle, and omitted wrist extension on the sign finish, executing only a forearm twist with a lax wrist.
Palm orientation
Child 2 produced 3 inward-outward palm orientation errors, signing the numbers six through eight with his palm turned inward rather than outward. He also produced the sign snack with the palm facing the midline rather than inward. The other three palm orientation errors were on the sign again, where the non-dominant hand faced down instead of towards the midline, the sign lion, where the palm faced upward rather than downward, and the sign finish, where the palms faced inward and did not flip outward.
2.2.3. Child 3 (age 6;6)
Child 3 produced 59 sign tokens; 35 (59.3%) contained one or more formational errors. He produced 23 movement errors, 4 palm orientation errors, 9 handshape errors, and 3 location errors. A complete list of the signs produced by Child 3 can be seen in Table 3.
Table 3.
Sign tokens and errors produced by Child 3 in Study 1
| Time | Gloss | Error, if any | Time | Gloss | Error, if any |
|---|---|---|---|---|---|
| 0:06 | HELLO | 7:56 | DANCE | 5-handshape instead of V | |
| 0:58 | wave | palm inward instead of outward | 8:04 | DANCE | 5-handshape instead of V |
| 1:02 | NAME-SIGN | 8:33 | DANCE | ||
| 1:18 | HORSE | 5-handshape instead of H-dot. Location on cheek instead of forehead. Movement reduced | 8:47 | DOWN | |
| 1:23 | DUCK | 8:55 | DANCE | ||
| 1:29 | DOG | 9:00 | DANCE | ||
| 1:45 | CHICKEN | 8-handshape instead of G, palm to side instead of out, movement reduced | 10:40 | point | |
| 1:47 | CHICKEN | palm | 11:16 | RED | |
| 2:07 | TURTLE | palm down instead of toward mid, all fingers move instead of thumb | 11:23 | YES | |
| 2:16 | STAR | lack of contact between the hands, location is lowered (at chest level) | 11:48 | THREE | palm downward instead of inward |
| 2:33 | BOOK | 13:25 | BLUE | ||
| 3:09 | SAD | 15:05 | GREEN | baby-C-handshape instead of G | |
| 3:09 | MONKEY | 15:12 | FLASHING-LIGHT | palm inward rather than outward; hand-internal movement omitted | |
| 3:12 | YOU | 15:18 | PURPLE | ||
| 3:30 | BEAR | 15:19 | BLUE | ||
| 3:32 | MONKEY | 15:20 | PURPLE | ||
| 3:44 | LION | movement is forward instead of backward | 15:22 | RED | |
| 3:56 | LION | movement is forward instead of backward | 15:31 | PURPLE | |
| 4:07 | ELEPHANT | movement omitted | 15:52 | YES | movement proximalized to elbow, slight sideways movement |
| 4:22 | GIRAFFE | movement omitted | 16:37 | ICE-CREAM | produced in neutral space, no movement. |
| 4:52 | MONKEY | 17:12 | BLUE | wrist movement wild | |
| 5:04 | MONKEY | 18:17 | point | ||
| 5:42 | point | 18:41 | RED | ||
| 5:48 | DANCE | 5-handshape instead of V; non-dominant hand omitted, wild movement | 18:42 | APPLE | A-handshape instead of X |
| 6:27 | HORSE | palms face midline instead of outward, location on cheeks instead of forehead | 19:14 | BLUE | |
| 7:11 | RABBIT | 4-handshape instead of H-dot. movement reduced | 19:15 | DOG | |
| 7:42 | BEAR | 19:18 | DOG | ||
| 7:45 | DANCE | 5-handshape instead of V, non-dominant hand omitted, wild movement | 19:19 | BLUE | |
| 7:51 | BEAR | 19:25 | ORANGE | location on knee rather than on chin | |
| 7:55 | PIG | 19:42 | YES | movement shows some forearm twist where there should be none |
Location
Child 3 produced several signs in locations lower than their citation forms: on the sign orange, he failed to raise his hand from the resting position in his lap and therefore made the sign in contact with his knee rather than his chin (confirmed by maternal repetition immediately afterward), and produced the sign ice-cream in neutral space rather than at the chin. Finally, he produced the sign star without contact between the hands, at chest level rather than chin/head level.
Handshape
Child 3 produced a 4-handshape instead of an H-dot handshape (i.e., a handshape with the first and second fingers extended and together, third and fourth fingers closed, and thumb extended) on rabbit, a 5-handshape instead of a V-handshape on dance (4 tokens), a baby-C-handshape instead of a G-handshape on the sign green, an A-handshape instead of an X-handshape on the sign apple, an 8-handshape instead of a G-handshape on the sign chicken, and a 5-handshape instead of an H-dot handshape on the sign horse.
Movement
The movement parameter was the source of the majority of Child 3's errors. He produced a forward movement (outward) rather than inward on the sign lion (two tokens) and computer (one token). He also did not execute a path movement on several signs that normally exhibit path movement (such as elephant and giraffe), and reduced movement on several signs that typically exhibit repeated cycles of movement (e.g., horse, duck, monkey, bear, and chicken). Child 3 deleted the movement segment entirely on the sign ice-cream. Other simplifications included the loss of the non-dominant hand on the sign dance as well as the dropping of one hand from a two-handed sign (bear, monkey). Several signs also exhibited wild, uncontrolled movement, which were only interpretable because the parent repeated the sign with the correct form; these included dance and blue. Finally, Child 3 produced the sign yes with a forearm rotation rather than with a nodding movement of the wrist (two tokens).
Palm orientation
Child 3's errors consisted of two substitutions of an inward orientation for an outward orientation (on the sign flashing-light as well as a wave gesture) and the substitution of a downward orientation for a midline-facing orientation (turtle) and for an inward orientation (three).
