Abstract
Objective
Left ventricular end systolic pressure (LV ESP) is important in assessing left ventricular performance. LV ESP is usually derived from prediction equations. It is unknown whether these equations are accurate at rest or following exercise in a young, healthy population.
Design
We compared measured LV ESP versus LV ESP values from the prediction equations at rest, 15 minutes and 30 minutes following peak aerobic exercise in 60 participants.
Methods
LV ESP was obtained by applanation tonometry at rest, 15 minutes post and 30 minutes post peak cycle exercise.
Results
Measured LV ESP was significantly lower (p<0.05) at all time points in comparison to the two calculated values. Measured LV ESP decreased significantly from rest at both the post15 and post30 time points (p<0.05) and changed differently in comparison to the calculated values (significant interaction; p<0.05). The two LV ESP equations were also significantly different from each other (p<0.05) and changed differently over time (significant interaction; p<0.05).
Conclusions
These data indicate that the two prediction equations commonly used did not accurately predict either resting or post exercise LV ESP in a young, healthy population. Thus, LV ESP needs to be individually determined in young healthy participants. Non-invasive measurement through applanation tonometry appears to allow for a more accurate determination of LV ESP.
Keywords: End systolic pressure, applanation tonometry, left ventricular function
Introduction
Left ventricular end systolic pressure (LV ESP) is the pressure at the completion of ventricular contraction and is important in assessing left ventricular performance1. LV ESP is used to determine the end-systolic pressure-volume relationship (ESPVR) providing a relatively load independent measure of left ventricular systolic performance2. LV ESP is also used to determine both arterial and left ventricular elastance (Ea and ELV, respectively)3. Ea and ELV are used to evaluate ventricular-arterial coupling, both at rest and following perturbations such as exercise4. Accurate determination of ESPVR and ventricular-vascular coupling is important as both are related to clinical outcomes in various patient subsets1, 5. It is also relevant to examine these measures in a young, healthy subset, especially in young populations with higher cardiovascular risk. It has been shown that early changes in Ea in young African American men contribute to an increase in left ventricular load, which may lead to left ventricular remodeling and an increased cardiovascular risk6.
LV ESP is directly determined using invasive techniques and is usually performed during heart catheterization, limiting its use and applicability5. To overcome this limitation, there are two prediction equations often used to derive LV ESP; Calc1: ([2 × systolic blood pressure (SBP) + diastolic blood pressure (DBP)] / 3) and Calc2: 0.9 × SBP7. These equations were shown to provide accurate estimations of LV ESP under resting conditions1, 7. However, the equations were derived from patients referred for atypical chest pain and the sample size was small (n=10). Although these equations have been validated in older patients (age 62 y) undergoing cardiac catheterization for chest pain or heart failure1, it is unknown if they also apply to a younger, healthier population. Furthermore, it is unknown if the equations can be used to evaluate the response to stress perturbations such as exercise. This is an important point as both LV ESPVR and ventricular-vascular coupling are often evaluated in response to stress2. Applanation tonometry provides a convenient, accurate and noninvasive way to estimate LV ESP, from measured pulse waves8. The pulse wave in the radial or carotid artery is used to construct the ascending aortic pulse waveform to identify cardiac systolic and diastolic cycles and to determine ventricular-vascular interaction9.
Therefore, the purpose of this study was to determine the accuracy of the LV ESP prediction equations in a young healthy population both at rest and following peak exercise by comparing the equation derived LV ESP to the noninvasive determined LV ESP using applanation tonometry.
Methods
Sixty (32 males and 28 females) healthy, nonsmoking, normotensive participants between the ages of 18 and 35 years participated in this study. Per questionnaire, participants were considered untrained based on self reported physical activity of less than 30 minutes one day a week for the previous 6 months. The participants were not taking any medication except for oral contraceptives. All participants were recruited from the local university population. The study followed the procedures for protection of human participants as provided in the declaration of Helsinki. Prior to any data collection, all participants signed informed consent and the study was approved by the University of Illinois at Urbana-Champaign institutional review board (Ethics Board).
