Abstract
Transplantation of syngeneic neural progenitor cells (NPCs) into mice persistently infected with the JHM strain of mouse hepatitis virus (JHMV) results in enhanced differentiation into oligodendrocyte progenitor cells (OPCs) that is associated with remyelination, axonal sparing, and clinical improvement. Whether allogeneic NPCs are tolerated or induce immune-mediated rejection is controversial and poorly defined under neuroinflammatory demyelinating conditions. We have used the JHMV-induced demyelination model to evaluate the antigenicity of transplanted allogeneic NPCs within the central nervous system (CNS) of mice with established immune-mediated demyelination. Cultured NPCs constitutively expressed the co-stimulatory molecules CD80/CD86 and IFN-γ treatment induced expression of MHC class I and II antigens. Injection of allogeneic C57BL/6 NPCs (H-2b background) led to a delayed type hypersensitivity (DTH) response in Balb/c (H-2d background) associated with T cell proliferation and IFN-γ secretion following co-culture with allogeneic NPCs. Transplantation of MHC-mismatched NPCs into JHMV-infected mice resulted in increased transcripts encoding the T cell chemoattractant chemokines CXCL9 and CXCL10 that correlated with increased T cell infiltration that was associated with NPC rejection. Treatment of MHC-mismatched mice with T cell subset-specific depleting antibodies increased survival of allogeneic NPCs without affecting commitment to an oligodendroyte lineage. Collectively, these results show that allogeneic NPCs are antigenic and T cells contribute to rejection following transplantation into an inflamed CNS suggesting that immunomodulatory treatments may be necessary to prolong survival of allogeneic cells.
Introduction
Multiple sclerosis (MS) is the most common cause of neurological disability in young adults1. The etiology of MS is thought to be multi-factorial including genetic, and environmental factors that may lead to initiation, maintenance and/or progression of disease2. For example, viral infection has long been considered a potential triggering mechanism involved in demyelination and numerous human viral pathogens have been suggested to be involved in eliciting myelin-reactive lymphocytes and/or antibodies that subsequently infiltrate the central nervous system (CNS) and damage the myelin sheath3–6. Therefore, viral models of demyelination are clearly relevant and have provided important insight into mechanisms associated with disease initiation, neuroinflammation, demyelination, and remyelination. An important clinical aspect related to the pathogenesis of MS is the eventual remyelination failure in chronic demyelinated plaques by endogenous oligodendrocyte progenitor cells (OPCs)7–9. With this in mind, cell-based therapies using neural progenitor cells (NPCs) have emerged as a potentially viable approach for promoting remyelination10,11. Our laboratory has recently demonstrated that transplantation of syngeneic mouse NPCs into mice persistently infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) is well tolerated and is associated with axonal sparing accompanied by extensive remyelination while not significantly dampening either neuroinflammation or T cell responses12,13. Evident from this work is the ability of engrafted NPCs to i) migrate to and colonize regions of demyelination by responding to the chemokine ligand CXCL1214 ii) preferentially differentiate into cells of an oligodendrocyte linage14,15, and iii) promote axonal sparing and remyelination15.
NPC transplantation offers a promising therapeutic approach for promoting remyelination in patients with demyelinating disease such as MS. However, similar to solid-organ transplantation, donor specific allogeneic responses are likely to occur that may require life-long immunosuppression that elevates susceptibility to opportunistic infections and tumors. Therefore, an important and clinically relevant question related to stem cell therapies revolves around the allograft rejection of implanted allogeneic stem cells as they may not be “self-derived.” This is particularly important when considering cellular transplantation for treatment of chronic neurodegenerative diseases as ongoing debate has centered on whether MHC matching is critical for successful engraftment into the CNS. Compelling evidence argues that unmatched grafts are well-tolerated within the CNS due to muted immunogenicity of NPCs and clinical studies supporting that transplantation of allogeneic NPCs results in prolonged survival16–18. However, the immunoprivileged status of NPCs has recently been questioned19 and more recent studies argue that MHC mismatching diminishes survival of NPCs and mutes endogenous neurogenesis, and this is associated with innate immune responses20. Moreover, transplantation of allogeneic NPCs in a model of spinal cord injury results in activation of the immune system and NPC rejection21. These findings indicate that in order for long-term engraftment of NPCs to be efficacious the use of immunomodulatory agents must be considered. With this in mind, it is imperative to determine if allogeneic NPCs are antigenic following transplantation into an environment with established inflammatory demyelinating disease. To this end, we provide evidence that allogeneic NPCs are recognized as foreign and infiltrating lymphocytes contribute to rejection following transplantation into JHMV-infected mice with established demyelination.
Materials and Methods
Animals and virus
Age-matched (5–7wk) C57BL/6 (H-2b, National Cancer Institute (NCI), Frederick, MD) and Balb/c (H-2d, NCI) mice were infected intracranially (i.c.) with 150 (C57BL/6) or 15,000 (Balb/c) plaque forming units (PFU) of MHV strain J2.2v-1 (JHMV) in 30 µl sterile HBSS for transplantation studies or intraperitoneally (i.p.) with 2.50×105 PFU of the DM strain of JHMV suspended in 200 µl sterile HBSS for T cell conditioned media (CM) preparation14. Mice were sacrificed at various days post infection (p.i.) and spinal cords were removed and processed for analysis. All experiments were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Cell culture, reagents, and transplantation
Enhanced Green Fluorescent Protein expressing NPCs (GFP-NPCs), derived from C57BL/6 mice, were cultured as previously described14. Undifferentiated GFP-NPCs were transplanted (2.5 × 105 in 2.5 µl HBSS/mouse) at spinal cord T9 at day 14 post-infection (p.i.) into C57BL/6 (syngeneic) and Balb/c (allogeneic) mice. As previously published, this time point for transplantation was chosen since virus and inflammation has waned while demyelination has peaked14. As a sham control, virally-infected mice were transplanted with HBSS alone15. Recombinant mouse IFN-γ was purchased from Cell Sciences (Canton, MA).