2.3. Discussion
The three children reported here all produced errors in multiple sign parameters during naturalistic observation, indicating that sign production accuracy is a problem in ASD. Moreover, the error rates appear to be higher than would be expected in typically-developing deaf children of the same age (Child 1, 72.3%; Child 2, 34.1%; Child 3, 59.3%). Despite this high overall error rate, not every parameter was equally problematic.
2.3.1. Location
The locations of signs were produced most successfully, with both Child 1 and Child 2 producing no location errors and Child 3 producing three location errors, all of which involved substituting a location lower on the body than the target form. Location is the parameter that has been reported to be acquired first and most successfully in the acquisition of signed languages by typically-developing children (Cheek et al., 2001; Conlin, Mirus, Mauk, & Meier, 1999; Marentette & Mayberry, 2000; Morgan, Barrett-Jones, & Stoneham, 2007; Siedlecki & Bonvillian, 1993) and in the acquisition of signs by hearing children with ASD (Seal & Bonvillian, 1997).
2.3.2. Handshape
It has been shown numerous times that handshape is the least accurate of all sign parameters in young children (Cheek et al., 2001; Clibbens & Harris, 1993; Karnopp, 1997; Marentette & Mayberry, 2000; Meier, 2006; Siedlecki & Bonvillian, 1993; Takkinen, 2003; von Tetzchner, 1984), probably due to the late development of fine motor control needed to produce handshapes accurately. Seal and Bonvillian (1997) also found very high error rates on handshape in their hearing participants with ASD, with more than one-third of target handshapes being produced with formational errors. Note, however, that the participants in Seal and Bonvillian's study were not exposed to sign natively: the earliest age of exposure was 4;3 and some children were not exposed to sign until 11;8. In our data, by contrast, handshape appeared to be a minor issue: Child 1 produced only one handshape error (1.5% of his total errors), Child 2 produced two (12.5% of his total errors), and Child 3 produced 9 (26% of his total errors).
2.3.3. Movement
The acquisition of movements has been reported to be difficult for both ASD and TD children (Marentette & Mayberry, 2000; Meier, 2006; Seal & Bonvillian, 1997; Siedlecki & Bonvillian, 1993). Seal and Bonvillian (1997) found that the movement parameter was difficult for ASD children and the source of many formational errors (36% error rate). In particular, children with ASD tended to reduce signs consisting of two or three sequential movements into a single movement. A similar phenomenon has been found for typical development: Morgan, Barrett-Jones and Stoneham (2007) reported that movement segments were commonly deleted by a typically-developing deaf British child between the ages of 19 and 24 months, especially when hand-internal movements were combined with a path (external) movement.
In our data, Child 1 produced 6 movement errors (9% of his total errors), including 2 movement reductions. Child 2 produced 7 movement errors (44% of his total errors), 6 of which were movement reductions. Child 3 produced 23 movement errors (66% of his total errors), most of which were reductions. Movements could be susceptible to the motor difficulties found in ASD, which must be considered as a possible source of these errors, especially the reduction or deletion of internal or path movements.
2.3.4. Palm orientation
Palm orientation errors accounted for more than half of all errors in the data. Child 1 produced 61 palm errors (90% of his total errors), Child 2 produced 7 (44% of his total errors), and Child 3 produced 4 (11% of his total errors). In particular, inward-outward palm orientation errors accounted for 56 of the 72 palm orientation errors (78%). Many of the palm orientation errors observed were produced on signs for letters, numbers, and days of the week. It is unsurprising that these particular vocabulary items occurred frequently in our sample, given that such vocabulary areas are common in elementary school classrooms. In ASL, these signs happen to be specified for inward or outward palm, making them more likely to be prone to error in ASD signing.
Such reversals have not been frequently reported in the literature on the typical acquisition of ASL (though palm orientation is not always reported as a separate parameter and is often conflated with hand configuration). To our knowledge, there are no documented examples of this kind of palm orientation error in the literature past the age of two. Cheek et al. (2001) found that palm orientation was produced relatively successfully (compared to handshapes and movements) in a study of four typically-developing deaf children between 9 and 17 months of age. Those errors that did occur in their data tended to involve the substitution of downward for upward orientation. However, they did not code for “inward” and “outward” palm orientations, instead coding for the pronation and supination of the forearm. Therefore, palm orientations were classified as “down” (pronated), “up” (supinated), or “mid” (neither pronated nor supinated). In this scheme, a “down” orientation could correspond to either an outward-facing palm orientation or a downward-facing palm orientation. Likewise, an “up” orientation could correspond to either an inward-facing or upward-facing palm orientation.
In order to further verify whether such palm orientation reversal errors occur in typical development, we examined 659 tokens in the database of children's early sign productions on which Cheek et al. based their report. This examination revealed six tokens involving an inward palm orientation where the adult citation form called for outward palm orientation. All six tokens were produced by one fourteen-month-old child and involved the sign bird. In addition, there were eight tokens in the database in which children appear to have produced an outward palm orientation when the adult citation form called for inward palm orientation: monkey (1 token), eat (3 tokens), red (2 tokens), and dog (2 tokens). Of these, dog is the most straightforward case to interpret, as it is produced in neutral space and does not have contact with the body; the other signs involve contact with the body and the resulting errors could be due to a preference for contact with a particular side of the hand. Overall there were 14 tokens (6 inward substitutions and 8 outward substitutions) of reversed inward-outward palm orientation in a database of 659 signs produced by typically-developing deaf children in the first year and a half of life.
Thus, a review of the literature as well as an analysis of a database of early signs yielded few clear examples of inward/outward palm orientation reversals. It therefore appears reasonable to conclude that such errors do not appear with much frequency in typical development, especially compared to the high error rates found on handshape and movement (Cheek et al., 2001; Clibbens & Harris, 1993; Karnopp, 1997; Marentette & Mayberry, 2000; Meier, 2006; Siedlecki & Bonvillian, 1993; Takkinen, 2003; von Tetzchner, 1984). The occurrence of such errors in the signing of three native-signing children with ASD between the ages of 4 and 8, as well as the pilot subject with ASD described in Section 1, is therefore quite notable.
