Abstract
Background
Ion channels play important roles in regulation of cellular proliferation. Ano1 (TMEM16A) is a Ca2+-activated Cl− channel expressed in several tumors and cell types. In the muscle layers of the gastrointestinal tract Ano1 is selectively expressed in interstitial cells of Cajal (ICC) and appears to be required for normal gastrointestinal slow wave electrical activity. However, Ano1 is expressed in all classes of ICC, including those that do not generate slow waves suggesting that Ano1 may have other functions. Indeed, a role for Ano1 in regulating proliferation of tumors and ICC has been recently suggested. Recently, a high-throughput screen identified a small molecule, T16Ainh-A01 as a specific inhibitor of Ano1.
Aim
To investigate the effect of the T16Ainh-A01 inhibitor on proliferation in ICC and in the Ano1-expressing human pancreatic cancer cell line CFPAC-1.
Methods
Inhibition of Ano1 was demonstrated by whole cell voltage clamp recordings of currents in cells transfected with full-length human Ano1. The effect of T16Ainh-A01 on ICC proliferation was examined in situ in organotypic cultures of intact mouse small intestinal smooth muscle strips and in primary cell cultures prepared from these tissues. ICC were identified by Kit immunoreactivity. Proliferating ICC and CFPAC-1 cells were identified by immunoreactivity for the nuclear antigen Ki67 or EdU incorporation, respectively.
Results
T16Ainh-A01 inhibited Ca2+- activated Cl− currents by 60% at 10 µM in a voltage-independent fashion. Proliferation of ICC was significantly reduced in primary cultures from BALB/c mice following treatment with T16Ainh-A01. Proliferation of the CFPAC-1 human cell-line was also reduced by T16Ainh-A01. In organotypic cultures of smooth muscle strips from mouse jejunum, the proliferation of ICC was reduced but the total number of proliferating cells/confocal stack was not affected, suggesting that the inhibitory effect was specific for ICC.
Conclusions
The selective Ano1 inhibitor T16Ainh-A01 inhibited Ca2+-activated Cl− currents, reduced the number of proliferating ICC in culture and inhibited proliferation in the pancreatic cancer cell line CFPAC-1. These data support the notion that chloride channels in general and Ano1 in particular are involved in the regulation of proliferation.
Keywords: Interstitial cells of Cajal, ICC, TMEM16A, GIST, cancer, intestine
1. Introduction
Alteration of membrane potential through changes in extracellular ion concentration is a crucial regulator of proliferation, performing important roles in the progression of cell cycle at multiple key checkpoints (reviewed in [1]). Many ions are involved in these processes; the role of chloride ions in regulating cell cycle has been studied mostly as a driving force in cytoplasmic condensation [2, 3]. Ano1 is a Ca2+ activated Cl− channel expressed in secretory epithelia of lung, salivary glands and kidney[4, 5, 6]. Expression of Ano1 is up-regulated in several cancers including esophageal cancer [7] and gastrointestinal stromal tumors (GISTs), the most common mesenchymal tumors in the gastrointestinal tract [8, 9]. In the muscle layers of the gastrointestinal tract Ano1 expression is restricted to interstitial cells of Cajal (ICC [10]), pacemaker and neuromodulator cells of the gut. Ano1 appears to be required for normal gastrointestinal function [11, 12] and has been proposed to play a key role in the pacemaker activity of ICC [12, 13]. Ano1 is expressed in all classes of ICC, including those that do not generate slow waves [10]suggesting that Ano1 may have other functions. A possible role of Ano1 in regulation of proliferation was suggested by its expression in GISTs. We investigated this possibility and showed that Ano1 activity contributes to the proliferation of Ano1 positive cells by acting at the G1/S phase of cell cycle. This was demonstrated by showing reduced proliferation of ICC in Ano1(−/−) mice, in the presence of chloride depleted culture medium and by the use of three structurally different chloride channel blockers [14]. Also, in a human head and neck squamous cell carcinoma cell line (UMSCC1) knockdown of Ano1 resulted in a decrease in xenograft growth in nude mice [15]. A significant problem in investigating the role of Ano1 in proliferation is the lack of selectivity of many Cl− channel inhibitors for Cl− channels and transporters and for Ano1. Compounds such as DIDS, niflumic acid and tamoxifen decrease ICC proliferation in culture; however, they are not selective and have multiple effects on other targets that can indirectly alter chloride secretion [16, 17, 18, 19].
Recently, a high throughput screen of 110,000 compounds revealed a novel small molecule, an aminophenylthiazole named T16Ainh-A01 as a specific inhibitor of Ano1 [20]. This compound was reported to inhibit Cl− efflux due to Ano1 (human) with an IC50 of 1.1 µM but to have little effect on CFTR and no effect on cytoplasmic calcium [20]. However, direct effects on Ano1-mediated Cl− currents have not been reported. The aim of this study was to confirm T16Ainh-A01’s inhibitory effect on Ano1-mediated Cl− currents in an heterologous expression system and to investigate the effect of the inhibitor on the proliferation of ICC and an Ano1-expressing human pancreatic cancer cell line.