Participants reported to the lab for one visit. All women were tested in the early follicular phase or during the placebo phase of oral contraceptives. Participants were instructed to be 4 hours postprandial and to abstain from caffeine and alcohol for at least 12 hours before testing. Initially, participants completed a physical activity and health history questionnaire and measurements of height and weight were taken. Participants then assumed a supine position and rested quietly for 5 min before systolic and diastolic blood pressure (BP) measurements were taken using an automated oscillometric cuff (HEM-907 XL; Omron, Japan). Brachial BP measurements were repeated and if both SBP and DBP values were within 5 mm Hg of each other, the average of the two values was used for analysis. If measurements were not within 5 mm Hg, readings were taken until two values within 5 mm Hg were obtained. American Heart Association guidelines were followed except all measurements were performed in the supine position to provide a more stable and reproducible blood pressure. Pulse wave analysis measurements were made using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, Australia). Radial artery pressure waveforms were obtained from a 10-s epoch using applanation tonometry (Miller Instruments, Houston, TX) and calibrated using diastolic and mean brachial BP. From this radial artery pressure wave form, a reconstructed central aortic pressure waveform was generated using a generalized validated transfer function8 to determine central BP and LV ESP. This technique has been validated and is reliable for use during exercise10, 11. The reconstructed arterial waveform is virtually identical to the invasively determined waveform, with almost identical values for aortic ESP10. Since our participants were young and healthy, and none had any history of a cardiovascular condition known to create an aortic valve gradient (such as aortic stenosis), it is reasonable to assume that the generated aortic ESP equals LVESP.
Following these cardiovascular measures, the participants underwent a VO2peak test. The VO2peak test was done on an upright, stationary cycle ergometer (Lode Excaliber Sport, Groningen, Netherlands). Participants were familiarized with the cycle ergometer and were allowed to practice before the start of the test. Although no standard familiarization technique was performed, post hoc analysis determined that adequate peak efforts were produced by all participants. Following a brief warm-up of unloaded cycling, participants began pedaling at 50 W. The resistance was then increased 30 W every 2 minutes until test termination. Heart rate (HR) was measured using a Polar Heart Rate Monitor (Polar Electro, Woodbury, NY) and expired air was collected and analyzed using a Quark b2 breath-by-breath metabolic system (Cosmed, Rome, Italy). The sampling interval used to determine VO2peak was the highest 10 second average VO2. All participants reached volitional fatigue or an inability to maintain pedal rate above 60 rpm, upon which the test was terminated. Following the VO2peak test, test results were examined and all participants were shown to have satisfied two or more of the following four criteria: a) a respiratory ratio of 1.1 or greater; b) a plateau in HR despite an increase in workload; c) a final rate of perceived exertion (RPE) score of 17 or greater on the Borg scale (scale 6–20); and/or d) an increase in work rate that elicited an increase in VO2 of ≤150 mL/min, indicative of a plateau in oxygen consumption12.
Post exercise measurements of blood pressure and pulse wave analysis were done at 15 and 30 minutes post VO2peak test.
Power analysis, due to a lack of information in the literature, was performed based on a small to medium effect size for the interaction between formula based and measured LV ESP at rest vs exercise. This yielded an estimated sample size of 24 to 54 participants, therefore 60 participants were included to satisfy the upper level criteria. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses and statistical significance was set at p < 0.05. Means and standard errors were calculated for all variables. To evaluate if a prediction equation for LV ESP could be developed based on data from this study, we applied block regression analysis. In block 1, brachial SBP and DBP were used as dependent variables; in block 2, age, height and weight were added; in block 3, gender was added. Since adding block 2 and 3 only explained an additional 2% of the variance, only results from the regression analysis with the dependent variables of SBP and DBP are reported. Mean differences between the estimated LV ESP, the prediction equation, and the two formulas calculated LV ESP over time was evaluated using a 3 × 3 [LV ESP measurement (estimated from pulse wave analysis and the two formulas) by time (rest, 15 and 30 min post exercise)] ANOVA with repeated measures followed by t-tests post hoc if indicated. Bonferroni corrections were applied to post hoc tests. To further evaluate the accuracy of LV ESP prediction from the two formulas compared to estimated LV ESP from the pulse wave analysis, we constructed Bland-Altman plots for each of the three time points. Bivariate correlations were applied to each Bland-Altman plot to evaluate the potential of systemic error.