Given that many of the palm orientation reversals observed in naturalistic observation were on fingerspelled letters, a follow-up study was conducted that specifically tested ASD and TD children's fingerspelling as a way to further examine the production of palm orientation in the signing of children with ASD.
3. Study 2: Fingerspelling Task
A task to elicit fingerspelled English words was devised for several reasons. First, the fingerspelling system is mastered later in development than are lexical signs and could be particularly challenging to learners, inasmuch as it requires some knowledge of English. Second, it exhibits a variety of articulatory properties that are not characteristic of the core ASL lexicon (see Padden, 1991, 2006) and thus may pose some difficulties for young learners. Finally, fingerspelling is in general produced with an outward palm orientation, unlike lexical signs, which are quite varied in their palm orientation.
3.1. Method
3.1.1. Participants
ASD Group
The three children from Study 1 participated in Study 2; however, Child 2 did not respond to any stimuli and therefore will not be discussed further. Two additional native-signing deaf children with ASD also participated in Study 2.
Child 4, a right-handed male age 5;8 at the time of observation, was evaluated for autism at age 2;4. His profound bilateral sensorineural deafness is hereditary, as both parents are deaf. He does not wear hearing aids and ASL is the primary means of expressive and receptive communication at home. He was diagnosed with ASD at age 2;4 by a major regional healthcare provider for children and adults with disabilities. His score on the CARS was 35, indicating mild-moderate autism, while his GARS-2 autism index was 106, indicating a very likely probability of autism. In prior testing he obtained a Leiter-R Full Scale IQ composite score of 143 (99th percentile), indicating that he is a highly intelligent, gifted child, and his score on the LPP-2 was a 32 (indicating language skills below the age of 4 in comparison to typically-developing deaf children). Autistic behaviors identified in files or observed by the investigator included: stereotyped behaviors, stiff/jerky movements, immediate and delayed echolalic signing, use of peripheral vision to watch signing, impaired eye contact, failure to recognize when people were present, looking away when his name is signed, failure to initiate conversations, flat affect, and impaired social/pragmatic language.
Child 5, a right-handed male age 7;2 at the time of observation, received a primary diagnosis of autism and ADHD after being evaluated at age 3;4 by a pediatric neuropsychologist. His profound bilateral sensorineural deafness is hereditary, as both parents as well as his two brothers are deaf. ASL is used at home as the primary expressive and receptive means of communication. At age 6, he received an autism index of 111 on the GARS-2, indicating a very likely probability of autism, and his LPP-2 score was 90 (indicating language skills within the normal range for deaf children). Autistic behaviors documented in files or observed by the investigator included: sign echolalia, repeating utterances out of context, responding inappropriately to commands, failure to initiate conversations, avoidance of eye contact, and failure to imitate others when requested.
Control Group
A control group of 12 typically-developing deaf children (henceforth TD children) ranging in age from 3;7 to 6;9 (M = 4;9) was recruited at two state schools for the Deaf. All 12 of the TD children had deaf parents. Given the small number of deaf children of deaf parents (5–10% of deaf children; Mitchell & Karchmer, 2004), it was not possible to match the ASD and TD children for chronological age or language age. The TD children were younger (M = 4;9; SD = 1;0) than their ASD counterparts (M = 6;8; SD = 0;9); a Mann-Whitney test found that this difference was significant, U(12,4) = 45, Z = 2.55, p < 0.01, two-tailed. The TD children also had higher LPP-2 scores (M = 90.25; SD = 17.07) than the ASD group (M = 50.0; SD = 28.89); a Mann-Whitney test found that this difference was significant, U(12,4) = 43, Z = 2.30, p < 0.05, two-tailed. Table 4 summarizes the characteristics of the participants in Study 2.
Table 4.
Participants in Study 2; all were deaf children of deaf parents except for ASD Child 3, who was the hearing child of deaf parents.
| Participant | Age | Sex | LPP-2 |
|---|---|---|---|
| ASD Child 1 | 7;5 | M | 52 |
| ASD Child 3 | 6;6 | M | 26 |
| ASD Child 4 | 5;8 | M | 32 |
| ASD Child 5 | 7;2 | M | 90 |
|
| |||
| TD Child 1 | 3;7 | F | 97 |
| TD Child 2 | 3;7 | F | 99 |
| TD Child 3 | 3;8 | M | 102 |
| TD Child 4 | 4;0 | F | 72 |
| TD Child 5 | 4;4 | F | 88 |
| TD Child 6 | 4;9 | M | 63 |
| TD Child 7 | 4;10 | F | 112 |
| TD Child 8 | 4;11 | F | 86 |
| TD Child 9 | 5;6 | F | 103 |
| TD Child 10 | 5;7 | M | 59 |
| TD Child 11 | 6;2 | M | 100 |
| TD Child 12 | 6;9 | M | 102 |
3.1.2. Stimuli
The English words bed, table, watch, telephone, cap, chair, door, shoes, book, and scissors were printed on 8” × 3.5” laminated cards. The order of presentation of stimuli was randomized.
3.1.3. Instructions
The researcher sat directly across the table from the child. The researcher simply held up each card and produced the sign fingerspell while pointing at the card. If the child needed additional prompting, the researcher pointed to each letter on the card individually and again produced the sign fingerspell. Some children read and understood the printed word and instead produced the corresponding lexical sign; in those cases they were again instructed to fingerspell the word.
3.1.4. Coding
Each handshape produced by the children was coded for the letter it represents as well as for palm orientation. A fingerspelled letter was counted as an error if the palm did not face outward toward the experimenter; misspellings were not coded as errors. A deaf signer of ASL re-coded four ASD children and four of the 12 TD children. Intercoder agreement was 90.1% for the ASD data and 97.3% for the TD data.
