Abstract
The bed nucleus of the stria terminalis (BNST) plays a critical role in regulating the behavioral response to stress. Stressors that activate the BNST also activate serotonergic (5-HT) systems. Hence, maladaptive changes of 5-HT receptor expression may contribute to stress-induced anxiety disorders. The BNST contains three neuronal types, Type I – III neurons. However, little is known about 5-HT receptor subtypes mRNA expression in these neurons, or whether it can be modulated by stress.
Whole-cell patch clamp recording from Type I – III neurons was used in conjunction with single cell RT-PCR to characterize 5-HT receptor mRNA expression, and examine the effects of stress on this expression. We report that Type I neurons expressed mRNA transcripts predominantly for 5-HT1A and 5-HT7 receptors. Type II neurons expressed transcripts for every 5-HT receptor except the 5-HT2C receptor. Type II neurons were divided into three sub-populations: Type IIA in which transcripts for 5-HT3 and 5-HT7 receptors predominate, Type IIB that mainly express 5-HT1B and 5-HT4 receptor transcripts, and Type IIC in which transcripts for 5-HT1A and 5-HT2A receptors predominate. Type III neurons were also subdivided into two sub-populations; one that predominantly expressed transcripts for 5-HT1A, 5-HT1B and 5-HT2A receptors, and another that mainly expressed transcripts for 5-HT2C receptor.
Unpredictable shock stress (USS) caused a long-lasting increase in anxiety-like behavior, and a concomitant decrease in 5-HT1A transcript expression in Type I – III neurons, as well as an up-regulation of a transcriptional repressor of 5-HT1A gene expression, deformed epidermal autoregulatory factor 1(Deaf-1). Significantly USS decreased 5-HT1A protein level, and increased the level of Deaf-1. USS also increased 5-HT1B transcript expression in Type III neurons, as well as 5-HT7 expression in Type I and II neurons. These data suggest that cell type-specific disruption of 5-HT receptor expression in BNSTALG neurons may contribute to stress-induced anxiety disorders.
Keywords: Bed nucleus of the stria terminalis, chronic stress, Deaf-1, serotonin receptors, single cell RT-PCR, 5-HT1A
Introduction
The stress response is an adaptive process essential for an animal to face challenging and threatening situations. Chronic stress, however, may produce maladaptive consequences such as anxiety disorders and depression (Lupien et al., 2009). Extra-hypothalamic responses to stress are mediated by several interconnected nuclei, including the bed nucleus of the stria terminalis (BNST), which play a key role in regulating the autonomic, neuroendocrine, and behavioral responses to stress (Walker et al., 2003, Choi et al., 2007, Crestani et al., 2010, Kuwaki, 2011). Importantly, the BNST is considered as the major integrative center of excitatory and inhibitory inputs that regulate the HPA axis (Forray and Gysling, 2004), and has been reported to mediate stress-responding and anxiety-like behavior resulting from persistent stress (Walker et al., 2009). Consistent with this observation, long-term plastic changes occur in the BNST in response to chronic stress, including alterations in the volume of the BNST that is accompanied by changes in the dendritic length and number of branch points in individual neurons (Vyas et al., 2003).
However, the BNST is a complex structure that is divided into anterior and posterior subdivisions by the fibers of the stria terminalis, into dorsal and ventral subdivisions by the fibers of the anterior commissure (De Olmos, 1985, Ju et al., 1989) and that contains approximately 16 different nuclei (Bota et al., 2012). Notably, the anterior BNST, and in particular the dorsolateral cell group (BNSTALG), contains a high density of neurons that express the stress neuropeptide, corticotropin releasing factor (Swanson et al., 1983) and has been reported to play a prominent role in modulating the behavioral response to chronic stressors (Dunn, 1987, Casada and Dafny, 1991, Shepard et al., 2006, Hammack et al., 2009, Davis et al., 2010, Christianson et al., 2011, Conrad et al., 2011). Recently, we identified three electrophysiolgically distinct cell types in the BNSTALG (Type I – III neurons, see Hammack et al., 2007) and we were interested to see how stress may affect the different cell types.
Stressors that activate the BNST also activate central serotonergic (5-HT) systems (Dilts and Boadle-Biber, 1995, Grahn et al., 1999b, Funada and Hara, 2001, Lowry, 2002, Summers et al., 2003, Takase et al., 2004). Interestingly, uncontrollable stress and anxiogenic drugs have been shown to activate a subset of serotonin (5-HT) containing neurons in the dorsal raphé nucleus (DRN) that preferentially target limbic forebrain regions such as the BNST (Grahn et al., 1999a, Lowry et al., 2000, Singewald et al., 2000). Previously, we have shown that activation of inhibitory 5-HTIA receptors in the BNSTALG has an anxiolytic action (Levita et al., 2004), and we have posited that activation of these receptors may function as an inhibitory-feedback mechanism to terminate the stress response (Hammack et al., 2009). Conversely, activation of excitatory 5-HT receptors, such as the 5-HT2A,2C,and7 receptors in BNSTALG neurons may have an opposing anxiogenic action (Guo et al., 2009). Hence, the net behavioral response to 5-HT release in the BNSTALG would be determined by the relative level of activation of these key receptor subtypes. It is noteworthy, therefore, that transgenic mice lacking the 5-HT1A receptor display decreased exploratory activity and increased fear of aversive environments compared to their wild type counterparts (Gordon et al., 2005), suggesting that reduced 5-HT1A receptor expression might result in heightened anxiety. Significantly, chronic stress, or chronic administration of stress hormones, has been shown to decrease 5-HT1A receptor expression and/or increase 5-HT2A receptor expression in other brain regions (Katagiri et al., 2001, Ossowska et al., 2001). A similar stress-induced alteration in the expression of either of these two receptors in the BNSTALG would be predicted to have a significant impact on stress-induced affective behavior.
Recent studies suggest that the 5-HT1A receptor gene is tightly regulated by an upstream dual repressor element (DRE) that inhibits gene expression in neuronal and non-neuronal systems (Ou et al., 2000). Subsequently, several novel DNA binding proteins have been shown to potently regulate 5-HT1A gene expression by binding to the DRE site, including hairy and enhancer of split 5 (Hes-5), five prime repressor element under dual binding protein (Freud-1) and deformed epidermal autoregulatory factor-1 (Deaf-1, also called NUDR for review see (Albert et al., 2011). Significantly, Deaf-1 is reported to selectively co-localize with 5-HT1A receptors in neurons of the DRN and limbic forebrain (Lemonde et al., 2003), and alterations in its expression have been associated with depression, suicide, and panic disorder (Szewczyk et al., 2009). However, nothing is known about the expression of the selective repressor elements in the BNSTALG, or whether their expression or that of any of the 5-HT receptor subtypes is altered following chronic stress.
Recently, we reported that mRNA transcripts for the 5-HT1A, 2A, 2C and7 receptor subtypes are differentially expressed in Type I–III BNSTALG neurons. Hence, the 5-HT1A receptor was seen to be expressed by all BNSTALG neurons, whereas 5-HT2A and 5-HT2C receptors were expressed exclusively by Type II, and Type III neurons, respectively. Similarly, 5-HT7 receptors were expressed mainly in Type I and II neurons but not in Type III neurons (Guo et al., 2009). Here, we extend these initial observations to examine 1) the mRNA expression pattern for all of the known 5-HT receptor subtypes in Type I–III BNSTALG neurons, and 2) the presence or absence of 5-HT1A receptor transcriptional repressors in these same neurons. We then further extend these studies to examine the effects of a repeated unpredictable shock stress (USS) paradigm on the expression of 5-HT receptor subtypes and/or 5-HT1A repressor elements in physiologically defined cell types in the BNSTALG.