2. Material and Methods
2.1 Animals
BALB/c mice were obtained from Harlan (Indianapolis, IN). Mice were killed by CO2 inhalation and cervical dislocation at post-natal day 3 (PND 3). The mice were maintained and the experiments were performed with approval from the Institutional Animal Care and Use Committee of the Mayo Clinic.
2.2 Cell cultures
Primary cultures enriched in ICC were obtained by enzymatic dissociation of the mouse small intestines and co-cultured in the presence of Sl/Sl4 mSCF248, murine stem cell factor–secreting fibroblasts as previously described [21]. M199 media without phenol red (Invitrogen) supplemented with 1% antibiotic-antimycotic (Invitrogen) was used for the co-cultures. Cells were allowed to incubate for 1 hour at 37°C/5% CO2 before adding 2 ml of the culture medium to the well.
CFPAC-1 is a human pancreatic duct cell line that endogenously expresses Ano1. This cell line was derived from a cystic fibrosis patient and as a consequence expresses the most common cystic fibrosis mutation in CFTR, a deletion of three nucleotides, resulting in the absence of phenylalanine at position 508. CFPAC-1 cells were grown in Iscove’s Modified Dulbecco’s Media (IMDM, ATCC) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
HEK293 cells were maintained in Minimum Essential Medium (MEM, Invitrogen) supplemented with 10% heat inactivated horse serum (Invitrogen), 1% sodium pyruvate (Invitrogen), 1% non-essential amino acids (Invitrogen) and1% penicillin/streptomycin. Cells were transfected with a vector bearing the full length Ano1 cDNA using LIPOFECTAMINE™ 2000 Reagent (Invitrogen).
2.3 Electrophysiology
Currents were recorded by standard whole cell voltage clamp recordings at room temperature (22 °C) from HEK293 cells expressing Ano1 and the fluorescent marker GFP. 2–5 MΩ glass patch clamp pipettes in standard whole cell configuration were used. Glucose-free N-methyl D-glucamine containing extracellular solutions (in mM: NMDG+ 149.2, K+ 4.74, Ca2+ 2.54, Gd3+ 0.01, Cl− 159, HEPES 5; pH 7.35, osmolality 290 mmol/kg) and CsCl and 500 nM free Ca2+ intracellular solutions (in mM: Cs+ 145, Na+ 5, Mg2+ 5, Ca2+ 1.27, Cl− 162.5, EGTA 2, HEPES 5; pH 7.25, osmolality 300 mmol/kg) were used. Data were collected and analyzed using an Axopatch 200B, Digidata 1322A, and pCLAMP 9 software (Molecular Devices). Free Ca2+ was calculated online at http://www.stanford.edu/~cpatton/CaMgATPEGTA-TS-Plot.htm. Under these conditions, Cs+NMDG+and Gd3+ block K+Na+, and non-selective cation currents, and equimolar Cl− results in a predicted reversal potential of 0 mV. Cells were held at −100 mV between 1 second long voltage steps from −100 to +120 mV. Start-to-start time between sweeps was 5 s. Data were analyzed using Clampfit and Excel (Microsoft). In current-voltage (I-V) relationships (Fig. 1), Cl− currents at 1 s are shown as a fraction of total cell capacitance (pA/pF). Previously we found non-transfected cells to have currents <2 pA/pF in 500 nM free Ca2+ [22]. Significance was determined by 1-way repeated measures ANOVA with Dunnett post-test. A P-value less than 0.05 was considered significant.