3.2. Results
Three of the four children with ASD – Child 1 (7;5), Child 3 (6;6), and Child 4 (5;8) – tended to reverse their palm orientation while fingerspelling, reversing palm orientation on 72 out of 179 tokens (40.2%); only Child 5 (7;2) did not produce any palm reversals. The results are reported in Table 5, with letters with reversed palm orientation bolded. Children 1, 3, and 4 produced fingerspelling reversals on most of the words they attempted (8 of 10 words, 7 of 9 words, and 6 of 6 words, respectively), but were not consistent in reversing all letters within a given word. For example, Child 4 fingerspelled d-o-o-r with the d palm in, the double o palm out, and the r palm in. Moreover, Children 1, 3, and 4 were not consistent in always reversing a specific letter. For example, Child 3 reversed the palm orientation on the letter t when fingerspelling `table' but produced a correct outward-facing palm orientation on the same letter when fingerspelling `watch.'
Table 5.
Performance of participants with ASD on Study 2 (Fingerspelling)
| Stimulus | Child 1 (7;5) | Child 3 (6;6) | Child 4 (5;8) | Child 5 (7;2) |
|---|---|---|---|---|
| bed | L-B-A-E-L-D | B-E-D | B-E-B-D | (no response) |
| table | I-T-A-L-D-E a | T-A-D-I-E | T-A-U-B-I-L-3-E | T-A-D-E-L |
| watch | W-A-T-C-H | W-A-T-C-H | (no response) | W-A-R-E-H |
| book | D-O-O-K | B-O-O-K | B-O-O-K | D-O-O-K |
| cap | C-A-P | C-A-K | C-A-P | C-A-P |
| chair | C-H-E-A-I-T-U | C-H-A-I-R | (no response) | C-D-H-A-I-R |
| shoes | S-H-O-E-S | A-S-H-O-E-S | S-H-O-E-S | S-H-O-E-S |
| door | D-B-O-D | D-O-O-R | D-O-O-R | D-O-O-D |
| telephone | T-E-L-E-P-H-O-N-E | T-E-I-?-H-O-N-E | (no response) | H-T-E-L-E-P-H-O-N-E |
| scissors | S-C-I-S-S-O-R-S | (obscured) | (no response) | S-C-I-S-S-S-O-R-S |
|
| ||||
| Percentage of letter handshapes reversed | 47.4% (27/57) | 60.5% (26/43) | 67.9% (19/28) | 0.0% (0/51) |
letters with reversed palm orientation bolded
There was a sharp contrast between the ASD and TD groups on this task. All 12 TD children completed the task, but the TD children did not produce any palm reversals. All 120 fingerspelled words and 588 fingerspelled letters were produced with the correct palm orientation. Thus, the children with ASD performed differently on this task from TD deaf children, even though the TD group was significantly younger than the ASD group.
We also compared the accuracy of the children on palm orientation with their accuracy in producing the correct hand configuration (letter). The ASD children all had difficulty with hand configuration (spelling) accuracy, but to a lesser degree than with palm orientation (with the exception of Child 5, who produced 7 hand configuration errors, i.e., 7 incorrect letters, but did not produce any palm orientation errors). Child 1 produced 11 hand configuration errors but 27 palm orientation errors, Child 3 produced 6 hand configuration errors but 26 palm orientation errors, and Child 4 produced 4 hand configuration errors but 19 palm orientation errors. Moreover, some words contained no hand configuration errors at all but did contain reversed palm orientations (e.g., Child 1's production of the words `scissors' and `telephone').
The TD children also produced hand configuration errors, despite not producing any palm reversals. TD Children 2 and 3 each produced 1 hand configuration error, TD Child 8 produced 2 hand configuration errors, and TD Children 5, 7, and 11 each produced 3 hand configuration errors. The majority of hand configuration errors produced by TD and ASD children alike reflected common errors in learning to read and write – e.g., confusion between the letters `b' and `d'. ASD Children 1, 3, and 5 all produced D for the `b' in `table'; TD Child 5 produced D for the `b' in `bed'; and ASD Children 1 and 4 and TD Children 3, 5, and 8 produced D for the `b' in `book'. TD Child 7 produced B for the `d' in `door' and `bed', and TD Child 2 produced B for the `p' in `cap'. This pattern was not consistent within subjects, however, with ASD Children 1, 3, and 4 all producing the B handshape correctly for the word `bed'.
3.3. Discussion
Three young ASD children showed a robust tendency to fingerspell with a reversed palm orientation. These errors have never before been reported in the literature on the acquisition of the fingerspelling system of ASL (Padden & Lemaster, 1985; Padden, 1991, 2006) or of any other sign language.3 Since fingerspelling is typically directed outward, such reversals again suggest that, in learning to fingerspell, children with ASD reproduce handshapes as they appear from their own perspective, failing to re-orient their own palm so that it faces their addressee. The children who participated in our study did not show evidence of looking at their hands while fingerspelling; if they had, this might have indicated that they were concentrating on producing the correct handshape.
TD children of similar chronological and language age to the four children with ASD did not reverse their palm while fingerspelling. Although the mean LPP-2 scores of the children with ASD were lower than the TD group, Children 6 and 10 in the TD group had LPP-2 scores (63 and 59, respectively) nearly two standard deviations below the mean of the TD group (M = 90.3), and close to the mean of the ASD group (M = 50). Crucially, neither of these children produced any palm orientation reversals. Therefore the palm reversals exhibited by the ASD children are unlikely to be the result of mere language delay, although it should be noted that the one child with ASD who did not produce any reversed fingerspelled letters (Child 5) also scored much higher on the LPP-2 (90).
4. General Discussion
In Study 1, deaf children with ASD exhibited various formational errors in their spontaneous signing. Of particular note were inward-outward palm orientation reversals, which are suggestive of a self-other mapping failure and do not appear to be frequent in typical development even in infancy. Study 2 revealed a strong tendency among three of four children with ASD to reverse palm orientation in fingerspelling, while a comparison group of TD children did not produce any palm reversals.