Experimental Procedures
Animals
All experiments were conducted on male Sprague-Dawley rats (Charles River, NC) aged between 35 and 45 days of age. All rats were housed four per cage and had unrestricted access to food and water. Care was taken to minimize the number of animals used, and all procedures were done in accordance with policy guidelines set by the National Institutes of Health and were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Single Cell RT-PCR
Whole cell patch clamp recordings were obtained from visually identified BNSTALG neurons in 350 μm brain slices, and individual cell types were determine as previously described (Hammack et al., 2007, Guo et al., 2009, Guo and Rainnie, 2010). Briefly, three electrophysiologically distinct cell types have been defined (Hammack et al., 2007); Type I neurons are characterized by a regular firing pattern in response to membrane depolarization, and a depolarizing sag in the voltage response to hyperpolarizing current injection that is mediated by the hyperpolarization-activated cation current, Ih. Type II neurons are characterized by a burst-firing pattern that is mediated by activation of the low-threshold calcium current, IT, and also express a prominent Ih. Type III neurons are characterized by a regular firing pattern, have no prominent Ih, and a pronounced fast hyperpolarization-activated voltage rectification indicative of the inwardly rectifying potassium current, IK(IR).
At the end of each recording session, the cell cytoplasm was aspirated into the patch recording pipette containing ~5 μl of RNase-free patch solution under visual control, by applying gentle negative pressure. The contents of the patch pipette were then expelled into a microcentrifuge tube containing 5 μl of the reverse transcription cocktail (Applied Biosystems, Foster City, CA). The RT product was amplified in triplicate and screened for 18S rRNA. Only those cell samples that were positive for 18S rRNA were subjected to amplification with primers. The procedure used to determine mRNA transcript expression in single cells has been described (Hazra et al, 2011). The sequence for the oligonucleotide primers of the 5-HT receptor subtypes used in this study have been reported previously (Guo et al, 2009). The primers used for Deaf-1 is forward 5'- GGT TTG TGC AGT GGT AGA TG-`3 and reverse 5'- GAG CGT GCC ACT GAT GTT C-`3 (Accession # NM_031801, 521bp); Freud-1 is forward 5'- CGC CAG CTG CAC TTC TAT AC -`3 and reverse 5'- CTC ACT CTC CAC CAG GTT CC -`3 (Accession # NM_001013869, 400bp); and Hes-5 is forward 5'- CGC ATC AAC AGC AGC ATT GAG -`3 and reverse 5'- TGG AAG TGG TAA AGC AGC TTC-`3 (Accession # NM_024383, 350bp). PCR products were visualized by staining with ethidium bromide and separated by electrophoresis in a 1% agarose gel.
Controls for the RT-PCR
PCR conditions were optimized using total RNA isolated from rat BNSTALG so that a PCR product could be detected from (250pg–1ng) of total RNA without contamination caused by non-specific amplification. For each PCR amplification, sterile water was used instead of cDNA as a control for contaminating artifacts. A second control with no RT present was also used in each amplification step. Both the controls gave negative results throughout the study. All primers were intron-spanning to exclude amplification of genomic DNA.
Acoustic startle test
In the acoustic startle response (ASR), rats were placed in an acoustic startle chamber for 5 minutes prior to testing to acclimatize the animals to the chambers. Four rats were tested simultaneously in identical 8 × 15 × 15 cm Plexiglas and wire mesh cages, each suspended between compression springs within a steel frame located within a custom-designed sound-attenuating chamber. Details of the recording apparatus have been reported previously (Walker and Davis, 1997, 2002, Sink et al., 2011). Subsequently, the ASR was measured following each of 30 acoustic stimuli. Startle responses were evoked by 50-ms 100, 105 and 110 dB white-noise bursts generated in a pseudorandom order by a Macintosh G3 computer sound file, amplified by a Radio Shack amplifier (100 W, Model MPA-200; Tandy, Fort Worth, TX), and delivered through speakers located 5 cm in front of each cage. Startle amplitude was defined as the maximum peak-to-peak voltage of the Instrument's output during the first 200 ms after each noise burst. The presentation and sequencing of all stimuli was under the control of a Macintosh G3 computer using custom designed software (The Experimenter; Glassbeads Inc.,Newton, CT). On day 10 a similar ASR paradigm was followed in both non stress (NS) and 4 day unpredictable shock stress rats (USS).
Repeated stress procedure and behavioral analysis
The USS paradigm used in these experiments was adapted from previous studies in rat and human (Walker et al., 2003, Moberg and Curtin, 2009). Here, rats were placed inside a modular operant conditioning chamber, 59.7 × 34.3 × 26.35 cm, with aluminum and polycarbonate walls (Lafayette Instruments, Lafayette, IN). The floor of the chamber is made of 0.4 cm diameter stainless steel bars spaced at 1.1 cm that conducts the electric shock. Before administering the USS, rats, 35 days of age, were first matched for their basal anxiety level using a standard acoustic startle paradigm (see above). In total, 32 rats were matched according to their initial startle response, and then divided into two groups, 16 NS rats and 16 USS rats. Eight rats (NS=4; USS=4) were used for total RNA isolation, eight rats for protein isolation, eight rats for single cell RT-PCR, and eight rats for contextual freezing. NS animals received exactly the same handling procedures as the USS group and were placed in the shock chamber for the same duration without being shocked. On day 1, the USS rats were placed in the shock chamber and allowed to habituate to their environment for 5 minutes. Rats then received two eight minute periods of eight randomly applied footshocks (0.5s, 0.5 mA) separated by an eight minute period of no shock. The USS paradigm was repeated on each of the following three days for a total of four consecutive days shock stress. Each rat was shocked at approximately the same time every day (9 AM) to control for diurnal hormone variations. Rats in both groups were then returned to their home cages for six days. On the tenth day, the rats were first measured for their post-stress startle response, and then placed in the chamber in which they received the shock and their contextual freezing behavior assessed over a 5 minute period, recorded on video, and analyzed off-line using the Freezescan software program (Cleversys Inc, VA). Rats were then immediately sacrificed; the brain removed, and processed for RNA and protein isolation using standard procedures (Dabrowska and Rainnie, 2010, Hazra et al., 2011).
Quantitative PCR measures of transcript expression
RNA Isolation
Total RNA was isolated from BNSTALG tissue by homogenizing each sample in Trizol (Invitrogen, Carlsbad, CA). The isolated RNA was then reverse transcribed using a cocktail containing 5 μl of 10×RT buffer, 10mM dNTP mix, 10× random hexanucleotide and Multiscribe RT 5U/ul and RNAase free water. The mixture was incubated in a thermal cycler at 25°C for 10 min and then at 37°C for 120 min, the resulting cDNA samples were stored at −20°C. All reagents were obtained from Applied Biosystems (Foster City, CA).
Quantitative PCR
Real-time PCR reactions were performed using an Applied Biosystems 7500 Fast-Real Time PCR system (Applied Biosystems, Foster City, CA). Here, 2μl of cDNA obtained from the isolated RNA were combined with Taqman probes specific for 18S rRNA (Accession # X03205), 5-HT1A (NM_012585), 5-HT1B (NM_022225), 5-HT1D (NM_012852), 5-HT1F (NM_021857), 5-HT2A (NM_017254), 5-HT2C (NM_012765),5-HT3 (NM_024394), 5-HT4 (NM_012853), 5-HT5 (NM_013148), 5-HT6(NM_024365),5-HT7 (NM_022938), Deaf-1 (NM_031801),Freud-1 (NM_001013869) and Hes5 (NM_024383) and 1×Taqman universal PCR Master Mix (Applied Biosystems). The reaction for each cell sample was performed in triplicate, and using a 40 cycle thermal cycling program: cycle 1– 20 min at 95°C; cycles 2 through 50– 95°C for 3 sec, followed by 60°C for 30 min. The relative levels of mRNA expression were normalized in all the samples with expression levels of 18S rRNA. The 2−ΔΔCt method of relative quantification was used to calculate the fold change in expression of genes. For statistical analysis, we used mean ΔΔCt ±SEM between NS and USS groups (Livak and Schmittgen, 2001).