Mixed Lymphocyte Reaction (MLR)
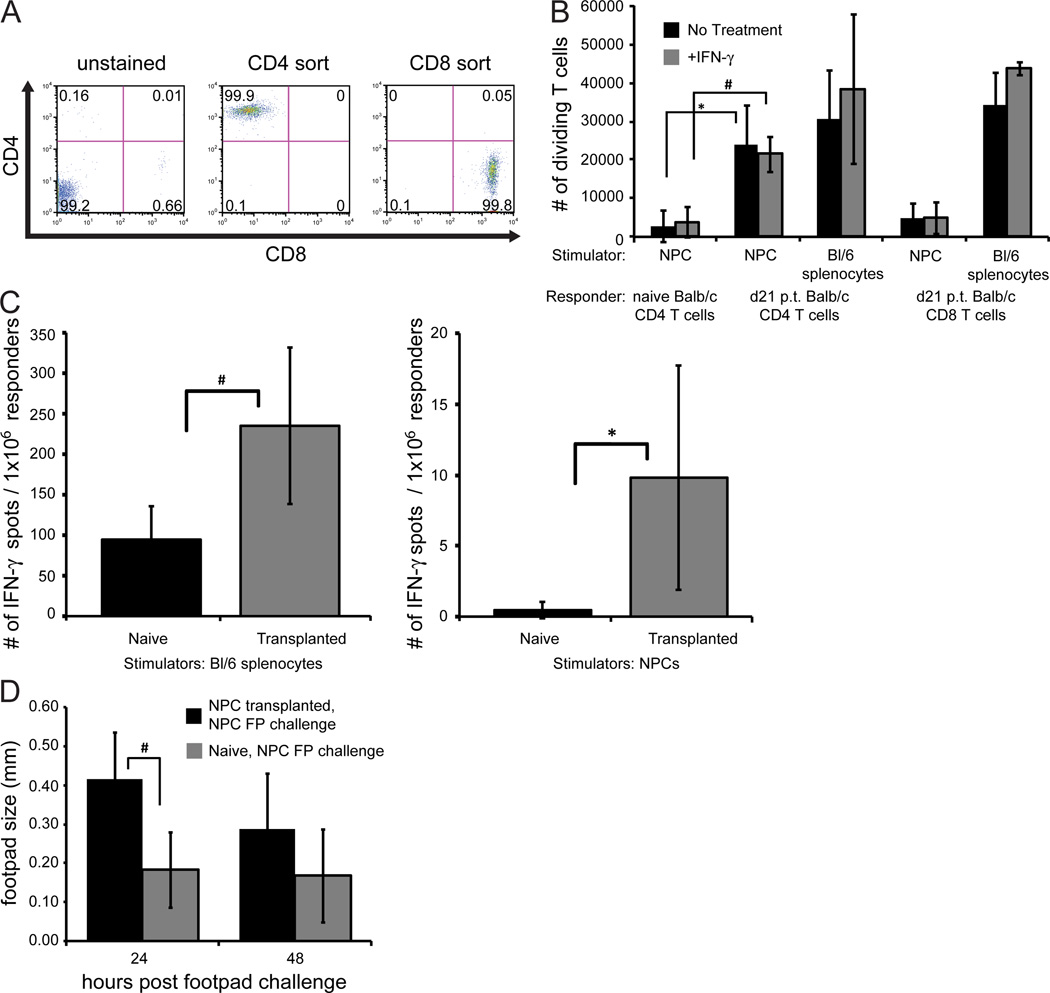
An MLR is an in vitro method for assaying T cell proliferation in response to alloantigen. Cells were isolated from 4–5 pooled spleens harvested from naive and infected mice at day 21 p.t. Responding T cell populations were purified by negative selection using a pan T cell isolation kit (Miltenyi Biotec, Auburn, CA) with an MS column, composed of ferromagnetic spheres capable of separating magnetically labeled and unlabeled cells when placed in the column attached to a magnet (Miltenyi Biotec). The loaded column was washed 5× with 3mL HBSS to remove and collect all unlabeled cells. Cells were further separated into CD4+ and CD8+ populations by staining with PE-conjugated CD4 (BD Biosciences, clone GK1.5) and PE-Cy7-conjugated CD8 (eBioscience, clone 53–6.6) antibodies and sorting with a FACSAria (BD Biosciences, Franklin Lakes, NJ). Aliquots of enriched T cells were stained for 8 min at room temperature with 5µm eFluor670 (EF670) labeling dye (eBioscience), which has an excitation of 647nm and is detected with an APC filter. As cells divide, dye expression is dampened by being evenly distributed into daughter cells. After staining, cells were immediately washed 2× with PBS + 2% fetal calf serum. Stimulating populations of cells included control splenocytes and NPCs that were treated with 50µg/ml mitomycin C (AG Scientific, San Diego, CA) for 30 min at 37°C. Cells were washed 5× with 15mL HBSS. 1×105 stimulators and 1×105 responders were plated together in a round bottom 96-well plate in the presence or absence of recombinant mouse IFN-γ (100U/ml). Co-cultures of cells were incubated for 5 days and number of dividing responder T cells isolated from spleens of Balb/c mice at day 21 p.t. was compared to number of dividing responder T cells isolated from spleens of naive Balb/c mice for statistical significance.