There is thus robust evidence for the this error type in that (a) these errors were produced by four of the five young signing children with ASD in our sample, and (b) these errors appeared in spontaneous signing as well as in experimentally-elicited fingerspelling. All children in this study were exposed to ASL from birth; consequently the observed errors cannot be attributed to impoverished language input. The frequency with which these errors appear is dramatic given that such errors have not been described in the literature on the typical or atypical acquisition of ASL beyond 18 months of age. These palm orientation reversals represent a new class of errors that has not been previously discussed in the literature on the acquisition of signed languages, and may therefore be a modality-specific marker of ASD in signing children.
4.1. Is the source of these errors motoric or perceptual?
Since fine motor difficulties are often associated with ASD (Ming, Brimacombe, & Wagner, 2007; Mostofsky, Burgess, & Gidley Larson, 2007), one alternative hypothesis is that these errors are purely articulatory. However, with the exception of Child 3, the children produced fewer errors in handshape, which is typically mastered latest in development, than in palm orientation or movement, and produced marked or difficult handshapes correctly (such as the ASL r handshape, in which the first and second fingers are crossed). It appears unlikely that motor difficulties could be the sole cause of these errors, since the correct production of handshape suggests good fine motor control.
Furthermore, there is no reason to think that inward-outward palm orientation reversals might be produced in order to ease articulation. Instead, signers with neuromotor disorders such as Parkinson's Disease sometimes produce neutral palm orientations toward the midline instead of upward or downward palm orientations, but do not produce inward-outward reversals (Brentari, Poizner, & Kegl, 1995). In our data, too, we find some signs produced with an orientation toward the midline rather than inward or outward (e.g., Child 1's production of wednesday, Child 2's production of snack). Given the motor problems associated with ASD, it is likely that some of the observed errors are consistent with motor control issues. However, the inward-outward palm orientation reversals cannot be explained in this way, but are instead suggestive of a perceptual deficit. Our conclusion is in line with other work that has found that impairments in the gesture of children with ASD cannot be accounted for by motor deficits alone (Dewey, Cantwell, & Crawford, 2007).
4.2. Implications for theory
Our findings are consistent with the hypothesis that a deficit in self-other mapping is characteristic of ASD. The fact that very similar errors occur in the signing of deaf children with ASD and in the gestural imitation of hearing children with ASD suggests that self-other mapping is a domain-general process that subserves the learning of linguistic and non-linguistic stimuli. Furthermore, this study adds to the previous findings of imitative gesture reversals in hearing children with ASD (Ham, Corley, Rajendran, Carletta, & Swanson, 2008; Hobson & Lee, 1999; Ohta, 1987; Smith & Bryson, 1998) by documenting that deaf children with ASD produce spontaneous (non-imitative) reversed signs. Thus, a self-other mapping deficit may have effects on the stored mental representations of linguistic symbols.
This study also demonstrates the striking difficulty of self-other mapping for ASD children with native exposure to a sign language, who presumably have far more practice imitating gestures than their hearing counterparts. It is interesting to note how frequently these errors appear in the signing of children with ASD, given their relative rarity in typical development. The persistence of such errors in the signing of children with ASD – despite life-long sign language exposure that might have been expected to attenuate the effects of a self-other mapping deficit – points to a persistent underlying neurobiological source. Dysfunction in ASD of the mirror neuron system through which children match their actions to those observed in others (cf. Dapretto et al., 2005; Iacoboni & Dapretto, 2006; Williams, Whiten, Suddendorf, & Perrett, 2001) is a plausible mechanism that is consistent with the errors described here.
From a cognitive perspective, self-other mapping is a complex process involving multiple component skills. It could be that the ASD child is unable to understand others' perspectives, reflecting a general deficit in theory of mind (e.g., Baron-Cohen, et al., 1985; Baron-Cohen, 2000); alternatively, the ASD child could lack the motivation to do so, thus revealing a failure to identify with others (Hobson & Hobson, 2007; Hobson & Lee, 1999; Meyer & Hobson, 2004). A third possibility is that a pragmatic deficit (cf. Tager-Flusberg, 1996) impedes the ASD child from understanding that signs must `face' one's addressee. In this sense, palm reversals in sign may be akin to the pronoun reversals of ASD speech (Bartak & Rutter, 1974; Charney, 1980; Chiat, 1982; Lee, Hobson, & Chiat, 1994; Evans & Demuth, 2012), whereby the ASD child uses the same pronoun to refer to himself as the one produced by his interlocutor (e.g., “you” in reference to self). In both cases, children with ASD reproduce linguistic symbols exactly as perceived, rather than shifting their forms appropriately (from “you” to “I” in speech, and by rotation of the palm in sign). Research is needed to determine whether signing children with ASD also reverse pronouns in sign, just as more work is needed to clarify whether the signing children who produce palm reversal errors also show evidence of specific deficits in other cognitive domains, such as theory of mind, identification with others, and pragmatics.
4.3. Implications for clinical practice
These results have clear implications for clinical practice. First, if palm reversals are indeed symptomatic of ASD signing, then instruments designed for detecting ASD in hearing children should be adapted for deaf children to reflect this phenomenon. It may be necessary to add new items or adapt existing items to fit the error pattern described here, and scoring algorithms may need to be adjusted. Deaf parents may be aware of these types of reversals in their children's signing. Since parent rating scales are often used as screening instruments for ASD (e.g., SCQ, SRS), it would be useful to incorporate items that ask parents about the specific sign errors documented here. Clinicians and educators who work with deaf children should also be made aware of the palm orientation error type and treat it as a possible red flag for further assessment for ASD in such children. Finally, in providing therapy for children who produce these error types, it may be helpful for the therapist to sit beside the child, rather than opposite him or her.