Western blotting
Western blots were used to determine the relative expression of 5-HT1A receptor and Deaf-1 protein in isolated BNSTALG samples using procedures mentioned previously (Dabrowska and Rainnie, 2010). In brief, 25 μg of protein per sample was loaded onto polyacrylamide-SDS mini-gels (Bio-Rad, Hercules, CA, USA), separated electrophoretically, blotted onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and blocked for 1 h in blocking buffer containing 2% nonfat dry milk, 0.1% Tween 20, 0.05 M NaCl, and 1 M HEPES (pH 7.4). To examine the relative expression of 5-HT1A and Deaf-1 the membranes were incubated with the following primary antibodies: rabbit polyclonal anti-5-HT1A (1:1000, AB 15350, Chemicon-Millipore, Billerica, MA), and rabbit monoclonal against Deaf-1 (1:1000, ab75792, Abcam, Cambridge, MA). On the following day, the immunoblots were incubated with an HRP-labeled specific secondary antibody (peroxidase conjugated anti-rabbit IgG antibody, Vector Labs, 1:2000) for 1 h at room temperature. The level of 5-HT1A and Deaf-1 proteins in the BNSTALG homogenates was determined using SuperSignal West Chemiluminescence (Pierce Biotechnology) and visualized with an Alpha Innotech Fluorochem imaging system (Alpha Innotech, San Leandro, CA).
Data analysis
Statistically significant differences were determined by Student's t test. The results are presented as mean ± SEM.
Results
Previously, we reported that mRNA transcripts for almost every 5-HT receptor subtype were expressed in whole tissue homogenates of the BNSTALG (Guo et al, 2009). Here, we extended our initial PCR observations to look at the expression profile for all of the 5-HT receptor subtypes in physiologically defined Type I – III BNSTALG neurons.
Transcriptome analysis of serotonin receptor subtypes in Type I – III BNSTALG neurons
For this study, we recorded the membrane properties and extracted the cytosolic mRNA from 75 visually identified neurons in the BNSTALG. Of these 75 neurons, 19/75 were Type I neurons, 34/75 were Type II neurons, and 22/75 were Type III neurons (see Methods for characterization criteria). It should be noted that the sample size of Type I and III neurons were inflated in order to allow comparisons between cell types, and do not represent their normal distribution in the general population (Hammack et al., 2007).
The relative transcriptome distribution for each of the 5-HT receptor subtypes in the three BNSTALG cell types is illustrated in Table 1. Here, Type I neurons (n=19) were seen to express mRNA transcripts predominantly for 5-HT1A (63%) and 5-HT7 (53%) receptors, at the expense of almost every other receptor subtype apart from 5-HT2C (5%) and 5-HT3 (11%) receptors. Phenotypically, there appear to be two sub populations of Type I neurons: one that expresses both 5-HT1A and 5-HT7 receptor transcripts (n = 10), and another that only expresses 5-HT1A receptor transcripts (n = 9).
Table 1.
Single-cell RT-PCR analysis of the relative distribution of 5-HT receptor subtypes mRNA in BNSTALG neurons.
| No of cell expressed | 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1F | 5-HT2A | 5-HT2C | 5-HT3 | 5-HT4 | 5-HT5 | 5-HT6 | 5-HT7 | Deaf-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I (19) | 63% | 0% | 0% | 0% | 0% | 5% | 11% | 0% | 0% | 0% | 53% | 21% |
| 10/19 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 |
| 9/19 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 4 |
| Type II (34) | 32% | 21% | 12% | 9% | 32% | 0% | 44% | 21% | 9% | 9% | 44% | 29% |
| A (15/34) | 0 | 0 | 2 | 1 | 0 | 0 | 15 | 0 | 0 | 2 | 15 | 10 |
| B(7/34) | 0 | 7 | 1 | 1 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 0 |
| C(11/34) | 11 | 0 | 1 | 1 | 11 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Type III (22) | 41% | 41% | 0% | 18% | 32% | 59% | 27% | 0% | 18% | 0% | 0% | 32% |
| 9/22 | 9 | 9 | 0 | 4 | 7 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| 13/22 | 0 | 0 | 0 | 0 | 0 | 13 | 6 | 0 | 0 | 0 | 0 | 7 |
The table shows the percentage of 5-HT receptor subtypes expression in different BNSTALG neurons (Type I–III). The number of neurons expressed 5-HT receptors subtypes and Deaf-1 mRNA in Type I–III subpopulation of BNSTALG neurons, has been shown.
Type II neurons (n = 34) expressed transcripts for almost all of the 5-HT receptor subtypes, with the exception of 5-HT2C receptors. There are, however, six 5-HT receptor subtypes that are predominantly expressed by Type II neurons; namely 5-HT1A (32%), 5-HT1B (21%), 5-HT2A (32%), 5-HT3 (44%), 5-HT4 (21%) and 5-HT7 (44%). In a previous paper we have argued that Type II neurons could be subdivided into 3 distinct subpopulations based on their ion channel mRNA expression pattern (Hazra et al, 2011). A similar tripartite division of Type II neurons could be seen in the current study, based on their 5-HT receptor subtype transcript expression pattern. One population (Type IIA) in which transcripts for 5-HT3 and 5-HT7 (n=15) receptors predominate, a second population (Type IIB) that mainly express 5-HT1B and 5-HT4 receptor transcripts (n=7), and a third population (Type IIC) in which transcripts for 5-HT1A and 5-HT2A receptors predominate (n=11). By comparison, expression of transcripts for 5-HT1D (12%), 5-HT1F (9%), 5-HT5 (9%), and 5-HT6 receptors (9%) appear to be uniformly distributed throughout the three sub-populations.
Finally, Type III neurons (n = 22) also expressed transcripts for multiple 5-HT receptor subtypes, with the exception of 5-HT1D, 5-HT4, 5-HT6 and 5-HT7 receptors. The most frequently expressed transcripts were those for the 5-HT1A (41%), 5-HT1B (41%), 5-HT2A (32%), 5-HT2C (59%), and 5-HT5 receptor subtypes (18%). Like Type I neurons, there appear to be two sub-populations of Type III neurons; a subpopulation that predominantly expressed transcripts for 5-HT1A, 5-HT1B and 5-HT2A receptors (n=9), and another that predominantly expressed transcripts for 5-HT2C receptor (n=13).
Together these data suggest that Type I–III neurons may represent heterologous cell populations that could be differentially affected by prolonged stress manipulations. Stress has been reported to alter the expression of several 5-HT receptor subtypes including 5HT1A and 5-HT7 receptors (Chaouloff et al., 1999, Chaouloff, 2000, Xu et al., 2011). Hence, we next examined the effects of repeated USS on anxiety-like behavior and looked to see if there were any correlated changes in the expression pattern of transcripts for the different 5-HT receptor subtypes in Type I – III neurons.
Effects of repeated USS on baseline startle and freezing behavior
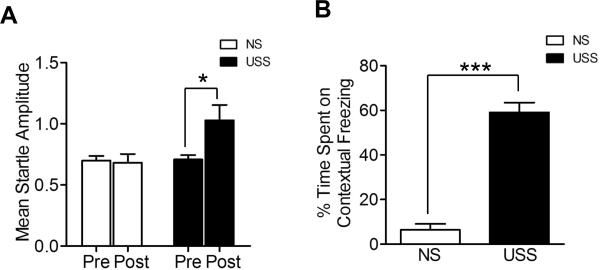
Rats were first tested for their baseline startle response prior to the repeated USS and again six days after the last shock presentation (Walker and Davis, 1997, 2002). No significant difference was observed between the NS and USS groups prior to training. However, as shown in Figure 1, rats receiving repeated USS (n =16, P<0.05) showed a significantly enhanced startle response compared to NS rats (n = 16). Moreover, when NS and USS rats were re-exposed to the shock chamber, as expected, the USS group showed significantly (P<0.001) more contextual freezing on day 10 than the NS group. Together these data strongly suggest that repeated USS induces a long-lasting increase in anxiety-like behavior and contextual fear.
Figure 1. Effect of unpredictable shock stress (USS) in startle and contextual freezing behavior.
Four daily sessions of USS causes a significant long-lasting enhancement of baseline startle and contextual freezing in Sprague-Dawley rats. Six days after the last presentation of the USS (A) Mean baseline startle amplitude were enhanced significantly (P<0.05) and (B) percentage of time spent on contextual freezing behavior was increased significantly (P<0.001) as well.