Results
Participants had a mean age of 24 ± 0.6 y, a mean height of 171.7 ± 1.3 cm, a mean weight of 78.7 ± 3.0 kg, a mean body mass index (BMI) of 26.5 ± 0.9 kg/m2, a mean VO2peak of 35.1 ± 1.1 ml/kg/min and a mean maximal HR of 185 ± 1.5 beats per minutes. The prediction equation (PredEq) that was developed based on data from this study was: ESP=(0.205*SBP)+(0.898*DBP)+0.4214 (r2=0.831, SEE = 4.2; p<0.0001). Measured LV ESP was significantly lower (p<0.05) at all time points in comparison to the two calculated LV ESPs from the literature. Although resting values were similar, the values from the equation derived from our sample were significantly different (p<.05) from measured LV ESP (p<0.05) for both post exercise time points (post15 and post30) (Figure 1). The prediction equation derived from our sample was also significantly different at all time points from the two calculated LV ESP equations from the literature (p<.05). Measured LV ESP was significantly different from rest for both the post15 and post30 time points (p<0.05) and changed differently in comparison to the estimated LV ESP . The two estimated LV ESP equations from the literature were also significantly different from each other (p<0.05) and changed differently over time (significant interaction; p<0.05). Calculated LV ESP equation 2 was significantly different from both rest and post 15 at the post 30 time point (p<0.05) whereas Calculated LV ESP equation 1 was only significantly different from rest at the post 30 time point (p<0.05).
Figure 1.
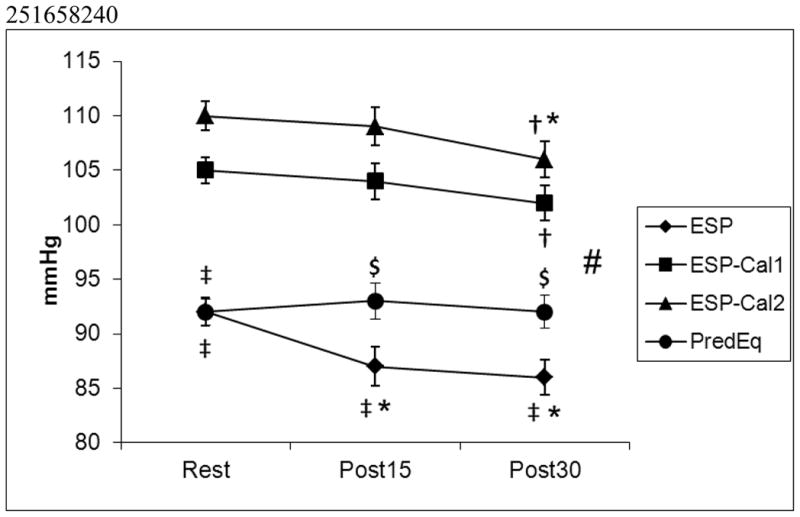
Comparisons of measured LV ESP, the LV ESP prediction equation developed from the subjects in this study (PredEq: (0.205*SBP)+(0.898*DBP)+4.214), and two calculations of LV ESP based on formulas from the literature7 (Calc 1: (2•SBP+DBP)/3; Calc 2: 0.9•SBP) LV ESP at rest and post15 and post30 minutes following an acute bout of exercise. # denotes an interaction. * p<0.05, significantly different from rest. † p<0.05, significantly different from post15. ‡ p<0.05, significantly different from both calculated LV ESP equations. $ p<0.05, significantly different from both calculated LV ESP equations and measured ESP.