4.4. Limitations
This study has several important limitations. First, the small sample size of native-signing children diagnosed with ASD makes a statistical analysis impossible. However, given the near-total absence of data on deaf children with ASD, we believe that the data presented here from five native signers (four of them deaf) represents a significant contribution. No prior study reports such a large sample of native-signing children with ASD.
Second, at the time of this writing there are no gold-standard instruments for diagnosing deaf children with ASD. Until the ADOS or ADI-R are translated and adapted for use with deaf children, we must rely on the judgment of qualified clinicians who are familiar with deaf children, sign language, and ASD. While we feel confident in the diagnoses of the children described in this paper, we acknowledge that the lack of instruments appropriate for deaf children is a limiting factor for this research.
Third, the children with ASD could not be closely matched with TD children for chronological or language age. It should be noted that it may be difficult to find native-signing TD deaf children with LPP-2 scores matching those of the ASD children; as it happens, the youngest TD deaf children in our sample were near ceiling on this measure. Importantly, two TD children with low scores on the LPP-2 language measure that fell within the range of our ASD subjects did not produce any palm orientation reversal errors.
Finally, an investigation of signing children with other types of intellectual disability (e.g., Down syndrome, Williams syndrome) and language disorders (e.g., Specific Language Impairment) could help clarify whether the errors reported here are specific to ASD.
4.5. Conclusions
Sign language provides a unique lens through which to view the effects of ASD on cognition. By documenting the sign production of children with ASD with native ASL exposure, we hope to shed new light on the deficits of ASD. As this study represents a first step towards describing the effects of ASD on language development in the visual-gestural modality, we suggest that future research focus on aspects of grammar that depend on self-other mapping skills (including the features described in this paper, but also inward-outward movements, verb agreement and classifier constructions; see Meier, 2002 and Emmorey, 2002 for reviews). Future work on the signing of children with ASD may reveal insights about not just sign language development specifically, but also about the effects of ASD on cognition more generally.
Highlights
We present the first reports on native-signing children with autism.
Children with ASD reverse inward- and outward-facing palm orientations.
Typically-developing native-signing children do not make such reversal errors.
Errors are similar to reversals in imitated gestures by hearing children with ASD.
These errors suggest a perceptual rather than motoric deficit.
Acknowledgments
Funding for this research was provided by Autism Speaks grant number 4721 to the first author and by NSF dissertation improvement grant BCS-0746009 to both authors. Support for this research was also provided by the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number F32DC011219. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank A. Rebecca Neal and Helen Tager-Flusberg for feedback on earlier versions of this manuscript, Lynn Hou for assistance with the coding of data, Annie Marks for creating and editing the sign photographs, Franky Ramont for serving as the model, and all of the parents, children, teachers, and school administrators at the Texas, Indiana, Ohio, and Iowa Schools for the Deaf, the Minnesota State Academy for the Deaf, and the Learning Center for Deaf Children.
APPENDIX
The handshapes of ASL.
This figure was generated using a font created by David Rakowski. This figure is licensed under a Creative Commons Attribution-ShareAlike 3.0 Unported License and may be reproduced freely with appropriate attribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Educational Objectives: The reader will: (a) learn about the gesture imitation deficit in autism; (b) be introduced to the four parameters of sign language articulation; and (c) understand how autism affects these parameters in native-signing children.
As is customary, ASL signs are indicated by English glosses written in SMALL CAPS.
The sample of 63 deaf Canadian and American children on which this norm is based was composed primarily of deaf children of hearing parents; only 3 children had deaf parents. We would expect that deaf children of deaf parents would pattern more closely with hearing children and thus reach ceiling levels earlier.
Indeed, the author of several papers on the acquisition of fingerspelling indicated that she had never seen a deaf child fingerspell with inward palm orientation (C. Padden, personal communication, December 3, 2009).
References
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:7–44. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Theory of mind and autism: A fifteen year review. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, editors. Understanding other minds: Perspectives from developmental cognitive neuroscience. second edition Oxford University Press; New York: 2000. pp. 3–20. [Google Scholar]
- Bartak L, Rutter M. The use of personal pronouns by autistic children. Journal of Autism and Developmental Disorders. 1974;4:217–222. doi: 10.1007/BF02115227. [DOI] [PubMed] [Google Scholar]
- Bebko JM, Calderon R, Treder R. The Language Proficiency Profile-2: Assessment of the global communication skills of deaf children across languages and modalities of expression. Journal of Deaf Studies and Deaf Education. 2003;8:438–451. doi: 10.1093/deafed/eng034. [DOI] [PubMed] [Google Scholar]
- Bebko JM, McKinnon EE. The Language Proficiency Profile. Department of Psychology, York University; Toronto, Canada: 1993. Available from Dr. James J. Bebko. [Google Scholar]
- Bonvillian JD, Blackburn DW. Manual communication and autism: Factors relating to sign language acquisition. In: Siple P, Fischer S, editors. Theoretical issues in sign language research, Vol. 2: Psychology. University of Chicago Press; Chicago: 1991. pp. 255–277. [Google Scholar]