Effects of repeated USS on the mRNA expression of 5-HT receptor subtypes in whole tissue BNSTALG and in Type I–III neurons
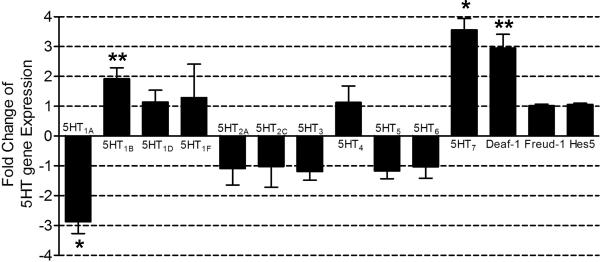
We next examined the effects of repeated USS on mRNA expression for all of the 5-HT receptor subtypes and the transcriptional regulators (Deaf-1, Freud-1 and Hes-5) in whole tissue homogenates of the BNSTALG using quantitative RT-PCR. As illustrated in Figure 2, exposure to repeated USS failed to cause any significant change in mRNA expression for the 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT5, and 5-HT6 receptor subtypes compared with the mRNA expression pattern in NS control rats. However, a 2.8 fold decrease in 5-HT1A receptor mRNA expression was observed, together with a concomitant 2 and 3.5 fold increase of 5-HT1B and 5-HT7 receptor mRNA expression, respectively.
Figure 2. Effect of unpredictable shock stress (USS) on 5-HT receptors and regulators gene expression in the BNSTALG tissue.
Quantitative RT-PCR was performed in whole BNSTALG tissue to determine the mRNA expression of 5-HT receptors (5-HT1A-7) and transcriptional regulators (Deaf-1, Freud-1, Hes-5) in USS rats. Each bar represents the relative fold change of a specific gene as determined by 2−ΔΔCt method of quantification in non-stress (NS) and USS rats (n=4). The data shows 5-HT1A receptor mRNA expression was down-regulated by 2.8 fold and 5-HT1B, 5-HT7 and Deaf-1 mRNA expression was up-regulated by 2, 3.5 and 3 fold respectively. Mean ΔΔCt ±SEM values show significant difference in 5-HT1A (P<0.05), 5-HT1B (P<0.01), 5-HT7 (P<0.05) and Deaf-1(P<0.01) mRNA expression in USS rats.
Given that we had previously shown selective expression for these receptor transcripts in distinct subpopulations of BNSTALG neurons, these data further suggested that repeated USS may preferentially disrupt mRNA expression in discrete subpopulations of BNSTALG neurons. However, whole tissue mRNA expression is derived not only from BNSTALG neurons but also from Schwann cells, glia, and vascular tissue in the surrounding neuropil. Consequently, we next examined the effects of repeated USS on 5-HT receptor subtype mRNA expression in Type I–III neurons using scRT-PCR. The results of these studies are summarized in Table 2. Here, 5-HT receptor mRNA transcripts in Type I – III neurons showed a similar subtype specific change in expression pattern to the changes seen in mRNA expression in tissue homogenates. Hence, no significant change was observed in the number of neurons showing mRNA transcript expression for the 5-HT1D, 5-HT1F, 5-HT2A, 5-HT3, 5-HT4, 5-HT5, and 5-HT6 receptor subtypes in Type I–III neurons. However, repeated USS caused a marked reduction in the number of neurons showing 5-HT1A receptor mRNA transcript expression, down from 63% to 10% of Type I neurons, from 32% to 16% of Type II neurons, and from 41% to 28% of Type III neurons. In contrast, repeated USS caused a significant increase in the number of Type III neurons showing 5-HT1B receptor mRNA expression (78%), without affecting the number of Type II neurons expressing the same receptor transcripts. Finally, the number of Type II neurons showing 5-HT7 mRNA transcript expression increased from 44% to 60% following USS, and the number of Type I neurons rose from 53% to 70%. Together these data suggest that repeated USS caused a widespread reduction in the expression of transcripts for the 5-HT1A receptor, and a cell type-specific modulation of expression for the 5-HT1B and 7 receptor subtypes in BNSTALG neurons.
Table 2.
Unpredictable shock stress changes the mRNA expression profile for 5-HT receptor subtypes in Type l-lll neurons.
| No of cells expressed | 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1F | 5-HT2A | 5-HT2C | 5-HT3 | 5-HT4 | 5-HT5 | 5-HT6 | 5-HT7 | Deaf-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I (10) | 10% | 0% | 0% | 0% | 0% | 0% | 20% | 0% | 0% | 0% | 70% | 40% |
| 10/10 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 7 | 4 |
| Type II (25) | 16% | 24% | 16% | 16% | 16% | 0% | 40% | 16% | 16% | 12% | 60% | 40% |
| A15/25 | 0 | 0 | 4 | 0 | 0 | 0 | 10 | 0 | 0 | 3 | 15 | 10 |
| B 6/25 | 0 | 6 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| C 4/25 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Type III (18) | 28% | 78% | 0% | 17% | 28% | 22% | 17% | 0% | 17% | 0% | 0% | 50% |
| 12/18 | 5 | 12 | 0 | 2 | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 6 |
| 6/18 | 0 | 2 | 0 | 1 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 3 |
The table shows the percentage of 5-HT receptor subtypes expression in USS BNSTALG neurons (Type I–III). The number of neurons expressed 5-HT receptors subtypes and Deaf-1 mRNA in Type I–III subpopulation of BNSTALG neurons, has been shown.
Effects of repeated USS on the mRNA expression for 5-HT1A transcriptional repressor elements in BNSTALG tissue and Type I–III neurons
We next determined whether concomitant changes could be observed in the expression of transcripts for the transcriptional repressors of 5-HT1A receptor gene expression. Following repeated USS, Deaf-1 mRNA expression was seen to be significantly, 3 fold, up-regulated in whole tissue homogenates (P<0.05). In contrast, repeated USS caused no significant change in Freud-1 or Hes-5 mRNA transcript expression (Figure 2). Because expression of Freud-1 and Hes-5 mRNA did not show any changes in whole tissue homogenates, we restricted our scRT-PCR screening of mRNA transcripts in single neurons to Deaf-1. Low level Deaf-1 mRNA expression was observed in all three cells types in NS control animals (Type I 21%, Type II 29%, and Type III 32%). Following repeated USS, Deaf-1 mRNA expression increased in all three cell types to 40%, 40%, and 50% respectively. The global decrease of 5-HT1A receptor mRNA expression and the concomitant increase in Deaf-1 mRNA expression in Type I – III neurons suggested a potential cause-effect relationship, which could induce a functional change in the response to local 5-HT release following repeated USS. However, these changes may be negated by post-translational modification of protein expression. Hence, we next determined if repeated USS could also induce changes in protein expression for these two key elements of 5-HT signaling in the BNSTALG.
Effects of repeated USS on 5-HT1A receptor and Deaf-1 protein expression in the whole tissue homogenates of the BNSTALG
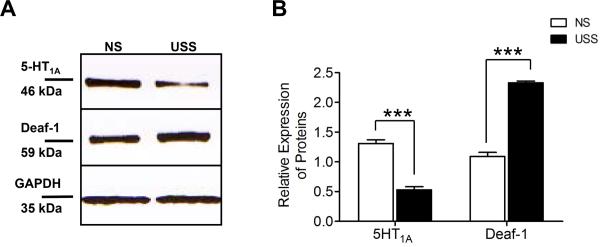
Here, the expression levels of 5-HT1A, and Deaf-1 peptides were determined using Western blot analysis of whole tissue BNSTALG extracts from NS and repeated USS rats. To normalize the data and control for variability in sample loading, protein expression was determined relative to the expression of a house-keeping peptide, GAPDH. As illustrated in Figure 3, single bands were observed for all three proteins at the predicted molecular weights. In agreement with results from our RT-PCR study, quantitative analysis revealed a significant decrease (P<0.001) in 5HT1A receptor protein levels in BNSTALG homogenates from repeated USS rats compared to the NS control group. Conversely, protein levels for Deaf-1 were significantly increased (P<0.001) in BNSTALG homogenates from the repeated USS group compared to the NS group.
Figure 3. Expression of 5-HT1A and Deaf-1 protein in unpredictable shock stress (USS) rats.