Enzyme linked immunosorbent spot (ELISPOT) assay
An ELISPOT assay captures secreted proteins on a specific antibody-coated microplate and can be used to determine memory T cell activation by detecting IFN-γ secretion. The frequency of alloreactive T cells was assessed by performing a 48 h MLR with purified T cells in 96 well Multiscreen-IP plates (Millipore, Billerica, MA). Responder T cells were purified from the spleens of naive Balb/c mice and Balb/c mice at day 21 p.t. by MACS sorting as described above. Stimulator cell populations were C57BL/6 splenocytes or GFP-NPCs. Stimulators and responders were incubated as previously described above with a minimum of 6 wells per experimental condition. Briefly, plates were coated with 4µg/ml IFN-γ capture antibody (eBioscience, Clone AN-18) prior to MLR. Plates were washed with 0.01% Tween 20/PBS, followed by a 2 h incubation at 37° C with 0.5µg/ml biotinylated IFN-γ detection antibody (eBioscience, clone R4-6A2). Plates were washed with PBS/Tween 20 and incubated with 1:1000 Streptavidin AP (Invitrogen, Carlsbad, CA) for 45 minutes at room temperature. Plates were washed with PBS only, and then incubated with 100µl per well of BCIP/NBT (Sigma, St. Louis, MO) until spots developed. Spots were counted using a dissection microscope and Repeated Measures ANOVA was performed for statistical analysis.
Delayed Type Hypersensitivity (DTH) Assay
A DTH assay was employed to determine if memory T cells were present in an antigen-sensitized animal. Naïve Balb/c or C57BL/6 mice were sensitized with a subcutaneous flank injection of C57BL/6 splenocytes (50×106 cells) or GFP-NPCs (15×106 cells) and 8 days later were challenged with a footpad injection of either 10×106 C57BL/6 splenocytes or 2.5×106 NPCs. Footpad swelling was measured at defined times post-footpad injection with a digital micrometer and measurements were normalized to measurements from sham-sensitized mice. Alternatively, JHMV-infected Balb/c mice at day 21 p.t. with GFP-NPCs were challenged with a footpad injection of either 10×106 allogeneic splenocytes or 2.5×106 allogeneic NPCs and footpad swelling determined.
Histopathology
Animals were euthanized by inhalation of halothane (Sigma, St. Louis, MO) and fixed by cardiac profusion. The spinal cord was extracted and processed for OCT and resin embedded sections as previously described12. The number of GFP-positive cells was counted on at least two sections 80µm apart from each tissue block for each animal. Counts from experimental mice were averaged and data presented as average+SD. For immunofluorescent staining, rat-anti-platelet derived growth factor α (PDGFRα, 1.67µg/ml; eBioscience, San Diego, CA) was used. Secondary antibody used for visualization was Alexa 594 goat anti-rat (Invitrogen). DAPI Fluoromount-G (Southern Biotech, Birmingham, AL) was used to visualize nuclei.
T cell conditioned media (CM)
C57BL/6 mice were infected with an i.p. injection of 2.5×105 PFU of DM virus. At day 8 p.i., CD4 and CD8 T cells were isolated from spleen by negative selection followed by FACS to enrich for T cell subsets. Antigen presenting cells (APCs) were isolated by collecting the column bound non-T cells. Enriched APCs were treated with 50µg/ml mitomycin-C (AG Scientific) and 35×106 APCs + 5µm CD4 specific [membrane (M) glycoprotein spanning amino acid residues 133–147 (M133-147), Bio-Synthesis, Lewisville, TX ] or 5µm CD8 specific [spike (S) glycoprotein spanning amino acid residues 510–518 (S510–518), Bio-Synthesis] viral peptide were co-cultured with 35×106 CD4+ or CD8+ T cells in a 25mm culture dish with 10ml GFP-NPC media for 48 h. Following incubation, CD4 and CD8 T cell CM was administered to GFP-NPCs for 18, 24, or 42 h.
Quantitative real-time PCR
Total RNA was extracted from homogenized spinal cord of JHMV infected, NPC or sham transplanted C57BL/6 and Balb/c mice at days 1, 8, and 21 p.t. and cDNA was generated as previously described22. Quantitative real-time Taqman analysis for HPRT and eGFP with previously described primers and probes23,24 was performed using a BioRad (Hercules, CA) iCycler instrument according to the manufacturer's instructions. Expression of eGFP was normalized to HPRT. Primers were purchased from Invitrogen and the probe was purchased from Integrated DNA Technologies (Coralville, IA). Bio-Rad iQ Supermix was used for the reactions. Data were analyzed using the Bio-Rad iCycler iQ version 3.0a software and quantified using the relative expression software tool, version 225. The real-time SYBR green analysis for GAPDH, CD4 and CD8 was performed using previously described primers26 with a BioRad SYBR green kit on a BioRad iCycler. The primers for CXCL9, CXCL10, and IFN-γ are: CXCL9 forward: TTT TCC TTT TGG GCA TCA TCT T, CXCL9 reverse: AGC ATC GTG CAT TCC TTA TCA CT; CXCL10 forward: TCA GCA CCA TGA ACC CAA G, CXCL10 reverse: CTA TGG CCC TCA TTC TCA CTG; IFN-γ forward: CTT TGG ACC CTC TGA CTT GAG, IFN-γ reverse: TCA ATG ACT GTG CCG TGG. CD4, CD8, CXCL9, CXCL10, and IFN-γ mRNA expression was normalized to GAPDH mRNA expression.
Lymphocyte enrichment from spinal cord
Cells were isolated from spinal cords from experimental mice as previously described27–30. Briefly, spinal cords were removed from PBS-perfused mice and isolated tissue was ground with the rubber end of a 5mL syringe plunger in a 100mm petri dish, followed by vigorous trituration in 7mL DMEM medium. Single cell suspensions were centrifuged for 30 min at 1200 × g at 4°C over a discontinuous percoll gradient at which point percoll and lipid layers were removed. Isolated cells were filtered, washed with 15mL DMEM, centrifuged at 1000 × g at 4°C, counted, and prepared for flow cytometry (see below). Cells isolated from spleens were used as positive controls.