- Bonvillian JD, Nelson KE, Rhyne JM. Sign language and autism. Journal of Autism and Developmental Disorders. 1981;11:125–137. doi: 10.1007/BF01531345. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Visual field assymetries for motion processing in deaf and hearing signers. Brain and Cognition. 2002;49:170–181. doi: 10.1006/brcg.2001.1498. [DOI] [PubMed] [Google Scholar]
- Brentari D, Poizner H, Kegl J. Aphasic and Parkinsonian signing: differences in phonological disruption. Brain and Language. 1995;48:69–105. doi: 10.1006/brln.1995.1003. [DOI] [PubMed] [Google Scholar]
- Carr EG. Teaching autistic children to use sign language: Some research issues. Journal of Autism and Developmental Disorders. 1979;9:345–359. doi: 10.1007/BF01531444. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevalence of autism spectrum disorders — Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morbidity and Mortality Weekly Report. 2012;61:1–19. [PubMed] [Google Scholar]
- Charney R. Pronoun errors in autistic children: Support for a social explanation. International Journal of Language & Communication Disorders. 1980;15:39–43. doi: 10.3109/13682828009011369. [DOI] [PubMed] [Google Scholar]
- Cheek A, Cormier K, Repp A, Meier RP. Prelinguistic gesture predicts mastery and error in the production of early signs. Language. 2001;77:292–323. [Google Scholar]
- Chess S, Fernandez P, Korn S. Behavioral consequences of congenital rubella. Journal of Pediatrics. 1978;93:699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- Chiat S. If I were you and you were me: The analysis of pronouns in a pronoun-reversing child. Journal of Child Language. 1982;9:359–379. doi: 10.1017/s0305000900004761. [DOI] [PubMed] [Google Scholar]
- Clibbens J, Harris M. Phonological processes and sign language development. In: Messer D, Turner G, editors. Critical influences on child language acquisition and development. Macmillan Press; New York: 1993. pp. 197–208. [Google Scholar]
- Conlin KE, Mirus GR, Mauk C, Meier RP. The acquisition of first signs: Place, handshape, and movement. In: Chamberlain C, Morford JP, Mayberry RI, editors. Language acquisition by eye. Lawrence Erlbaum Associates; Mahwah, NJ: 1999. pp. 51–70. [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Western Psychological Services; Los Angeles: 2002. [Google Scholar]
- Cunningham M, Cox EO. Hearing assessment in infants and children: Recommendations beyond neonatal screening. Pediatrics. 2003;111:436–440. doi: 10.1542/peds.111.2.436. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2005;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark T. Do deaf children with Autism Spectrum Disorder show deficits in the comprehension and production of emotional and linguistic facial expressions in British Sign Language (Doctoral dissertation) University College London; London, United Kingdom: 2011. [Google Scholar]
- Dewey D, Cantwell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit disorder. Journal of the International Neuropsychological Society. 2007;13:246–256. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Emmorey K. Language, cognition, and the brain: Insights from sign language research. Lawrence Erlbaum Associates; Mahwah, NJ: 2002. [Google Scholar]
- Evans K, Demuth K. Individual differences in pronoun reversal: Evidence from two longitudinal case studies. Journal of Child Language. 2012;39:162–191. doi: 10.1017/S0305000911000043. [DOI] [PubMed] [Google Scholar]
- Frost LA, Bondy AS. The Picture Exchange Communication System: Training manual. Pyramid Educational Consultants; Newark, DE: 1994. [Google Scholar]
- Fulwiler RL, Fouts RS. Acquisition of American Sign Language by a noncommunicating autistic child. Journal of Autism and Childhood Schizophrenia. 1976;6:43–51. doi: 10.1007/BF01537941. [DOI] [PubMed] [Google Scholar]
- Gallaudet Research Institute . Regional and national summary report of data from the 2009–2010 annual survey of deaf and hard of hearing children and youth. GRI, Gallaudet University; Washington, DC: 2011. [Google Scholar]
- Gilliam JE. Gilliam Autism Rating Scale. Second Edition. Pro-Ed; Austin, TX: 2006. [Google Scholar]
- Grossman RB, Tager-Flusberg H. Reading faces for information about words and emotions in adolescents with autism. Research in Autism Spectrum Disorders. 2008;2:681–695. doi: 10.1016/j.rasd.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham HS, Corley M, Rajendran G, Carletta J, Swanson S. Brief report: Imitation of meaningless gestures in individuals with Asperger Syndrome and High-Functioning Autism. Journal of Autism and Developmental Disorders. 2008;38:569–573. doi: 10.1007/s10803-007-0417-x. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Brindley R, Frith U. Visual perspective taking impairment in children with autistic spectrum disorder. Cognition. 2009;113:37–44. doi: 10.1016/j.cognition.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Hobson RP. Identification: The missing link between joint attention and imitation? Development and Psychopathology. 2007;19:411–431. doi: 10.1017/S0954579407070204. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A. Imitation and identification in autism. Journal of Child Psychology and Psychiatry. 1999;40:649–659. [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Jure R, Rapin I, Tuchman R. Hearing-impaired autistic children. Developmental Medicine and Child Neurology. 1991;33:1062–1072. doi: 10.1111/j.1469-8749.1991.tb14828.x. [DOI] [PubMed] [Google Scholar]
- Karnopp LB. Phonological acquisition in sign languages. Letras de Hoje. 1997;32:147–162. [Google Scholar]
- Kemper AR, Downs SM. A cost-effectiveness analysis of newborn hearing screening strategies. Archives of Pediatric and Adolescent Medicine. 2000;154:484–488. doi: 10.1001/archpedi.154.5.484. [DOI] [PubMed] [Google Scholar]
- Lacroix A, Guidetti M, Rogé B, Reilly J. Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Research in Developmental Disabilities. 2009;30:976–985. doi: 10.1016/j.ridd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Lee A, Hobson RP, Chiat S. I, you, me, and autism: An experimental study. Journal of Autism and Developmental Disorders. 1994;24:155–176. doi: 10.1007/BF02172094. [DOI] [PubMed] [Google Scholar]
- Liddell SK. American Sign Language syntax. Mouton; The Hague: 1980. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Austism Diagnostic Observation Schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Marentette PF, Mayberry RI. Principles for an emerging phonological system: A case study of early ASL acquisition. In: Chamberlain C, Morford JP, Mayberry RI, editors. Language acquisition by eye. Lawrence Erlbaum Associates; Mahwah, NJ: 2000. pp. 71–90. [Google Scholar]