(A) Representative western blot analysis of BNSTALG tissue revealed reduced expression of 5-HT1A (46 kDa) protein following USS. Consequently Deaf-1 protein expression, showing distinct band at 59 kDa, was enhanced considerably after USS. Lower bands represent GAPDH loading controls. (B) A bar chart showing the quantitative data of relative protein expression in all western blots experiments (n=4) performed. A significant decrease in 5-HT1A (P<0.001) and a significant increase in Deaf-1 (P<0.001) protein expression was noted in the BNSTALG of USS rats relative to their non-stress counterparts.
Discussion
The results of our study have confirmed and extended our original observations of neuronal diversity in the BNSTALG and revealed that subgroups of Type I – III neurons differentially express specific combinations of 5-HT receptor subtypes. Hence, two subpopulations of Type I neurons were identified, one of which expressed transcripts for 5HT1A receptors only, and one which expressed transcripts for both 5-HT1A and 5-HT7 receptors. Three Type II subpopulations were identified; for comparative purposes we have named these subpopulations Type IIA – IIC. Type IIA neurons predominantly expressed transcripts for 5-HT3 and 5-HT7 receptors. Type IIB neurons predominantly expressed transcripts for 5-HT1B and 5-HT4 receptors, and Type IIC neurons predominantly expressed transcripts for 5-HT1A and 5-HT2A receptors. Lastly two subpopulations of Type III neurons were identified, one of which expressed transcripts for 5-HT1A, 5-HT1B and 5-HT2A receptors, and one which only expressed transcripts for 5-HT2C receptors. Intriguingly, Deaf-1 transcript expression in all BNSTALG neurons from NS control animals was low (~20–30%), and restricted primarily to those subpopulations that did not normally express transcripts for the 5-HT1A receptor, with the exception of the Type I subpopulation.
Consistent with our previous observation (Hammack et al., 2009), repeated USS caused a long-lasting increase in anxiety-like behavior as measured, 6 days after the last shock delivery, by an elevated baseline startle response and enhanced contextual freezing. Using quantitative RT-PCR we then showed that the increase in anxiety-like behavior and contextual fear was associated with 1) a decrease in transcript expression of the inhibitory 5-HT1A receptor, 2) an increase in transcript expression of excitatory, 5-HT2C, and 5-HT7 receptors, and 3) an increase in 5-HT1B receptor expression in BNSTALG homogenates. Repeated USS also caused a significant increase in the mRNA and protein expression for the 5-HT1A receptor repressor element, Deaf-1, in the BNSTALG. Moreover, our scRT-PCR study further revealed that whereas the reduction in 5-HT1A receptor transcript expression and increase in Deaf-1 expression was observed across the board in Type I – III neurons, stress-induced alterations in 5-HT1B, 5-HT2C, and 5-HT7 receptor transcript expression were cell type-specific. Hence, 5-HT1B receptor transcript expression only increased in one of the two Type III neuron subpopulations, and 5-HT2C expression only decreased in the other. Intriguingly, 5-HT7 transcripts appeared to increase in the same subpopulation of Type I neurons in which 5-HT1A receptor expression decreased, and also increased in Type IIA neurons, which do not normally express 5-HT1A receptor transcripts.
Together these data strongly suggest that repeated stress exposure would markedly affect the response of Type I – III neurons to local release of 5-HT in the BNSTALG. Globally, inhibitory control by postsynaptic 5HT1A receptor activation would be reduced in Type I – III neurons, whereas excitatory drive would increase in subpopulations of Type I and IIA neurons through 5-HT7 receptor activation, and in Type III neurons through 5-HT2C receptor activation. Given that electrical stimulation of the BNSTALG increases anxiety-like behavior (Shaikh et al., 1986, Casada and Dafny, 1991, Dunn and Williams, 1995) a net increase in 5-HT-induced excitation in the BNSTALG may contribute to the long-lasting anxiety-like behavior induced by repeated USS.
Functional correlates of 5-HT receptor subtype expression in the BNSTALG
Our study is the first to systematically investigate baseline mRNA expression for all of the known 5-HT receptor subtypes (5-HT1A-5-HT7) in whole BNSTALG tissue homogenates as well as in individual BNSTALG neurons (Type I–III). As previously reported, 5-HT can both inhibit and excite neurons of the BNSTALG (Levita et al., 2004; Guo et al., 2009). Inhibition was the most prevalent response via activation of 5-HT1A receptors, whereas excitation was mediated by activation of 5-HT2A, 5-HT2C, and/or 5-HT7 receptors. However, most neurons within the BNSTALG responded to 5-HT with a mixed response whereby an initial inhibition was followed by excitation. In these neurons, the 5-HT1A receptor-mediated inhibitory response was followed by excitation mediated by either 5-HT2A or 5-HT7 receptor activation. The results of our present study are consistent with these initial observations in that our single cell RT-PCR data revealed that ~ 50% of all BNSTALG neurons (32/65) expressed mRNA transcripts for the 5-HT1A receptor. We have shown that selective in vivo activation of 5-HT1A receptors in the BNSTALG elicits an anxiolytic-like behavioral response in rats (Levita et al., 2004). Activation of 5-HT1A receptors in the BNST has been reported to mediate the facilitation of the baroreflex response by induced cannabinoids in response to blood pressure increases (Alves et al., 2010, Gomes et al., 2011). Together these data suggest that activation of a distinct population of BNSTALG neurons comprised of the majority of Type I neurons, Type IIC neurons, and a subpopulation of Type III neurons may play a critical role in the acute response to adverse environmental stimuli. Consistent with this premise, Type I neurons co-expressed mRNA transcripts for the 5-HT7 receptor, whereas Type IIC neurons and Type III neurons co-expressed transcripts for the 5-HT2A receptor. Recent studies have suggested that activation of 5-HT2A and 5-HT7 receptors may facilitate anxiety-like behavior (Delgado et al., 2005, Hedlund, 2009), and that ligands with mixed 5-HT1A receptor agonist and 5-HT2A receptor antagonist properties may make more effective anxiolytics (Delgado et al., 2005).
Significantly, Type IIA, Type IIB, and the remainder of the Type III neurons never expressed mRNA transcripts for the 5HT1A receptor. Notably, the Type IIA neurons expressed transcripts for the 5-HT3 and 5-HT7 receptor subtypes, whereas the subpopulation of Type III neurons lacking 5-HT1A receptor transcripts expressed transcripts for the 5-HT3 and 5-HT2C receptor subtypes suggesting that this population of neurons could respond to local 5-HT release with a rapid excitation mediated by 5-HT3 receptor activation (Farber et al., 2004) as well as a slower excitation mediated by 5-HT2C/7 receptor activation (Guo et al., 2009). Like activation of 5-HT7 receptors, activation of 5-HT2C and 5-HT3 receptors has been reported to have anxiogenic-like actions (Delgado et al., 2005, Harada et al., 2006, Dekeyne et al., 2008) suggesting that these neurons may play a role in the rapid anxiogenic response to acute stressors. However, a caveat to this hypothesis is that the BNSTALG is primarily a GABAergic system and it is possible that a subset of these neurons act as local circuit inhibitory interneurons, and function to inhibit the activity of BNSTALG output neurons.
Intriguingly, the subpopulation of Type III neurons that expressed mRNA transcripts for 5-HT1A and 5-HT2A receptors also expressed 5-HT1B receptor transcripts. 5-HT1B receptors not only act as autoreceptors to modulate serotonergic transmission, but also act as heteroreceptors to modulate release of other neurotransmitters (Morikawa et al., 2000). Several studies have reported high levels of 5-HT1B receptor binding sites in the BNST (Bonaventure et al., 1997, Cloez-Tayarani et al., 1997, Cloez-Tayarani et al., 1998), and we have shown that activation of presynaptic 5-HT1B receptors reduced glutamate transmission in the BNSTALG (Guo et al., 2010). However, it is most likely that any protein resulting from transcription of the 5-HT1B receptor mRNA would be shipped to the axon terminals of these neurons to regulate release of their endogenous neurotransmitters. It is interesting to note, therefore, that 5-HT1B receptor knockout mice show an exaggerated autonomic response to stress (Bouwknecht et al., 2000, Groenink et al., 2003) just like 5-HT1A receptor knockout mice (Sibille and Hen, 2001). Hence, activation of 5-HT1A and 5-HT1B receptors in these neurons may act synergistically to limit transmitter release to downstream targets.