Flow Cytometry
Lymphocytes isolated from the spinal cord were immunophenotyped with fluorescent antibodies (1:200) for the following cell surface markers:, PE-conjugated CD4 (GK1.5; BD Biosciences) and PE-Cy7-conjugated CD8 (Ly-2; BD Biosciences). Appropriate isotype controls were used for each antibody. Cells were run on a FACStar flow cytometer (BD Biosciences) or LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, OR). NPCs were trypsinized with 0.05% trypsin (Invitrogen) and immunophenotyped with fluorescent antibodies (1:200) specific for the following cell surface markers: PE-conjugated MHC class I (eBioscience), MHC class II (BD Biosciences), CD80 (eBioscience) and CD86 (eBioscience). All cells for flow cytometry were FC blocked with anti-CD16/32 (1:200; BD Biosciences) for 20 min at 4°C.
Antibody Treatment
JHMV-infected mice were intraperitoneally (i.p.) treated with 100µg /mouse of either a depleting monoclonal antibody for CD4 [GK1.5, American Type Culture Collection (ATCC, Rockville, MD) TIB 207] or CD8 (Ly-2.2, 2.43, ATCC TIB 210), or control Rat IgG (Sigma) in 150µl sterile saline at days -1, 1, 3, 5, 12, and 19 p.t. The initial time point was chosen to ensure depletion occurs after viral clearance but begins prior to NPC transplantation. Delivery of antibody every other day allowed for efficient depletion of cells which was then switched to once a week for maintenance of cell depletion. These time points followed previously published guidelines31. Various concentrations were tested and sufficient depletion as determined by flow analysis of spleen was achieved at 100µg/mouse/time point.
Statistical Analysis
Statistical analysis was carried out by student's T-test, one-way Anova, or Repeated Measures ANOVA and p≤0.05 was considered significant.
Results
NPCs express MHC class I and II in response to IFN-γ treatment
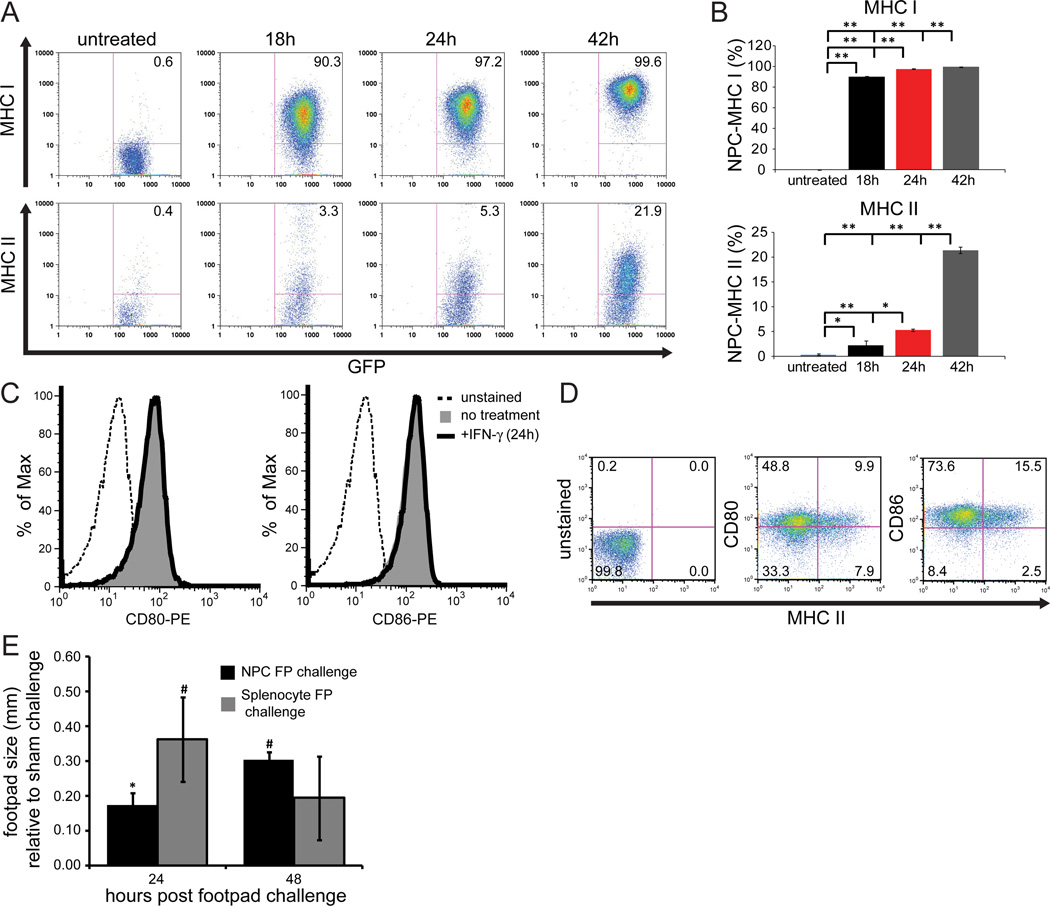
Cultured GFP-NPCs (derived from C57BL/6 mice, H-2b background) were treated with either medium alone or IFN-γ (100U/ml) and expression of MHC class I and II antigens were measured by flow cytometry. Expression of both MHC class I and II antigens increased over time as determined by staining at 18, 24, and 42 hours post-treatment when compared to medium only-treated cultures (Figure 1). Medium only-treated GFP-NPCs expressed <1% MHC class I and II. The kinetics of expression in response to IFN-γ treatment were different between the two surface antigens with 89.9±0.7% of cells expressing MHC class I by 18 h and ultimately peaking to 99.5+0.2% at 42 h (Figures 1A and B). In contrast, 2.2+0.9% of IFN-γ-treated cells expressed detectable MHC class II by 18 h and this reached 21.4+0.6% by 42 h (Figures 1A and B). These data demonstrate that while cultured NPCs are capable of expressing both MHC class I and II, expression of MHC class I is markedly more rapid and robust when compared to MHC class II. Examination of CD80 and CD86 expression revealed that cultured NPCs constitutively expressed co-stimulatory molecules and exposure to IFN-γ did not augment expression of either of these molecules (Figures 1C and D). We next evaluated whether allogeneic NPCs could evoke a T cell-mediated response. Balb/c mice (H-2d background) were immunized with splenocytes derived from C57BL/6 mice (H-2b background) and challenged via footpad injection (8 days post-immunization, p.i.) with either NPCs or splenocytes derived from either C57BL/6 mice or Balb/c mice. Within 24 h post-challenge, immunized Balb/c mice exposed to MHC-mismatched NPCs or splenocytes displayed increased footpad swelling with splenocyte challenged mice displaying greater swelling (0.36±0.12mm, p<0.01, n=3) as compared to NPC challenged mice (0.18±0.04mm, p<0.05, n=3). By 48 h, swelling had decreased in splenocyte-challenged mice yet increased in NPC-challenged animals (0.31±0.02mm, p<0.01), indicating that although the memory response following splenocyte immunization was stronger against splenocytes than NPCs, NPCs were capable of inducing a memory T cell response (Figure 1E).