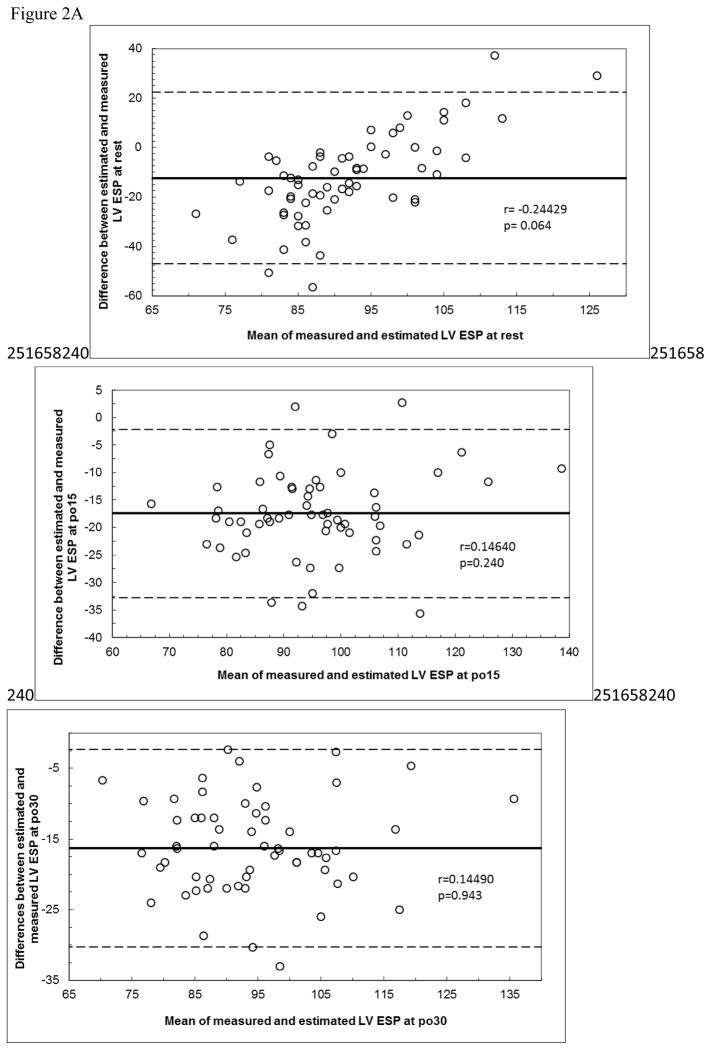
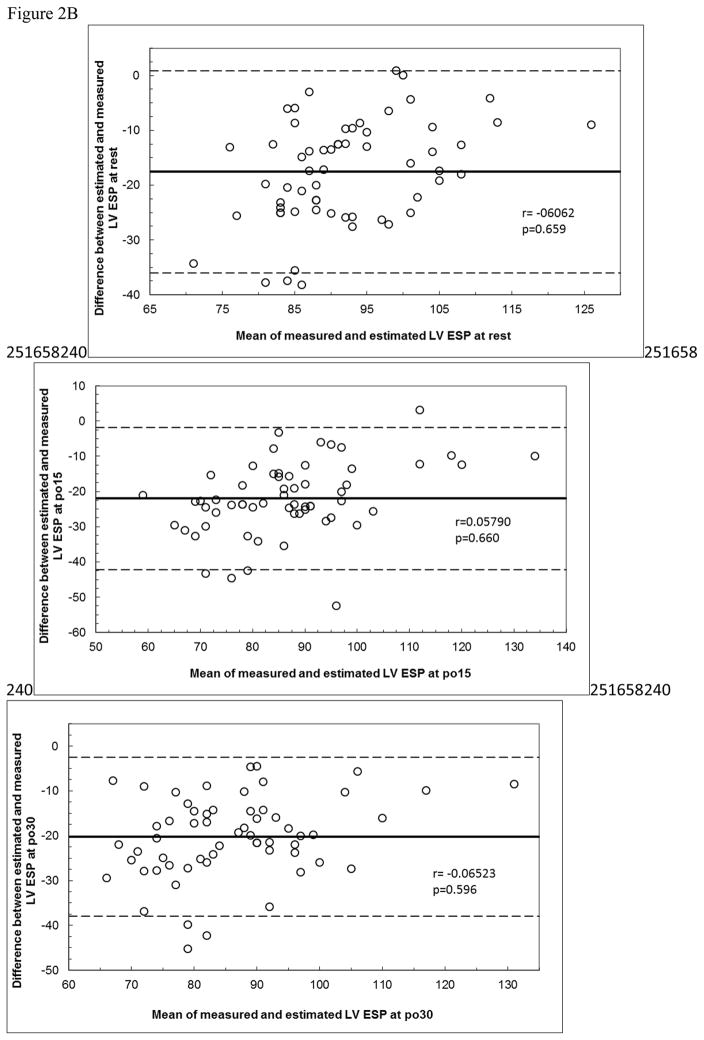
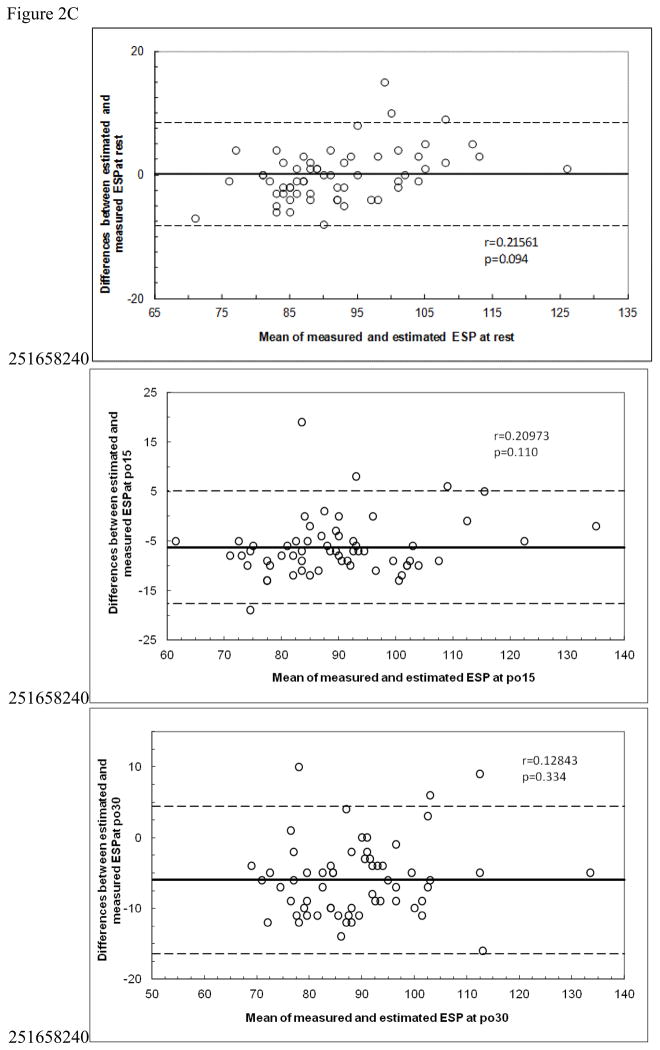
Bland Altman plots are shown in Figure 2. There were no significant correlations for any of the plots. However, in Figure 2A, the top panel shows near significance (p=0.064) and visual inspection suggest systematic bias. Both calculated equations (from the literature) overpredicted measured LV ESP with very large limits of agreement, and the prediction equation developed from our data also overpredicted post exercise values.
Figure 2.
Figure 2A. Bland Altman Plots for Equation 1.
Figure 2B: Bland Altman plots for Equation 2.
Figure 2C: Bland Altman plots for Prediction Equation
Resting and post exercise blood pressures and heart rates are shown in Table 1. Only brachial SBP changed significantly from rest (p<0.05).
Table 1.
Participant pressure characteristics.
| REST | PO15 | PO30 | |
|---|---|---|---|
| SBP (mmHg) | 122 ± 1.5 | 121 ± 1.9 | 118 ± 1.9ab |
| DBP (mmHg) | 70 ± 1.2 | 71 ± 1.5 | 71 ± 1.4 |
| centralSBP (mmHg) | 104 ± 1.3 | 103 ± 1.7 | 102 ± 1.6 |
| centralDBP (mmHg) | 71 ± 1.2 | 74 ± 1.5 | 73 ± 1.4 |
| centralMAP (mmHg) | 86 ± 1.2 | 86 ± 1.6 | 86 ± 1.5 |
| HR (bpm) | 61 ± 1.3 | 60 ± 1.3 | 60 ± 1.2 |
Data are mean ± SEM.
p<0.05 vs rest
p<0.05 vs PO15.