5-HT receptor subtype mRNA expression is altered after USS
Previously, we have shown that acute CRF receptor activation or a mild stress (one week isolation housing) could facilitate the 5-HT1A-mediated inhibitory response in BNSTALG neurons (Hammack et al, 2009), whereas repeated restraint stress facilitated anxiety-like behavior and altered whole tissue expression of selected 5HT receptor subtypes. Moreover, Chattarji and colleagues have shown that chronic stress can result in dendritic remodeling in BNST neurons (Vyas et al., 2003). Hence, stress intensity and/or duration appear to be critical factors in regulating affective behavior by promoting neuronal plasticity and/or remodeling within the BNST. The results of the current study extend these observations and show that repeated USS caused a prolonged increase in anxiety-like behavior that was associated with a 2.8 fold reduction in 5-HT1A mRNA expression in BNSTALG tissue homogenates and a concomitant decrease in 5-HT1A mRNA expression in Type I – III BNSTALG neurons. These data are consistent with previous studies showing that chronic stress or corticosterone administration resulted in reduced 5HT1A receptor expression in other brain regions (Ferretti et al., 1995, McKittrick et al., 1995, Crayton et al., 1996, Fernandes et al., 1997, Takao et al., 1997, Lopez et al., 1998, Maines et al., 1999). Moreover, 5-HT1A knockout mice show increased anxiety-like behavior compared to their wild-type litter mates (Parks et al., 1998), and show increased freezing and tachycardia in response to footshock (Gross et al., 2000, Alves et al., 2010). As noted above, activation of 5-HT1A receptors in the BNST is thought to facilitate the baroreflex response to increased blood pressure (Alves et al., 2010). Hence, reduced 5-HT1A receptor expression in response to repeated USS may directly contribute to the enhanced anxiety-like behavior observed in these animals. A similar down-regulation of 5-HT1A receptor expression has been observed in the prefrontal cortex in response to chronic social defeat (Kieran et al., 2010). Significantly, dysfunction of the 5-HT1A receptor has been associated with anxiety and major depressive disorder in humans (Savitz et al., 2009), and stress is known to be a major precipitating factor in the etiology of both of these disorders (Heim and Nemeroff, 2002).
It is important to note that unpredictable shock has also been shown to cause a prolonged and elevated release of 5-HT in the limbic forebrain (for review see (Maier and Watkins, 2005), and that this response was dependent on CRF release within the dorsal raphe nucleus (DRN). We have shown that CRF neurons in the BNSTALG of transgenic mice that selectively express green fluorescent protein (GFP) in this cell population (Martin et al., 2010) have identical physiological properties to those of Type III neurons of the rat BNSTALG (Rainnie DG, 2010). Here, we show that USS caused a significant reduction in the expression of 5-HT1A receptor transcripts in Type III neurons of the rat, suggesting that USS may result in a dis-inhibition of BNSTALG CRF neurons. The BNST has been shown to send afferent projections to the DRN (Peyron et al., 1998) and hence dis-inhibition of BNST CRF neurons may contribute to the hyper-activation of the DRN induced by unpredictable stress. Significantly, USS also reduced 5-HT1A mRNA transcript expression in Type I and Type IIC neurons and the resultant dis-inhibition of these neurons may also contribute to the heightened anxiety-like behavior observed following USS. Studies are in progress to determine the neuropeptide phenotype of these subpopulations of BNSTALG neuron. Consistent with the results presented here, a recent human study has shown that tryptophan depletion increases anxiety, but not fear, and that this response may result from reduced serotonergic inhibition of CRF neurons in the BNST (Robinson et al., 2012).
Dis-inhibition is not the only mechanism by which USS may increase the output of BNST neurons. As noted above, 5-HT release is increased in the forebrain following USS, and we have shown that the expression of transcripts for several 5-HT receptor subtypes that could potentially mediate excitation of BNST neurons is unaffected by USS and in some cases expression is up-regulated. Thus, activation of excitatory 5-HT receptors together with prolonged 5-HT release may also contribute to the heightened anxiety-like behavior. For example, in the subpopulation of Type III neurons that normally express 5-HT1A and 5-HT2C receptor transcripts, USS reduced the number of neurons expressing 5-HT1A transcripts but had no effect on the number of neurons expressing 5-HT2C receptor transcripts. Our Western blot data suggest that alterations in mRNA expression are mirrored in receptor protein expression; hence, the inhibitory-excitatory balance in these Type III neurons would shift heavily in favor of excitation. Similarly, USS also caused a significant reduction in the number of Type I neurons expressing transcripts for the 5-HT1A receptor (63% NS–vs-10% USS) and a concomitant increase in the number of these neurons expressing transcripts for the 5-HT7 receptor (53% NS–vs-70% USS). We have shown that activation of 5-HT7 receptors causes a depolarizing shift in the membrane potential of BNSTALG neurons (Guo et al., 2009); hence, USS would also cause a shift in the inhibitory-excitatory balance in these neurons to favor 5-HT-induced excitation. Significantly, 5-HT7 knockout mice show anti-depressant-like activity in the Porsolt forced swimming test (Guscott et al., 2005), and in the tail suspension test (Hedlund et al., 2005), as well as impaired contextual fear conditioning (Roberts et al., 2004), suggesting a potential role for 5-HT7 receptors in both depression or anxiety-like behavior. Consistent with this premise, chronic treatment with antidepressants has been shown to down-regulate 5-HT7 receptor binding (Sleight et al., 1995, Mullins et al., 1999).
Another receptor that is up-regulated following USS is the 5-HT1B receptor. In non-stressed animals transcripts for 5-HT1B receptor was detected in 21 % of Type IIB neurons, and in 41% of Type III neurons. Following USS the number of Type IIB neurons expressing mRNA for the receptor remains constant, but the number of Type III neurons expressing transcripts for the 5-HT1B receptor increased from 41% to 78%. Consistent with this observation, Ferguson and co-workers reported that chronic mild stress increased the expression of 5-HT1B receptors on the terminals of nucleus accumbens neurons that project to the ventral tegmental area (Ferguson et al., 2009).
In this study, we did not see any significant changes in the expression of 5-HT2A, 5-HT2C, and 5-HT3 receptor transcripts following USS. However, resistance to modulation by stress does not necessarily imply that these receptors do not contribute to the enhanced anxiety-like behavior following USS. As noted above, removal of inhibitory control by 5-HT1A receptor down-regulation may unmask an anxiogenic profile for these receptors. Indeed, Weisstaub and co-workers reported that 5-HT2A receptor knockout mice show a decrease in anxiety-like behavior in several behavioral tasks (Weisstaub et al., 2006). Similarly, gene knockout of 5-HT2C receptors leads to a decrease in anxiety-like behavior (Heisler et al., 2007), an effect that is mimicked by administration of 5-HT2C receptor antagonists (Dekyne et al., 2008; Harada et al., 2006). Conversely, selective over expression of 5-HT2C receptors in the forebrain resulted in increased anxiety and hypoactivity (Kimura et al., 2009), suggesting that activation of these receptors contributes to the expression of anxiety-like behavior even under conditions of basal 5-HT release. It remains to be determined if activation of 5-HT2C receptors in the BNSTALG contribute to the altered behavioral state. Finally, systemic administration of 5-HT3 receptor antagonists, such as ondansetron and tropisetron, have also been shown to have anxiolytic–like effects in rodent behavioral assays (Griebel, 1995, Millan et al., 2003, Costall and Naylor, 2004). Hence, by reducing 5-HT1A receptor expression USS may favor the expression of anxiety-like behavior as a result of local activation of any combination or permutation of these excitatory 5-HT receptors.