Figure 1. GFP-NPCs express MHC antigens and are antigenic.
Cultured GFP-NPCs were treated with IFN-γ (100U/ml) for defined periods of time and MHC class I and II as well as CD80/86 determined by flow cytometry. (A) Representative flow analysis for MHC class I and II shown and (B) quantification of both class I and II induction. Data represents three independent experiments and data is shown as average+SD. (C) Representative histogram for CD80/86 staining show that IFN-γ does not increase expression of co-stimulatory antigens. (D) Dual expression of MHC class II and CD80/86 on IFN-γ-treated (24 h) GFP-NPCs. (E) Balb/c mice sensitized with subcutaneous injection of C57BL/6 splenocytes were challenged with either GFP-NPCs or C57BL/6 splenocytes and footpad swelling determined at 24 and 48 h post-injection. Footpad swelling in experimental mice was compared to unsensitized but footpad challenged mice. Data presented represents three independent experiments and data is presented as average+SD; * p<0.05, # p<0.01, ** p<0.001.
NPCs express MHC class I and II in response to treatment with conditioned media (CM) from antigen-sensitized T cells
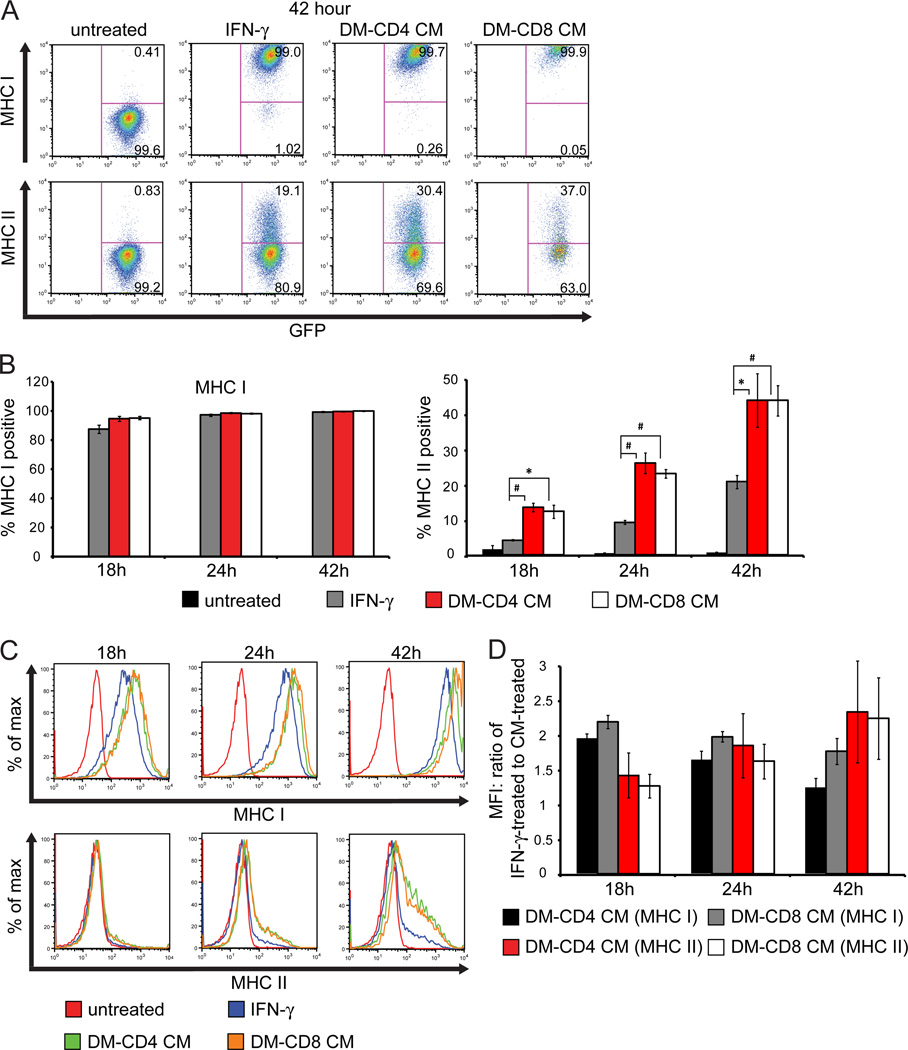
CD4+ and CD8+ T cells were isolated from the spleens of C57BL/6 mice at 8 days p.i. with JHMV. Enriched T cell subsets were co-cultured with antigen presenting cells (APCs) from uninfected C57BL/6 mice pulsed with viral peptides corresponding to the CD4 specific epitope within the membrane (M) glycoprotein spanning amino acid residues 133–147 (M133–147) or CD8 specific epitope within the spike (S) glycoprotein spanning amino acid residues 510–518 (S510–518) in order to stimulate virus-specific T cells. After 48 h, media was collected and cultured GFP-NPCs were treated for defined periods of time and expression of MHC class I and II measured by flow cytometry. Similar to IFN-γ treatment, CM from antigen sensitized CD4 and CD8 T cells induced MHC class I expression on cultured GFP-NPCs at 18, 24, and 42 h (Figures 2A and B). In addition, there was a significant increase in the frequency of MHC class II-positive NPCs following treatment with CM from viral peptide stimulated populations of CD4+ and CD8+ T cells relative to IFN-γ treatment (Figures 2A and B). While MHC class I expression was not appreciably increased on GFP-NPCs following incubation with CD4+ and CD8+ T cell conditioned media relative to IFN-γ, MFI analysis determined that the level of MHC class I expression per cell was increased approximately 2-fold compared to IFN-γ treatment, although this