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; bpm, beats per minute; LV ESP, left ventricular end systolic pressure; mmHg, millimeters of mercury; PO15, 15 minutes following an acute bout of exercise; PO30, 30 minutes following an acute bout of exercise.
Discussion
Our study is the first to compare the accuracy of two commonly used13, 14 LV ESP prediction equations with a validated estimation using non-invasive applanation tonometry in a young, healthy population at rest and following exercise. The main finding was that these often used prediction equations15, 16 overpredict LV ESP in this population. Furthermore, changes in LV ESP following the exercise perturbation were inaccurate using either prediction equation. We also developed a prediction equation for LV ESP from the data on our participant sample, which although accurate at rest, did not accurately predict he post exercise values. These novel findings suggest that LV ESP should not be predicted from these commonly used formulas in the literature in young healthy individuals. Instead, resting LV ESP can be accurately predicted from the formula we developed. However, exercise alters LV ESP differentially from brachial blood pressure, resulting in inaccurate prediction of the response to exercise using any of these formulas.
We used the formulas developed by Kelly et al.7; (2 × SBP + DBP) / 3 and 0.9 × SBP. These formulas were developed at rest in a small population of individuals with symptoms of cardiovascular disease7 and they were validated in older adults with heart failure or chest pain1. These equations use brachial blood pressure measurements to determine LV ESP, however, SBP and pulse pressure (PP) may differ between brachial and central arteries17. The association between central and peripheral SBP can vary significantly between individuals and brachial SBP measurements may give an inadequate estimate of central SBP11. These inaccuracies can be misleading as it has been shown that 32% of men and 10% of women with normotensive brachial BP have aortic SBP similar to aortic SBP of people with brachial hypertension18. Thus, LV ESP equations using brachial pressures could be inaccurate because brachial BP may not accurately reflect aortic BP. Our data supports this notion, as the formula developed on the participant population in our study also did not accurate predict post exercise LV ESP. This appeared to be due to differential effects of the exercise on LV ESP and brachial blood pressure.
Central and brachial SBP can differ because of demographic and hemodynamic differences that can affect arterial wave reflection19. For example, it has been shown that young healthy African Americans have similar brachial SBP but higher aortic SBP compared to age, BMI and fitness matched Caucasians20. There are also differences between young and older populations, as aging induces significant increases in peripheral and central PP21. This could be due to the stiffening of the aorta with age21, 22 or age related changes in arterial length23, 24 which causes an increase in pulse wave velocity due to a distal shift of the arterial wave reflection site potentially due to an attempt to match impedance of central and peripheral arterial stiffness25.
We estimated LV ESP from a central pulse wave form using applanation tonometry where the central pulse wave form is created using a validated transfer function9. This transfer function has also been validated against invasive measures during and following exercise11. Sharman et al.11 showed that at rest and during exercise there was a high correlation between invasive and transfer function derived measures of LV ESP (p<0.001), and the mean LV ESP was almost exactly the same.
Exercise is another confounding factor when using these equations as the LV ESP prediction equations have not been validated during exercise3. As seen in Table 1, brachial SBP significantly decreased whereas aortic SBP did not change significantly following exercise. HR increases significantly during exercise and the central to peripheral SBP difference is intensified such that pressure differences can surpass 80 mmHg26. Central BP changes during exercise are different compared with brachial BP changes indicating peripheral BP response is not a good reflection of the actual pressure load at the heart11. Furthermore, Munir et al.27 showed that with exercise there was a change in morphology of the radial and digital pressure pulse waveforms leading to a reduction in late systolic and diastolic augmentation of the peripheral pressure pulse. This continued for 60 minutes into recovery and was present even when HR, stroke volume and pulse wave velocity had returned to baseline. LV ESP is estimated at the closure of the aortic valve at the dicrotic notch and these waveform changes that occur with exercise will likely alter LV ESP. These confounding factors are likely why the prediction equation we developed from our data was able to accurately predict resting LV ESP in a young, healthy population but was unable to predict changes in LV ESP following a bout of exercise. Therefore, using applanation tonometry instead of the estimation formulas appears to be more accurate when determining LV ESP following exercise since it accounts for these waveform changes.