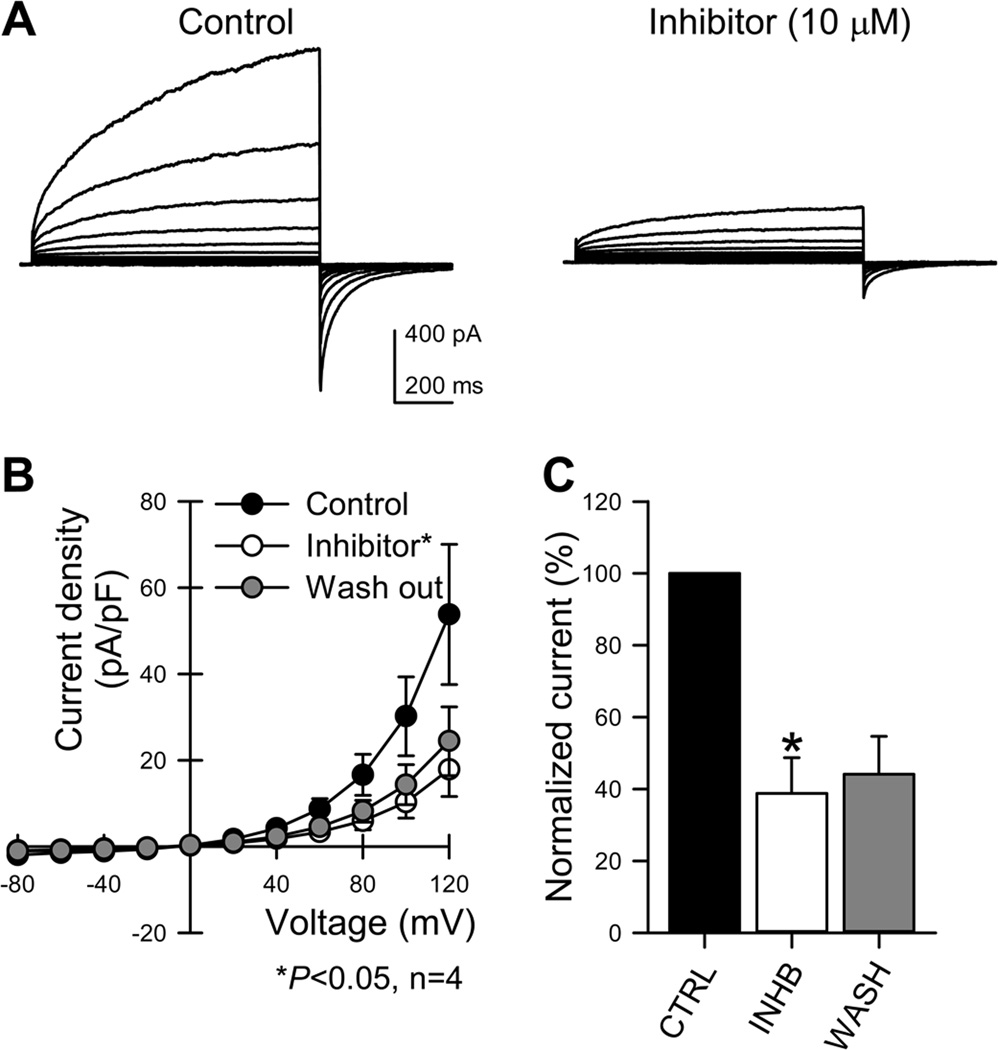
Figure 1. T16Ainh-A01 inhibits Ano1 currents in transfected cells.
A, representative traces of Ano1-mediated currents evoked by depolarization from the holding potential of −100 mV. Outwardly rectifying chloride currents were recorded from HEK293 cells transfected with full-length human Ano1 in control conditions or in the presence of T16Ainh-A01 inhibitor (10 µM). B, current-voltage graph for chloride currents. Note reduced current densities after exposure to T16Ainh-A01. C, Graph showing quantification of normalized currents. Data are means ± SEM, n=4, *p<0.05.
2.4 Organotypic cultures
The tunica muscularis from the jejunum of PND 3 mice was quickly dissected out, flushed with ice-cold calcium-free Hanks balanced salt solution (Invitrogen, Carlsbad, CA) and pinned onto a sterile Sylgard lined petri dish. Whole-mount preparations were incubated for 24h in the presence of vehicle (DMSO) or T16Ainh-A01 inhibitor diluted to a final concentration of 10 µM in medium composed of M199 without phenol red supplemented with 4.5 g/l of glucose (Sigma-Aldrich), 10% FBS and 2% antibiotic-antimycotic (Invitrogen).
2.5 Immunohistochemistry
For primary cultures, immunohistochemistry was performed as previously described [23] and proliferating ICC were identified for immunoreactivity for the nuclear antigen, Ki67 (Abcam). Proliferating CFPAC-1 were identified by detection of incorporated 5-ethynyl- 2’-deoxyuridine (EdU) using the “Click-iT” technology (Invitrogen) according to the manufacturer’s instructions. Immunostained cultures were examined with the use of a fluorescence microscope (BX51WI, Olympus). Proliferating ICC were identified by co-immunoreactivity for Kit and Ki67, while proliferating CFPAC-1 cells were identified by positivity to EdU. Cells were counted using a 20X objective on two separate coverslips per condition.