diminishes after prolonged exposure to IFN-γ (Figures 2C and D). Conversely, while MHC class II was expressed a greater frequency of GFP-NPCs following incubation with CD4+ and CD8+ T cell CM, the amount of MHC class II per cell was not appreciably increased. Although the MFI of MHC class II increased at the later time points relative to IFN-γ this was likely due to increased number of cells expressing MHC class II, as shown by the histogram in Figure 2C. These data demonstrate that antigen sensitized CD4+ and CD8+ T cells are capable of inducing MHC class I and MHC II expression on NPCs and the overall increased expression represents the presence of additional proinflammatory cytokines working synergistically with IFN-γ being secreted from virus-specific T cells.
Figure 2. NPCs express MHC class and II in response to treatment with conditioned media from antigen sensitized T cells.
Conditioned media (CM) was collected following exposure of T cells isolated from JHMV-immunized mice with defined CD4+ (M133-147) and CD8+ (S510-518) viral peptides for 48 h. Cultured GFP-NPCs were treated with either CD4 CM, CD8 CM, IFN-γ (100U/ml) or control media (untreated) for 18, 24, or 42 h and expression of MHC class I and II determined by flow cytometry. (A) Representative flow analysis at one time point (42 h) and (B) quantification of MHC I and II at 18, 24, and 42 h. Statistics are calculated for MHC expression on GFP-NPCs following treatment with CD4 or CD8 CM relative to treatment with IFN-γ; *p<0.05, #p<0.01. (C) Representative histogram of MHC class I and II expression at 18, 24, and 42 h. (D) The MFI for MHC I and II expression on GFP-NPCs following incubation with CD4 or CD8 CM was determined and divided by the MFI for MHC I and II following IFN-γ treatment to determine the fold increase in MFI.
Allogeneic NPCs are rejected following transplantation into JHMV-infected mice
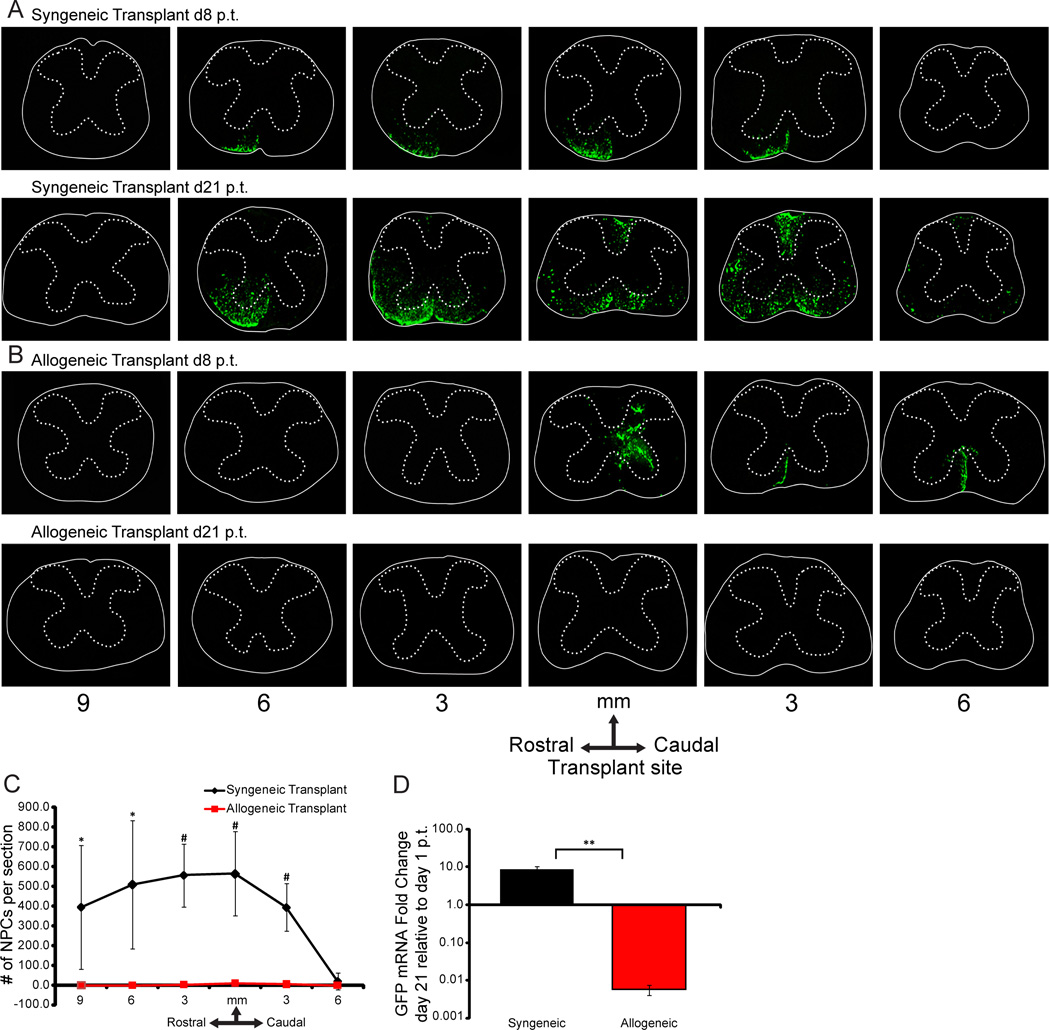
Infection of susceptible mice with JHMV induces an acute encephalomyelitis followed by an immune-mediated demyelinating disease that results in clinical and histological disease with similarities to the human demyelinating disease MS27,32–35. C57BL/6 (H-2b background) and Balb/c (H-2d background) mice were infected i.c. with JHMV and subsequently transplanted by intraspinal injection with GFP-NPCs (H-2b background) at day 14 p.i. which represents a time in which demyelination is established14,15,36,37. Recipient mice were not treated with any immunomodulatory drugs and animals were sacrificed at 8 days and 3 weeks p.t.. Survival