The LV ESP calculated equations are typically used in arterial-ventricular coupling, which shows that left ventricular performance is influenced by arterial load and arterial properties are influenced by left ventricular performance3. Arterial-ventricular coupling is important in the determination of cardiovascular performance and myocardial energetics28, 29. Due to the discrepancies between the calculated equations and estimated LV ESP, measured LV ESP should be used when evaluating arterial-ventricular coupling in a young, healthy population.
These results are also clinically relevant because there are changes within the arterial tree due to age and cardiovascular risk factors that influence central hemodynamics during exercise. Men with hypercholesterolemia exhibited similar brachial SBP changes but had increases in augmentation index (AIx) and attenuated PP amplification during exercise when compared with controls with normal cholesterol levels. This suggests that individuals with high cholesterol have systemic arterial stiffening with exercise30 and brachial BP does not accurately demonstrate myocardial afterload. Central pressures more consistently predict mortality, regulate left ventricular hypertrophy and are independently correlated with cardiovascular events, making the central BP response to exercise a better predictor of cardiovascular risk31–33.
There are several limitations to our findings. No actual invasive LV ESP measurements were made in this study, therefore we can only assume that the estimated LV ESP determined through applanation tonometry is an accurate reflection of LV ESP. However, the tonometry determined LV ESP has been validated against invasively measured LV ESP11, thus it is likely our tonometry determined LV ESP is a fairly accurate determination. Noninvasive brachial BP was used to calibrate the radial waveforms and any errors in this estimation would affect the true estimation of LV ESP. However, this would not affect the comparison of LV ESP methods because both rely on brachial BP. We also did not determine if participants had aortic stenosis or any other condition causing an aortic valve gradient through participant history and aortic gradients were not measured in our study. A significant aortic valve gradient would cause discrepancies between LV ESP and aortic ESP rendering our estimated LV ESP inaccurate. However, the group of individuals studied were young and healthy, none of whom had any history of any cardiovascular complications. Thus it is reasonable to assume that aortic and LV ESP would not differ in our participants. Considering we chose a young healthy population the findings presented can only be applied to comparable populations.
Conclusion
Our study showed that the two commonly used LV ESP prediction equations were not accurate in a young, healthy population and also did not reflect the LV ESP response to a perturbation such as exercise when compared to estimated measures using applanation tonometry. This is potentially due to the population used when these equations were developed and differences between changes in central compared to peripheral BP. A LV ESP prediction equation was developed due to the data resulting from this study, but although it accurately predicted resting LV ESP, it did not accurately predict changes following exercise. It appears that these formulas could accurately predict post exercise changes in LV ESP because brachial blood pressure changes differentially from LV ESP following exercise.
Practical Implications.
Resting LV ESP can be accurately predicted from brachial blood pressures in young healthy individuals when using the prediction equation developed from our participant sample.
The LV ESP response to exercise needs to be measured and cannot be accurately determined from prediction formulas.
Non-invasive radial pressure wave form measurements appear to offer an acceptable alternative to evaluate ESP both at rest and following exercise. This allows for non-invasive determination of both arterial and ventricular elastance which can provide insight regarding cardiovascular function and risk beyond traditional measures.