For whole-mount staining, following incubation with either vehicle or T16Ainh-A01 inhibitor, tissues were incubated with EdU (10 µM diluted in culture medium) for 2 hours and then fixed in 4% paraformaldehyde for 30 minutes, washed with PBS and permeabilized in 0.3% Triton X-100 for 30 min. After washing, tissues were incubated in “Click-iT” reaction buffer for 2 hours at room temperature protected from light, washed in PBS and then incubated with 10% normal donkey serum (NDS, Jackson Immunoresearch Laboratories) and 0.3% Triton-X-100 (Sigma-Aldrich) in PBS (4°C, overnight) to minimize nonspecific antibody binding. Tissues were then incubated with Kit antibody (0.4 µg/ml in 5% NDS, R&D Systems) for 8 hours at 4°C. Following washings in PBS, the tissues were incubated with donkey anti-goat IgG conjugated with CY3 (Jackson Immunoresearch Laboratories, 1.8 µg/ml in 2.5% NDS, 4°C, 24 hours). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 0.01 µg/ml in dH2O, 30 min). The labeled tissues were mounted using Slowfade mounting medium (Invitrogen). Whole-mounts were imaged using an FV1000 confocal microscope (Olympus). Four image stacks (field size: 211.5 µm×211.5 µm) representing the entire thickness of the muscle were collected from each tissue using a 60X (NA 1.2) objective.
2.6 T16Ainh-A01 inhibitor
T16Ainh-A01 inhibitor compound was provided by Dr. Verkman (UCSF). Stock solutions (5 mM) were made in DMSO (Sigma-Aldrich) and stored at −20°C. The compound was diluted fresh on the day of the experiment to a final concentration of 10 µM. DMSO was used at a final concentration of 1:500 for the vehicle control.
3. Results
3.1 T16Ainh-A01 inhibits Ano1 currents in transfected cells
To test if the T16Ainh-A01 inhibitor blocked Ano1 currents we recorded whole cell patch clamp currents from HEK293 cells transfected with a plasmid containing the full-length human Ano1 in the presence or absence of the inhibitor. As previously shown in Mazzone &al [22], transfection with the Ano1 vector resulted in expression of channels that produced Ca2+- and voltage-dependent, outwardly rectifying currents (30.2±9.1 pA/pF at 100 mV, Fig1A). When the transfected cells were treated with the Ano1 inhibitor at a final concentration of 10 µM, current densities markedly decreased (10.3±3.7 pA/pF at 100 mV, p < 0.05, student t-test, n=4, Fig1A). Treatment with the inhibitor reduced the current density of Ano1 at all the voltages examined (Fig1B, p < 0.05, n=4). The effect of T16Ainh-A01 was relatively voltage-independent with inhibition ranging from 44±7% at - 100mV to 67±5% at −20 mV and 53.7±3.4% at 20 mV to 63±7% at 100 mV (Fig. 1C). Washing out the inhibitor did not restore the original current density (14.3±4.6 pA/pF at 100 mV, n=4, Fig1B,C).
3.2 T16Ainh-A01 reduces the number of proliferating ICC in primary cultures from mouse small intestine
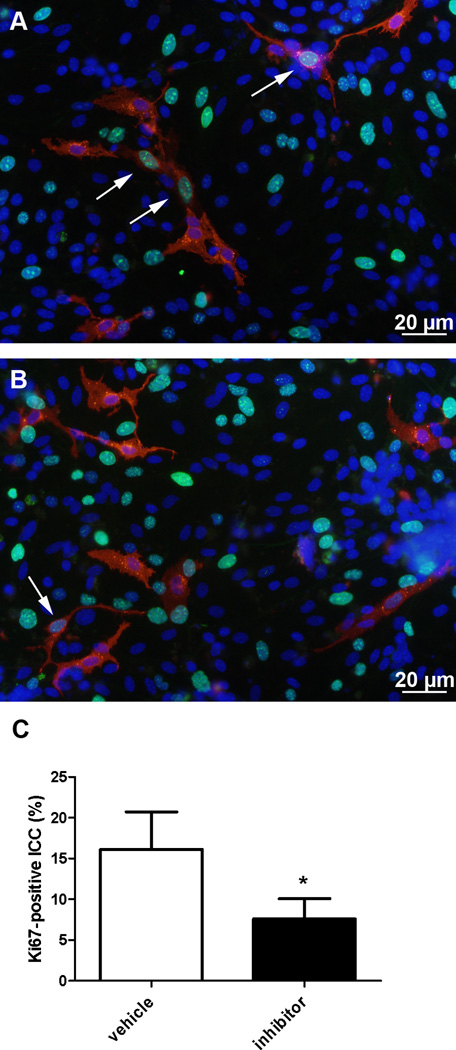
To study the effect of the T16Ainh-A01 inhibitor on the proliferation of ICC we tested the effect of this inhibitor in primary cultures. Proliferation of ICC was significantly reduced in primary cultures from BALB/c mice following treatment with T16Ainh-A01 (10 µM, Fig2B), when compared to controls (Fig2A). This was observed as a decrease in number of ICC displaying nuclear immunostaining for Ki67 (arrows Fig 1A) from 16.14 ± 2.3% per field-of-view to 7.5 ± 3.5% (p < 0.03, paired t-test, n = 4, Fig2C).
Figure 2. T16Ainh-A01 reduces the number of proliferating cells in primary cultures.