- Meier RP. The acquisition of verb agreement: Pointing out arguments for the linguistic status of agreement in signed languages. In: Morgan G, Woll B, editors. Directions in sign language acquisition. John Benjamins; Amsterdam: 2002. pp. 115–141. [Google Scholar]
- Meier RP. The form of early signs: Explaining signing children's articulatory development. In: Schick B, Marschark M, Spencer PE, editors. Advances in the sign language development of deaf children. Oxford University Press; New York: 2006. pp. 202–230. [Google Scholar]
- Meyer JA, Hobson RP. Orientation in relation to self and other: The case of autism. Interaction Studies. 2004;5:221–244. [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain and Development. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Mitchell RE, Karchmer MA. Chasing the mythical ten percent: Parental hearing status of deaf and hard of hearing students in the United States. Sign Language Studies. 2004;4:138–163. [Google Scholar]
- Morgan GD, Barrett-Jones S, Stoneham H. The first signs of language: Phonological development in British Sign Language. Applied Psycholinguistics. 2007;28:3–22. [Google Scholar]
- Morgan GD, Smith NV, Tsimpli I, Woll B. Language against the odds: the learning of British Sign Language by a polyglot savant. Journal of Linguistics. 2002;38:1–41. [Google Scholar]
- Morgan GD, Smith NV, Tsimpli I, Woll B. Classifier learning and modality in a polyglot savant. Lingua. 2007;117:1339–1353. [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Newport EL, Meier R. The acquisition of American Sign Language. In: Slobin DI, editor. The crosslinguistic study of language acquisition. Volume 1: The data. Lawrence Erlbaum Associates; Hillsdale, NJ: 1986. pp. 881–938. [Google Scholar]
- Ohta M. Cognitive disorders of infantile autism: A study employing the WISC, spatial relationship conceptualization, and gesture imitations. Journal of Autism and Developmental Disorders. 1987;17:45–62. doi: 10.1007/BF01487259. [DOI] [PubMed] [Google Scholar]
- Padden CA. The acquisition of fingerspelling by deaf children. In: Siple P, Fischer SD, editors. Theoretical issues in sign language research, Vol. 2: Psychology. University of Chicago Press; Chicago: 1991. pp. 191–210. [Google Scholar]
- Padden CA. Learning to fingerspell twice: Young signing children's acquisition of fingerspelling. In: Schick B, Marschark M, Spencer PE, editors. Advances in the sign language development of deaf children. Oxford University Press; New York: 2006. pp. 189–201. [Google Scholar]
- Padden C, Lemaster B. An alphabet on hand: The acquisition of fingerspelling in deaf children. Sign Language Studies. 1985;14:161–172. [Google Scholar]
- Poizner H, Klima ES, Bellugi U. What the hands reveal about the brain. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Quinto-Pozos D, Forber-Pratt AJ, Singleton JL. Do developmental communication disorders exist in the signed modality? Perspectives from professionals. Language, Speech, and Hearing Services in the Schools. 2011;42:423–443. doi: 10.1044/0161-1461(2011/10-0071). [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales, Fifth Edition: Technical Manual. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised. Stoelting; Wood Dale, IL: 1997. [Google Scholar]
- Rosenhall U, Nordin V, Sandström M, Ahlsén G, Gillberg C. Autism and hearing loss. Journal of Autism and Developmental Disorders. 1999;29:349–357. doi: 10.1023/a:1023022709710. [DOI] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS. The development of emotion recognition in individuals with autism. Child Development. 2009;80:1434–1447. doi: 10.1111/j.1467-8624.2009.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Schick B, de Villiers P, de Villiers J, Hoffmeister R. Language and theory of mind: A study of deaf children. Child Development. 2007;78:376–396. doi: 10.1111/j.1467-8624.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) Journal of Autism and Developmental Disorders. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Seal BC, Bonvillian JD. Sign language and motor functioning in students with autistic disorder. Journal of Autism and Developmental Disorders. 1997;27:437–466. doi: 10.1023/a:1025809506097. [DOI] [PubMed] [Google Scholar]
- Siedlecki T, Bonvillian JD. Location, handshape & movement: Young children's acquisition of the formational aspects of American Sign Language. Sign Language Studies. 1993;78:31–52. [Google Scholar]
- Smith IM, Bryson SE. Gesture imitation in autism I: Nonsymbolic postures and sequences. Cognitive Neuropsychology. 1998;15:747–770. doi: 10.1080/026432998381087. [DOI] [PubMed] [Google Scholar]
- Smith NV, Tsimpli I, Morgan GD, Woll B. The signs of a savant: Language against the odds. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- Stokoe WC. Sign language structure: An outline of the visual communication systems of the American deaf. Studies in linguistics: Occasional papers (No. 8) Dept. of Anthropology and Linguistics, University of Buffalo; Buffalo: 1960. [Google Scholar]
- Szymanski C. Characteristics and symptomotology of autism in children who are deaf and hard of hearing (Doctoral dissertation) Gallaudet University; Washington, DC: 2010. [Google Scholar]
- Szymanski CA, Brice PJ, Lam KH, Hotto SA. Deaf children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1452-9. DOI: 10.1007/s10803-012-1452-9. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Current theory and research on language and communication in autism. Journal of Autism and Developmental Disorders. 1996;26:169–72. doi: 10.1007/BF02172006. [DOI] [PubMed] [Google Scholar]
- Takkinen R. Variation of handshape features in the acquisition process. In: Baker AE, van den Bogaerde B, Crasborn O, editors. Cross-linguistic perspectives in sign language research. Signum Verlag; Hamburg: 2003. pp. 81–91. [Google Scholar]
- Volker MA, Lopata C, Smith DA, Thomeer ML. Facial encoding of children with high-functioning Autism Spectrum Disorders. Focus on Autism and Other Developmental Disabilities. 2009;24:195–204. [Google Scholar]
- von Tetzchner S. First signs acquired by a Norwegian deaf child with hearing parents. Sign Language Studies. 1984;44:225–257. [Google Scholar]
- Williams JH, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34:285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]