Acknowledgments
This study was supported in part by National Institutes of Health HL093249-01A1 (Fernhall). We are grateful to the study volunteers for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 2.Hausdorf G, Gluth J, Nienaber CA. Non-invasive assessment of end-systolic pressure-length and stress-shortening relationships in normal individuals: significance of different loading conditions induced by methoxamine and angiotensin II. Eur Heart J. 1987;8(10):1099–1108. doi: 10.1093/oxfordjournals.eurheartj.a062175. [DOI] [PubMed] [Google Scholar]
- 3.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105(4):1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar SS, Schulman SP, Gerstenblith G, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44(3):611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara H, Yokota M, Sobue T, et al. Relation between ventriculoarterial coupling and myocardial energetics in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1994;23(2):406–416. doi: 10.1016/0735-1097(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 6.Heffernan KS, Fernhall B. A systematic appraisal of ventricular-aortic load in African American men. Artery Research. 2009;3:65–72. [Google Scholar]
- 7.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86(2):513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 8.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14(5):S147–157. [PubMed] [Google Scholar]
- 10.Holland DJ, Sacre JW, McFarlane SJ, et al. Pulse wave analysis is a reproducible technique for measuring central blood pressure during hemodynamic perturbations induced by exercise. Am J Hypertens. 2008;21(10):1100–1106. doi: 10.1038/ajh.2008.253. [DOI] [PubMed] [Google Scholar]
- 11.Sharman JE, Lim R, Qasem AM, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47(6):1203–1208. doi: 10.1161/01.HYP.0000223013.60612.72. [DOI] [PubMed] [Google Scholar]
- 12.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8(1):73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- 13.Tartiere-Kesri L, Tartiere JM, Logeart D, et al. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59(5):455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 14.Chantler PD, Melenovsky V, Schulman SP, et al. Use of the Frank-Starling mechanism during exercise is linked to exercise-induced changes in arterial load. Am J Physiol Heart Circ Physiol. 2012;302(1):H349–358. doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122(18):1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gayat E, Mor-Avi V, Weinert L, et al. Noninvasive quantification of left ventricular elastance and ventricular-arterial coupling using three-dimensional echocardiography and arterial tonometry. Am J Physiol Heart Circ Physiol. 2011;301(5):H1916–1923. doi: 10.1152/ajpheart.00760.2011. [DOI] [PubMed] [Google Scholar]
- 17.Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension: experts' opinion and review of the data. Hypertension. 2009;54(2):375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 18.McEniery CM, Yasmin, McDonnell B, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51(6):1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 19.Nichols W, O'Rourke M. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London, United Kingdom: Edward Arnold; 1998. [Google Scholar]
- 20.Heffernan KS, Jae SY, Wilund KR, et al. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008;295(6):H2380–2387. doi: 10.1152/ajpheart.00902.2008. [DOI] [PubMed] [Google Scholar]
- 21.McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46(9):1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 23.Wenn CM, Newman DL. Arterial tortuosity. Australas Phys Eng Sci Med. 1990;13(2):67–70. [PubMed] [Google Scholar]
- 24.Sugawara J, Hayashi K, Yokoi T, et al. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1(6):739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara J, Hayashi K, Tanaka H. Distal shift of arterial pressure wave reflection sites with aging. Hypertension. 2010;56(5):920–925. doi: 10.1161/HYPERTENSIONAHA.110.160549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowell LB, Brengelmann GL, Blackmon JR, et al. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation. 1968;37(6):954–964. doi: 10.1161/01.cir.37.6.954. [DOI] [PubMed] [Google Scholar]
- 27.Munir S, Guilcher A, Kamalesh T, et al. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;51(1):112–118. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- 28.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46(1):185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 29.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J. 1993;125(6):1659–1666. doi: 10.1016/0002-8703(93)90756-y. [DOI] [PubMed] [Google Scholar]
- 30.Sharman JE, McEniery CM, Dhakam ZR, et al. Pulse pressure amplification during exercise is significantly reduced with age and hypercholesterolemia. J Hypertens. 2007;25(6):1249–1254. doi: 10.1097/HJH.0b013e3280be5911. [DOI] [PubMed] [Google Scholar]
- 31.Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39(3):735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 32.Westerhof N, O'Rourke MF. Haemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical therapy. J Hypertens. 1995;13(9):943–952. doi: 10.1097/00004872-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 34.Casey DP, Nichols WW, Braith RW. Impact of aging on central pressure wave reflection characteristics during exercise. Am J Hypertens. 2008;21(4):419–424. doi: 10.1038/ajh.2007.74. [DOI] [PubMed] [Google Scholar]