Representative pictures of freshly dissociated ICC from PND2-3 BALB/c mice after 24 hours in culture in the presence of vehicle (A) or 10 µM concentration of T16Ainh-A01 inhibitor (B). Kit (red) was use as a marker of ICC and Ki67 (green) as a marker of proliferation. The cells nuclei were identified by DAPI staining (blue). Arrows indicates proliferating ICC. Treatment with T16Ainh-A01 inhibitor (10 µM) significantly reduced the percentage of proliferating ICC (C, *p<0.03, Paired t test, n=4). Data are means ± SEM.
3.3 T16Ainh-A01 reduces the number of proliferating cells in the human pancreatic cancer cell line, CFPAC-1
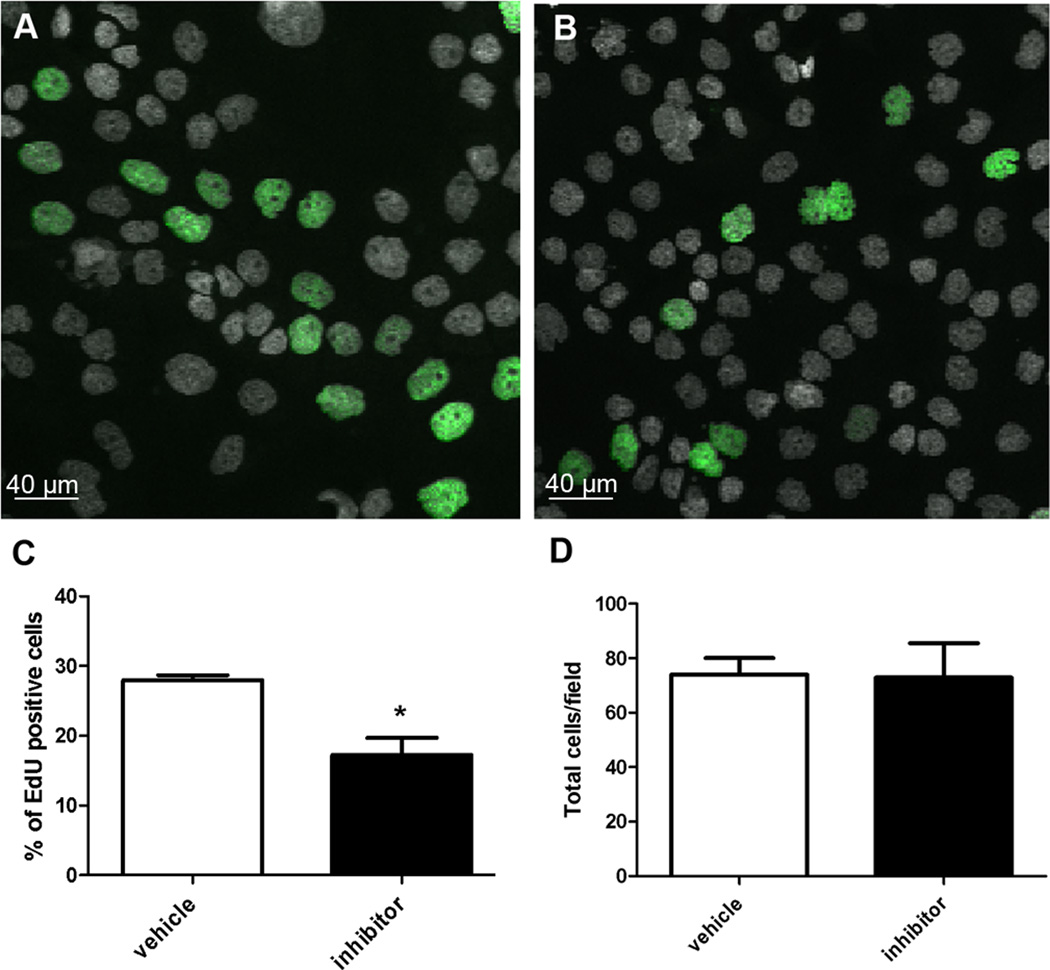
We also tested the effect of the T16Ainh-A01 inhibitor on CFPAC-1, a human pancreas cell line endogenously expressing Ano1. To assess proliferation we counted the number of nuclei that incorporated EdU in each field. Proliferation of CFPAC-1 cells was significantly reduced when cultured in the presence of 10 µM T16Ainh-A01 compared to vehicle (Vehicle: 27.9 ± 0.4, Fig3A; inhibitor: 17.2 ± 1.2, Fig3B; p < 0.002, paired t-test, n = 4, Fig3C). The total number of cells per field was not different between the two conditions (Vehicle: 73. ± 3, inhibitor: 72.9 ± 6.2, p = 0.77, t-test, n = 4, Fig3D).
Figure 3. T16Ainh-A01 reduces the number of proliferating cells in human cell line CFPAC-1.