of engrafted cells determined by visualizing expression of GFP from transplanted cells14 and PCR amplification of GFP mRNA transcripts. Syngeneic transplant of GFP-NPCs into infected C57BL/6 mice resulted in extensive migration along the spinal cord both rostral and caudal to implantation site and preferentially colonized within areas of white matter damage (Figure 3A). Conversely, allogeneic GFP-NPCs, transplanted into JHMV-infected Balb/c mice, were detected at day 8 p.t. but not at day 21 p.t. (Figure 3B). Quantification of transplanted cells, as determined by counting GFP-positive cells in defined areas rostral and caudal to the implantation site, revealed a significant difference in the number of GFP-NPCs present in the spinal cords of syngeneic transplanted mice compared to allogeneic transplanted mice at day 21 p.t. (Figure 3C). Further, determination of GFP expression by mRNA by quantitative PCR revealed a >180-fold decrease in signal intensity in allogeneic transplant at day 21 p.t. relative to day 1 p.t. as compared to syngeneic recipients in which there was an ~9-fold increase (p≤0.001) in GFP transcript levels at day 21 p.t. relative to day 1 p.t., consistent with earlier findings that engrafted cells proliferate (Figure 3D)14.
Figure 3. Transplanted allogeneic GFP-NPCs are rejected following intraspinal injection into JHMV-infected mice.
(A, B) Representative coronal spinal cord sections obtained at defined locations rostral and caudal to the transplantation site from JHMV-infected mice receiving either syngeneic (A) or allogeneic (B) NPCs. Experimental mice were sacrificed at either 8 or 21 days p.t. and migration/survival of transplanted cells evaluated by visualization of GFP-expression from transplanted cells. (C) At day 21 p.t. migration/survival of transplanted was cells enumerated. Dual-positive DAPI and GFP NPCs were counted in coronal sections 9mm rostral and 6mm caudal to transplant site at 3mm intervals from allogeneically transplanted (n=6) and syngeneically transplanted (n=7) mice. Arrow indicates transplantation site. Increased numbers (* p<0.05; # p<0.01) of GFP-NPCs were present within the spinal cords of syngeneic recipients compared to allogeneic. (D) GFP expression (determined by quantitative RT-PCR) is increased (** p<0.001) within the spinal cords of mice following syngeneic versus allogeneic transplant. The fold change of GFP expression was determined at d21 p.t. relative to d1 p.t. in syngeneically transplanted (day 1, n=3, d21: n=4) and allogeneically transplanted (day 1, n=3, d21: n=3) mice.