A potential mechanism for stress-induced down-regulation of 5-HT1A receptor expression
Transcriptional regulation of 5-HT1A receptor expression is an important determinant of the basal response of BNSTALG neurons to local 5-HT release. Here, expression of the 5-HT1A gene is regulated by a TATA-driven promoter and also by upstream repressors that inhibit gene expression (Parks and Shenk, 1996, Ou et al., 2000) including Deaf-1, Freud-1, and Hes-5 (Albert and Lemonde, 2004). Here, we show that under basal conditions mRNA transcripts for all three repressors were expressed in BNSTALG tissue homogenates suggesting that 5-HT1A receptor expression may be tightly regulated in BNSTALG neurons. However, USS failed to alter the expression levels of Freud-1 and Hes5, but caused a significant up-regulation of Deaf-1 mRNA transcripts. These data were consistent with the significant reduction in 5-HT1A receptor expression following USS, and suggest that Deaf-1 may be the principal regulator of 5-HT1A receptor expression in BNSTALG neurons. Differential regulation of 5-HT1A gene repressor expression has also been observed in the PFC (Iyo et al., 2009). Here chronic restraint stress caused a significant reduction in Freud-1 mRNA and protein expression but had no effect on Deaf-1 expression. These data suggest that transcriptional regulation of Deaf-1 and Freud-1 expression, and by extension 5-HT1A receptor expression, may be brain region and context-specific. Intriguingly, chronic social defeat was observed to reduce 5-HT1A mRNA expression but did not reduce either Deaf-1 or Freud-1 expression levels (Kieran et al., 2010). It should be noted that Deaf-1, Freud-1, and Hes-5 are not the only transcriptional regulators of 5-HT1A gene expression. The 5-HT1A receptor promoter also contains a glucocorticoid response element (GRE) that can bind heterodimers of type 1 (mineralocorticoid, MR) and type 2 (glucocorticoid, GR) receptors and repress 5-HT1A receptor expression (Ou et al., 2001). The BNSTALG contains moderate to high levels of both MR and GR (Ahima and Harlan, 1990, Pietranera et al., 2001) and it is possible that stress-induced glucocorticoid release may also contribute to the down-regulation of 5-HT1A receptor expression following USS.
At the single cell level, Deaf-1 expression was detected in distinct subpopulations of BNSTALG neurons. Significantly, Deaf-1 expression was observed only in those subpopulations of Type I and III neurons that did not show basal mRNA expression for the 5-HT1A receptor. Similarly, Deaf-1 was highly expressed in Type IIA neurons, which did not express 5-HT1A receptor transcripts. These data raise the intriguingly possibility that this population of neurons may be capable of expressing 5-HT1A receptor transcripts, but that even under basal conditions gene expression is strongly repressed by Deaf-1. Conversely, Deaf-1 transcripts were never observed in Type I – III BNSTALG neurons that expressed 5-HT1A receptor mRNA transcripts, suggesting that these cells either do not co-express Deaf-1, or that under basal conditions Deaf-1 was itself repressed. Consistent with the latter premise, following USS the number of Deaf-1 expressing neurons increased in Type I – III neurons. Significantly, Deaf-1 expression was now observed in some Type I and Type III neurons that also expressed transcripts for the 5-HT1A receptor. These data are consistent with studies in the prefrontal cortex which report co-expression of Deaf-1 and 5-HT1A receptor protein in cortical neurons (Szewczyk et al., 2009). Our results suggest that enhanced expression of Deaf-1 may bind to its repressor sequence on the promoter region of the 5-HT1A receptor gene and inhibit transcription. Hence, induction of Deaf-1 in select subpopulations of BNSTALG neurons may contribute to the prolonged elevation of anxiety-like behavior following repeated USS. Selective targeting of factors that regulate Deaf-1 induction and/or translation may offer novel avenues of approach for the development of new pharmacotherapeutics for anxiety disorders and depression.
Questions still to be answered
Notwithstanding decades of research into the putative role of the serotonergic system in the behavioral response to stress stimuli, no clear picture has emerged thus far. Understanding the role of selective expression of the different 5-HT receptor subtypes in subpopulations of BNSTALG neurons seems a more promising approach to unraveling the role these receptors in the etiology of anxiety. However, a key issue that has yet to be addressed is how activation of the different cell types modulates the output activity of the BNSTALG as a whole.
Highlights
Cell type specific distribution of 5-HT receptors in BNSTALG neurons.
5-HT receptors and transcriptional regulators are modulated by stress.
Stress decreased 5-HT1A mRNA and protein in the BNSTALG.
Stress up-regulates the transcriptional repressor of the 5-HT1A gene, Deaf-1.
Acknowledgements
This work was supported by National Institute of Mental Health (MH072908) to DGR, and the Yerkes National Primate Research Center base grant RR-00165 awarded by the Animal Resource Program of National Institutes of Health.
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- 5-HT1A
serotonin 1A receptor
- 5-HT1B
serotonin 1B receptor
- 5-HT1D
serotonin 1D receptor
- 5-HT1F
serotonin 1F receptor
- 5-HT2A
serotonin 2A receptor
- 5-HT2C
serotonin 2C receptor
- 5-HT3
serotonin 3 receptor
- 5-HT4
serotonin 4 receptor
- 5-HT5
serotonin 5 receptor
- 5-HT6
serotonin 6 receptor
- 5-HT7
serotonin 7 receptor
- ASR
acoustic startle response
- BNST
the bed nucleus of the stria terminalis
- BNSTALG
anterolateral cell group of the bed nucleus of the stria terminalis
- CRF
corticotrophin releasing factor
- Deaf-1
deformed epidermal autoregulatory factor 1
- DRN
dorsal raphe nucleus
- Freud-1
five prime repressor element under dual binding protein 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GR
glucocorticoid receptors
- GRE
glucocorticoid response element
- Hes5
hairy and enhancer of split 5
- MR
mineralocorticoid receptors
- NS
non stress
- PCR
polymerase chain reaction
- PFC
prefrontal cortex
- scRT-PCR
single cell reverse transcriptase polymerase chain reaction
- USS
unpredictable shock stress
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Albert PR, Le Francois B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Alves FH, Crestani CC, Gomes FV, Guimaraes FS, Correa FM, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis modulates baroreflex activity through 5-HT1A receptors. Pharmacol Res. 2010;62:228–236. doi: 10.1016/j.phrs.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using [3H]alniditan, a new radioligand. Receptors Channels. 1997;5:225–230. [PubMed] [Google Scholar]
- Bota M, Sporns O, Swanson LW. Neuroinformatics analysis of molecular expression patterns and neuron populations in gray matter regions: the rat BST as a rich exemplar. Brain Research. 2012;1450:174–193. doi: 10.1016/j.brainres.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Dirks A, Maes RA, Hen R, Geyer MA, Olivier B. Startle responses, heart rate, and temperature in 5-HT1B receptor knockout mice. Neuroreport. 2000;11:4097–4102. doi: 10.1097/00001756-200012180-00037. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Research Bulletin. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Cardona A, Rousselle JC, Massot O, Edelman L, Fillion G. Autoradiographic characterization of [3H]-5-HT-moduline binding sites in rodent brain and their relationship to 5-HT1B receptors. Proc Natl Acad Sci U S A. 1997;94:9899–9904. doi: 10.1073/pnas.94.18.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloez-Tayarani I, Cardona A, Sarhan H, Rousselle JC, Massot O, Edelman L, Fillion G. Mapping of 5-HT-moduline binding sites in guinea-pig brain by film and digital autoradiography. Brain Research. 1998;798:311–315. doi: 10.1016/s0006-8993(98)00393-x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Louderback KM, Gessner CP, Winder DG. Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav. 2011;104:248–256. doi: 10.1016/j.physbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. 5-HT3 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:27–37. doi: 10.2174/1568007043482624. [DOI] [PubMed] [Google Scholar]
- Crayton JW, Joshi I, Gulati A, Arora RC, Wolf WA. Effect of corticosterone on serotonin and catecholamine receptors and uptake sites in rat frontal cortex. Brain Research. 1996;728:260–262. doi: 10.1016/0006-8993(96)00189-8. [DOI] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct. 2010;6:30. doi: 10.1186/1744-9081-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Rainnie DG. Expression and distribution of Kv4 potassium channel subunits and potassium channel interacting proteins in subpopulations of interneurons in the basolateral amygdala. Neuroscience. 2010;171:721–733. doi: 10.1016/j.neuroscience.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Olmos JS, Alheid GF, Beltramino CA. Amygdala. In: Paxinos G, editor. The Rat Nervous System: Forebrain and Midbrain. vol. 1. 1985. p. 223. [Google Scholar]