Representative pictures of CFPAC-1 cells after 24 hours in culture in the presence of vehicle (A) or 10 µM T16Ainh-A01 Ano1 inhibitor (B). EdU incorporation was used as a marker of proliferation (green). Nuclei were identified by DAPI staining (gray). Treatment with T16Ainh-A01 reduced proliferation of CFPAC-1 cells (C, *p<0.002, paired t test, n = 4), but had no effect on the total number of cells per field (D). Data are means ± SEM.
3.4 T16Ainh-A01 reduces the number of proliferating mouse ICC in intact muscle strips
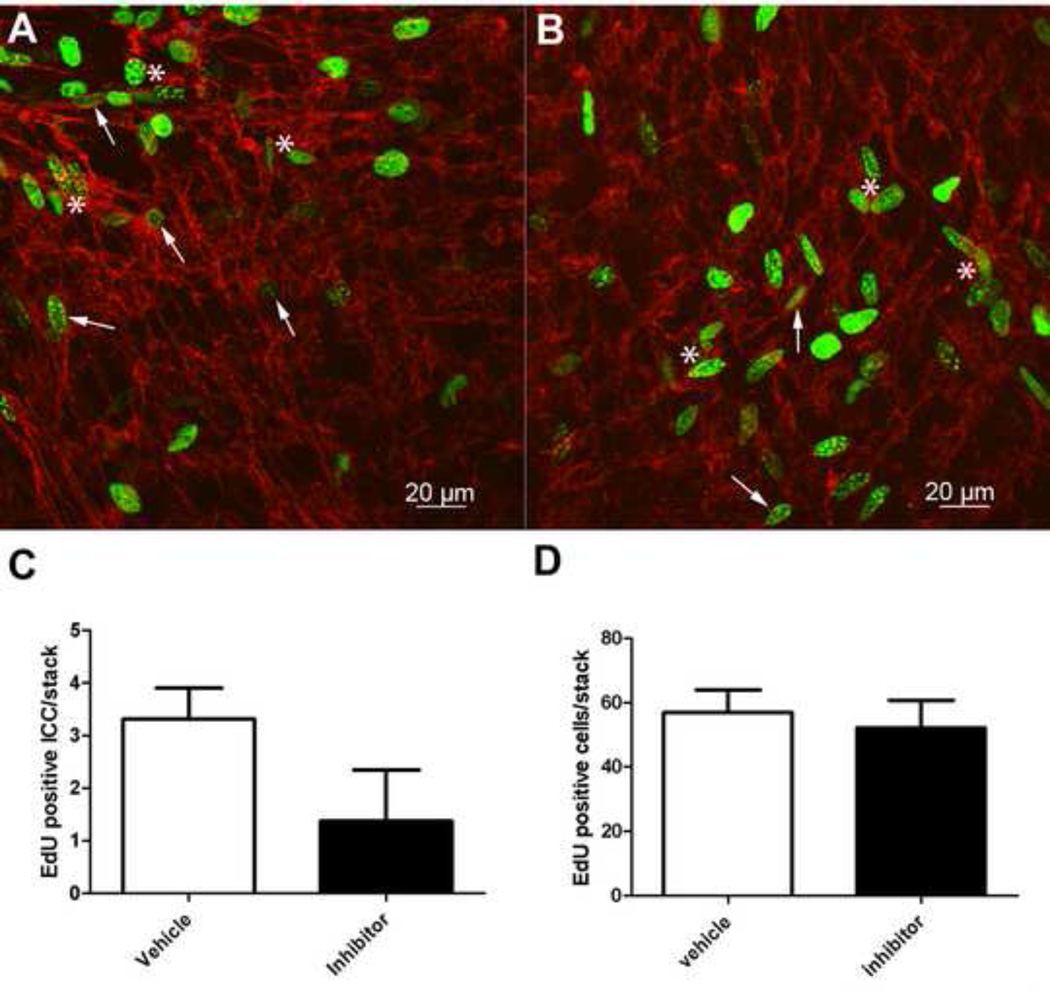
Next, we tested the effect of the T16Ainh-A01 inhibitor on the proliferation of ICC in situ in mouse jejunal tissues maintained in organotypic culture. For this purpose muscle strips were freshly dissociated from the jejunum of PND 3 mice and cultured for 24 hours in the presence of either vehicle or inhibitor (10 µM). After fixation and labeling to identify proliferating cells and ICC, high-resolution confocal stacks were acquired to visualize the EdU positive nuclei both in ICC (arrow) and other cell types (asterisks in Fig 4A,B). Treatment with the inhibitor significantly reduced the number of proliferating ICC as assessed by EdU incorporation (Vehicle: 3.3 ± 0.3 per image stack, inhibitor: 1.4 ± 0.5, p < 0.03, paired t test, n=4, Fig4C). This effect appeared to be specific only for ICC, the cells that expressed Ano1, because the total number of proliferating cells in each stack was not statistically different between the two conditions (Vehicle: 56.9 ± 3.5, inhibitor: 52.2 ± 4.3, p = 0.12, paired t test, n=4, Fig4D).
Figure 4. T16Ainh-A01 reduces the number of proliferating ICC in intact smooth muscle strips.
Representative images from immunolabeled muscle strips dissected from PND3 BALB/c mice after 24 hours in culture in the presence of vehicle (A) or 10 µM T16Ainh-A01 Ano1 inhibitor (B). Kit (red) was used as a marker of ICC and EdU incorporation (green) to identify proliferating nuclei. Arrows indicate proliferating ICC and asterisks indicate the proliferating nuclei of other, unidentified cell types. Treatment with T16Ainh-A01 inhibitor significantly reduced the percentage of proliferating ICC (C, *p < 0.03, Paired t test, n=4 mice). There was no difference in the total number of ICC per field between the two conditions (D). Data are means ± SEM.
4. Discussion
In the present study we show that the inhibitor T16Ainh-A01 blocked human Ano1 mediated Cl− currents and that this more selective inhibitor decreased proliferation of Ano1-expressing mouse ICC in dissociated culture and intact tissue. Furthermore, the compound also inhibited proliferation in the human pancreatic cancer cell line CFPAC-1. This supports the work published previously using non-selective Cl− channel inhibitors, modulation of Cl− concentration and Ano1 knockout mice to interrogate the role of Ano1 in cellular proliferation [14]. It also addresses the issue regarding the use of broad-spectrum blockers. In the previous study, DIDS, niflumic acid and tamoxifen caused a decrease in ICC proliferation in culture. However, they are not selective and have multiple effects on other targets, including but not limited to effects on Cl− transport and secretion. For example niflumic acid alters intracellular Ca2+ release and inhibits voltage-gated K+ channels and tamoxifen is a high-affinity ligand for the estrogen receptor [16, 17, 18, 19]. Structurally, the T16Ainh-A01 compound is unrelated chemically to previously reported chloride channel or CFTR inhibitors. Moreover, it has been shown that T16Ainh-A01 at a concentration of 10 µM nearly completely inhibited Cl− flux (using the fluorescence of YFP as an indicator for intracellular Cl concentration) and Cl− currents, but had little effect on the CFTR transporter and did not alter cytoplasmic calcium, thus differentiating this inhibitor from DIDS and niflumic acid [20]. We also determined that the T16Ainh-A01 significantly reduced Ca2+-activated Cl− currents generated by transfection of cells with a vector bearing the full length cDNA encoding human Ano1. The effect on these characteristically outward rectifying currents [6] was robust in the presence of 500 nM intracellular Ca2+. The inhibition was voltage-dependent of and the inhibitor did not affect the kinetics of the current during the voltage steps. The inhibition was not reversible within the time frame studied (10 min washout). These data indicate that T16Ainh-A01 is a direct and potent inhibitor of Ano1 but do not clarify the mechanism of action of the molecule.
The use of different but complementary experiments to determine proliferation (Ki67 staining and EdU incorporation) in multiple experimental paradigms including mouse primary cultures enriched in ICC, the human pancreatic cancer cell line, CFPAC-1 and intact mouse muscle strips in organotypic cultures also strengthen the interpretation of our data. Our results show that in all these approaches the use of T16Ainh-A01 reduced the proliferation of ICC and Ano1 expressing cells when compared to control conditions treated with vehicle only.
In primary cells and tissue strips the T16Ainh-A01 inhibitor had no effect on the total number of proliferating cells. The lack of effect of T16Ainh-A01 on the proliferation of Kit-negative cells in the cultured mouse muscle strips is further confirmation that the effects of the compound on proliferation are a consequence of inhibiting Ano1. These findings suggest that the effect of T16Ainh-A01 is specific for the cells expressing Ano1 in all the substrates studied.
We previously showed that the role of Ano1 in cell proliferation is important for the progression of the cell cycle at the G1/S checkpoint [14]. Consistent with our results, a recent work in a model of head and neck squamous cell carcinoma showed that expression of Ano1 was directly correlated to proliferation of the tumor cells [15]. In this model the mechanism of action of Ano1 appeared to take place through induction of cyclin D1 (which is a key gene in the G1/S checkpoint) and activation of the MAP/ERK pathway and in particular ERK1/2. These results are particularly interesting given the high expression of Ano1 in GIST [8] [9]. It has already been suggested that Ano1 could be a new potential target to pursue to arrest proliferation in GIST [24]. Based on these novel results, T16Ainh-A01 and similar inhibitors represent a further step in this direction. However, a recent paper on vascular tissue described an opposite effect of Ano1 as a negative regulator of cell proliferation in a model of hypertensive rat smooth muscle cells [25]. In this model Ano1 expression affects the proliferation of smooth muscle cells by arresting them at the G0/G1 checkpoint through reduction of cyclin D1 and cyclin E expression [25]. It is thus possible that Ano1 might have different roles in different cell types or in different physiological contexts.