- Dekeyne A, Mannoury la Cour C, Gobert A, Brocco M, Lejeune F, Serres F, Sharp T, Daszuta A, Soumier A, Papp M, Rivet JM, Flik G, Cremers TI, Muller O, Lavielle G, Millan MJ. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology. 2008;199:549–568. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- Delgado M, Caicoya AG, Greciano V, Benhamu B, Lopez-Rodriguez ML, Fernandez-Alfonso MS, Pozo MA, Manzanares J, Fuentes JA. Anxiolytic-like effect of a serotonergic ligand with high affinity for 5-HT1A, 5-HT2A and 5-HT3 receptors. European Journal of Pharmacology. 2005;511:9–19. doi: 10.1016/j.ejphar.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly presented inescapable sound. Neurosci Lett. 1995;199:78–80. doi: 10.1016/0304-3940(95)12027-2. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Research. 1987;407:327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Williams TJ. Cardiovascular responses to electrical stimulation of the bed nucleus of the stria terminalis. Journal of Comparative Neurology. 1995;352:227–234. doi: 10.1002/cne.903520206. [DOI] [PubMed] [Google Scholar]
- Farber L, Haus U, Spath M, Drechsler S. Physiology and pathophysiology of the 5-HT3 receptor. Scand J Rheumatol Suppl. 2004;119:2–8. [PubMed] [Google Scholar]
- Ferguson SM, Sandygren NA, Neumaier JF. Pairing mild stress with increased serotonin-1B receptor expression in the nucleus accumbens increases susceptibility to amphetamine. Eur J Neurosci. 2009;30:1576–1584. doi: 10.1111/j.1460-9568.2009.06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Blengio M, Gamalero SR, Ghi P. Biochemical and behaviour changes induced by acute stress in a chronic variate stress model of depression: the effect of amitriptyline. European Journal of Pharmacology. 1995;280:19–26. doi: 10.1016/0014-2999(95)00172-h. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Funada M, Hara C. Differential effects of psychological stress on activation of the 5-hydroxytryptamine- and dopamine-containing neurons in the brain of freely moving rats. Brain Res. 2001;901:247–251. doi: 10.1016/s0006-8993(01)02160-6. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimaraes FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology. 2011;213:465–473. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Lacefield CO, Kentros CG, Hen R. State-dependent alterations in hippocampal oscillations in serotonin 1A receptor-deficient mice. J Neurosci. 2005;25:6509–6519. doi: 10.1523/JNEUROSCI.1211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn RE, Maswood S, McQueen MB, Watkins LR, Maier SF. Opioid-dependent effects of inescapable shock on escape behavior and conditioned fear responding are mediated by the dorsal raphe nucleus. Behav Brain Res. 1999a;99:153–167. doi: 10.1016/s0166-4328(98)00101-6. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Research. 1999b;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Griebel G. 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther. 1995;65:319–395. doi: 10.1016/0163-7258(95)98597-j. [DOI] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Rainnie DG. Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience. 2010;165:1390–1401. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, Bromidge F, Owens AP, Huscroft I, Myers J, Rupniak NM, Patel S, Whiting PJ, Hutson PH, Fone KC, Biello SM, Kulagowski JJ, McAllister G. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. Journal of Neurophysiology. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, Ishibashi K, Matsuoka N. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. European Journal of Pharmacology. 2006;553:171–184. doi: 10.1016/j.ejphar.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Ryan SJ, Jasnow AM, Dabrowska J, Rainnie DG. A transcriptomic analysis of type I–III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci. 2011;46:699–709. doi: 10.1016/j.mcn.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology. 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry. 2002;7:147–159. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC. Differential regulation of the serotonin 1 A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. The Journal of comparative neurology. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, Morinobu S, Yamawaki S. Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1269–1281. doi: 10.1016/s0278-5846(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Kieran N, Ou XM, Iyo AH. Chronic social defeat downregulates the 5-HT1A receptor but not Freud-1 or NUDR in the rat prefrontal cortex. Neuroscience Letters. 2010;469:380–384. doi: 10.1016/j.neulet.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Stevenson PL, Carter RN, Maccoll G, French KL, Paul Simons J, Al-Shawi R, Kelly V, Chapman KE, Holmes MC. Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci. 2009;30:299–306. doi: 10.1111/j.1460-9568.2009.06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–596. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maines LW, Keck BJ, Smith JE, Lakoski JM. Corticosterone regulation of serotonin transporter and 5-HT1A receptor expression in the aging brain. Synapse. 1999;32:58–66. doi: 10.1002/(SICI)1098-2396(199904)32:1<58::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, Nemeroff CB, Owens MJ. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Veiga S, Girardon S, Brocco M. Blockade of serotonin 5-HT1B and 5-HT2A receptors suppresses the induction of locomotor activity by 5-HT reuptake inhibitors, citalopram and fluvoxamine, in NMRI mice exposed to a novel environment: a comparison to other 5-HT receptor subtypes. Psychopharmacology. 2003;168:397–409. doi: 10.1007/s00213-003-1389-y. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 2001;108:311–319. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- Ou XM, Jafar-Nejad H, Storring JM, Meng JH, Lemonde S, Albert PR. Novel dual repressor elements for neuronal cell-specific transcription of the rat 5-HT1A receptor gene. J Biol Chem. 2000;275:8161–8168. doi: 10.1074/jbc.275.11.8161. [DOI] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Shenk T. The serotonin 1a receptor gene contains a TATA-less promoter that responds to MAZ and Sp1. J Biol Chem. 1996;271:4417–4430. doi: 10.1074/jbc.271.8.4417. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pietranera L, Saravia FE, McEwen BS, Lucas LL, Johnson AK, De Nicola AF. Changes in Fos expression in various brain regions during deoxycorticosterone acetate treatment: relation to salt appetite, vasopressin mRNA and the mineralocorticoid receptor. Neuroendocrinology. 2001;74:396–406. doi: 10.1159/000054706. [DOI] [PubMed] [Google Scholar]
- Rainnie DGGJ, Hazra R, Dabrowska J, Ressler KJ. Characterization of the physiological and genetic phenotype of CRF expressing neuron in the bed nucleus of the stria terminalis. 40th Annual Meeting of Society for Neuroscience; San Diego, CA. 2010. [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT receptors show specific impairments in contextual learning. Eur J Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Allen PS, Pine DS, Grillon C. Acute Tryptophan Depletion Increases Translational Indices of Anxiety but not Fear: Serotonergic Modulation of the Bed Nucleus of the Stria Terminalis? Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MB, Brutus M, Siegel HE, Siegel A. Regulation of feline aggression by the bed nucleus of stria terminalis. Brain Research Bulletin. 1986;16:179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Sibille E, Hen R. Serotonin(1A) receptors in mood disorders: a combined genetic and genomic approach. Behav Pharmacol. 2001;12:429–438. doi: 10.1097/00008877-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Singewald N, Kouvelas D, Kaehler ST, Sinner C, Philippu A. Peripheral chemoreceptor activation enhances 5-hydroxytryptamine release in the locus coeruleus of conscious rats. Neuroscience Letters. 2000;289:17–20. doi: 10.1016/s0304-3940(00)01241-6. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31:1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingelstein C, Bourson A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. Mol Pharmacol. 1995;47:99–103. [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. J Neuroendocrinol. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K, Nagatani T, Kitamura Y, Yamawaki S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. European Journal of Pharmacology. 1997;333:123–128. doi: 10.1016/s0014-2999(97)01126-6. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Baratta M, Bland ST, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav Brain Res. 2004;153:233–239. doi: 10.1016/j.bbr.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacology, Biochemistry & Behavior. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang C, Wang R, Govindarajan SS, Barish PA, Vernon MM, Fu C, Acharya AP, Chen L, Boykin E, Yu J, Pan J, O'Donnell JM, Ogle WO. Corticosterone induced morphological changes of hippocampal and amygdaloid cell lines are dependent on 5-HT7 receptor related signal pathway. Neuroscience. 2011;182:71–81. doi: 10.1016/j.neuroscience.2011.02.042. [DOI] [PubMed] [Google Scholar]