In conclusion, these data add to the emerging evidence that chloride channels in general and Ano1 in particular are involved in the regulation of proliferation. The role of Ano1 in the progression of the cell cycle is confirmed by the effects of the more selective inhibitor T16Ainh-A01. T16Ainh-A01 is also shown to directly inhibit Ano1-mediated Cl− currents. Small molecules of this class with less off-target effects may be novel therapeutic tools for arresting proliferation of Ano1-positive tumors such as GISTs.
Highlights.
T16Ainh-A01 blocked Ano1 currents in HEK cells expressing Ano1
T16Ainh-A01 reduced proliferation in ICC primary cultures and CFPAC-1 cell line
T16Ainh-A01 reduced proliferation of ICC in intact smooth muscle strips
Acknowledgments
We would like to thank Dr. Verkman from UCSF for providing the T16Ainh-A01 compound. We would also like to thank Kristy Zodrow for secretarial assistance and Gary Stoltz for technical assistance. This work was supported by NIH grant DK57061.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jirsch J, Deeley RG, Cole SP, Stewart AJ, Fedida D. Inwardly rectifying K+ channels and volume-regulated anion channels in multidrug-resistant small cell lung cancer cells. Cancer research. 1993;53:4156–4160. [PubMed] [Google Scholar]
- 3.Shuba YM, Prevarskaya N, Lemonnier L, Van Coppenolle F, Kostyuk PG, Mauroy B, Skryma R. Volume-regulated chloride conductance in the LNCaP human prostate cancer cell line, American journal of physiology. Cell physiology. 2000;279:C1144–C1154. doi: 10.1152/ajpcell.2000.279.4.C1144. [DOI] [PubMed] [Google Scholar]
- 4.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 5.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap MK, Marimuthu A, Kishore CJ, Peri S, Keerthikumar S, Prasad TS, Mahmood R, Rao S, Ranganathan P, Sanjeeviah RC, Vijayakumar M, Kumar KV, Montgomery EA, Kumar RV, Pandey A. Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer biology & therapy. 2009;8:36–46. doi: 10.4161/cbt.8.1.7090. [DOI] [PubMed] [Google Scholar]
- 8.West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. The American journal of pathology. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. The American journal of surgical pathology. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract, American journal of physiology. Gastrointestinal and liver physiology. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21413–21418. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. The Journal of physiology. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. The Journal of physiology. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanich JE, Gibbons SJ, Eisenman ST, Bardsley MR, Rock JR, Harfe BD, Ordog T, Farrugia G. Ano1 as a regulator of proliferation, American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G1044–G1051. doi: 10.1152/ajpgi.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM. TMEM16A Induces MAPK and Contributes Directly to Tumorigenesis and Cancer Progression. Cancer research. 2012;72:3270–3281. doi: 10.1158/0008-5472.CAN-12-0475-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiological reviews. 1999;79:S109–S144. doi: 10.1152/physrev.1999.79.1.S109. [DOI] [PubMed] [Google Scholar]
- 17.Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast Cancer. British journal of pharmacology. 2006;147(Suppl 1):S269–S276. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrugia G, Rae JL, Szurszewski JH. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. The Journal of physiology. 1993;468:297–310. doi: 10.1113/jphysiol.1993.sp019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liantonio A, Giannuzzi V, Picollo A, Babini E, Pusch M, Conte Camerino D. Niflumic acid inhibits chloride conductance of rat skeletal muscle by directly inhibiting the CLC-1 channel and by increasing intracellular calcium. British journal of pharmacology. 2007;150:235–247. doi: 10.1038/sj.bjp.0706954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. The Journal of biological chemistry. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ordog T, Gibbons SJ, Farrugia G. Altered expression of Ano1 variants in human diabetic gastroparesis. The Journal of biological chemistry. 2011;286:13393–13403. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wouters MM, Roeder JL, Tharayil VS, Stanich JE, Strege PR, Lei S, Bardsley MR, Ordog T, Gibbons SJ, Farrugia G. Protein kinase C{gamma} mediates regulation of proliferation by the serotonin 5-hydroxytryptamine receptor 2B. The Journal of biological chemistry. 2009;284:21177–21184. doi: 10.1074/jbc.M109.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardsley MR, Horvath VJ, Asuzu DT, Lorincz A, Redelman D, Hayashi Y, Popko LN, Young DL, Lomberk GA, Urrutia RA, Farrugia G, Rubin BP, Ordog T. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, Du YH, Lv XF, Liu J, Zhou JG, Guan YY. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]