Elevated T cell inflammation into the spinal cords of allogeneic recipients
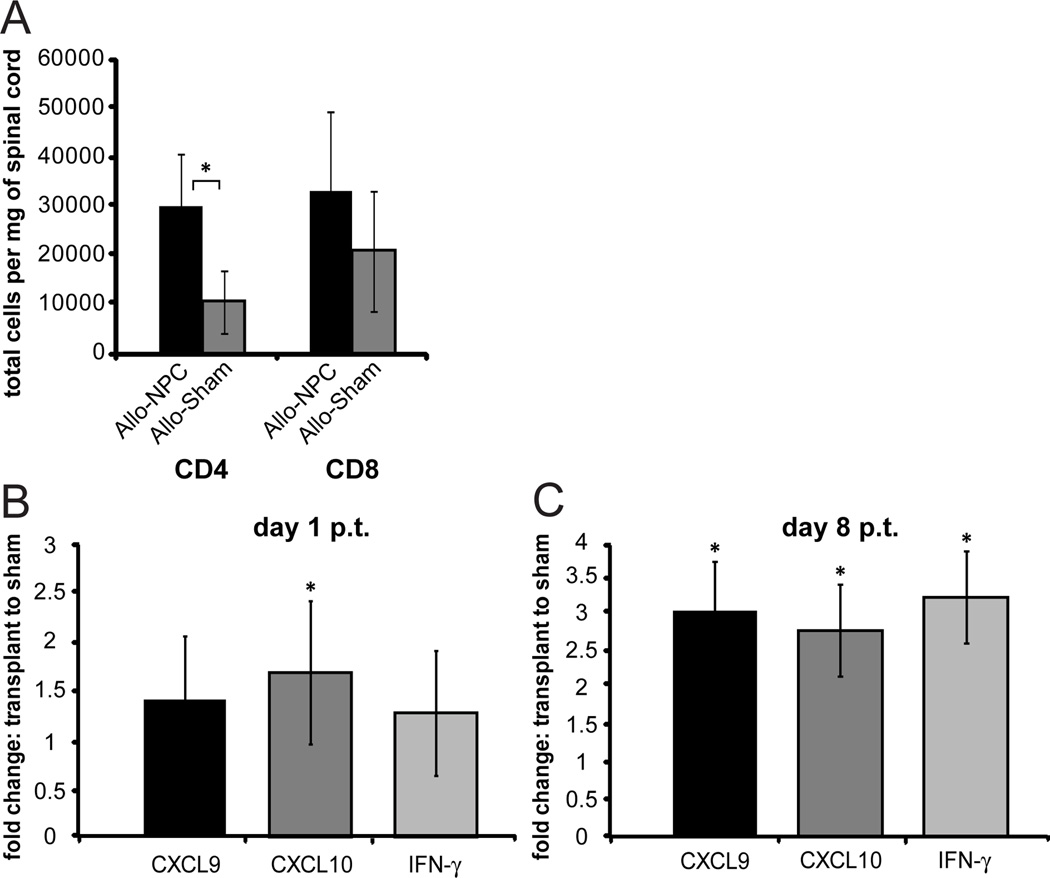
Spinal cords from mice receiving either syngeneic or allogeneic NPCs were isolated and lymphocyte infiltration was determined. By day 8 p.t., both CD4+ (p<0.05) and CD8+ T cell infiltration was elevated in comparison to non-transplanted JHMV-infected mice as determined by flow cytometric analysis (Figure 4A). There was not a significant increase of either CD4 or CD8 T cells following syngeneic transplant (data not shown). We next determined if transcripts associated with T cell infiltration were elevated in allogeneic transplanted mice compared to non-transplanted mice. At day 1 p.t. transcripts for the T cell chemoattractant CXCL10 (Figure 4B) and day 8 p.t. the chemokines CXCL9 and CXCL10 and the cytokine IFN-γ (Figure 4C) were significantly increased (p<0.05) in the allogeneically transplanted spinal cord relative to sham transplant. There was no significant difference in CXCL9, CXCL10, or IFN-γ following syngeneic transplant (data not shown).
Figure 4. Increased CD4+ T cells in spinal cord of allogeneically transplanted mice.
A) Flow cytometric analysis was performed on mononuclear cells isolated from spinal cord (9mm rostral and caudal to transplant site was used) of mice 8 days following allogeneic transplantation of NPCs or sham transplantation. Two spinal cords were pooled for each cell isolation. Cells were stained with PE-conjugated CD4 and PE-Cy7-conjugated CD8. The number of CD4+ and CD8+ T cells was determined in each NPC transplanted and sham transplanted group and normalized to mg of isolated spinal cord. B,C) CXCL9, CXCL10, and IFN-γ mRNA from spinal cords of mice allogeneically transplanted with GFP-NPC (n=9) or sham transplanted (n=7) at day 1 p.t. (B) and day 8 p.t. (C) was analyzed by qRT-PCR. Ct values were normalized to GAPDH and the ratio of each mRNA from allogeneic transplant to sham transplant at 8 p.t. was determined (ΔΔCt). ΔCt for allogeneic transplant compared to ΔCt for sham transplant was used for calculating statistical significance, *p<0.05. Standard error is presented as ΔΔCt.
T cells are sensitized to allogeneic NPCs
We next determined if T cells isolated from transplanted mice were sensitized to allogeneic NPCs by measuring T cell proliferation in response to NPC co-culture through us of an MLR assay. To ensure T cells proliferation was not caused by potential contamination of APCs during the isolation, T cells were enriched from the spleens of recipient mice and subsequently sorted by FACS to isolate specific CD4+ and CD8+ T cell populations (>99% purity, Figure 5A). Co-cultures of T cell subsets from mice at day 21 p.t. mice showed increased CD4+ T cell proliferation in response to exposure to either MHC-mismatched splenocytes as well as NPCs treated in medium alone or IFN-γ as compared to CD4+ T cells from naïve non-transplanted Balb/c mice (Figure 5B). Notably, IFN-γ treatment did not significantly alter T cell proliferative response. Although CD8+ T cells exhibited a similar response to MHC-mismatched splenocytes as CD4+ T cells, there was a comparatively muted response to NPCs (Figure 5B). Further, ELISPOT analysis revealed increased (p<0.05) numbers of IFN-γ-producing T cells following co-culture with either C57BL/6 splenocytes or NPCs used as stimulators compared to T cells obtained from naive non-transplanted mice (Figure 5C). We next tested whether there was an antigen-recall response to transplanted allogeneic NPCs in vivo by measuring a DTH response. Naive non-transplanted Balb/c mice or GFP-NPC-transplanted Balb/c mice received a footpad injection of GFP-NPCs at day 21 p.t. The majority of injected GFP-NPC transplanted mice (75%; 6 out of 8) exhibited increased footpad swelling relative to naive non-transplanted controls by 24 h post footpad injection, and this increased to ~90% of mice responding by 48 h following injection. At 24 h, the footpad swelling in mice that received intraspinal NPCs (0.42±0.12mm) was significantly (p<0.01) greater than swelling in naive non-transplanted control mice (0.18±0.10mm, Figure 5D). As controls, naïve Balb/c or C57BL/6 mice received a subcutaneous injection of GFP-NPCs and 8 days later challenged with GFP-NPCs and footpad swelling measured at days 1, 2, and 4 post-injection. Challenge of Balb/c mice injected with allogeneic GFP-NPCs resulted in a significant (p<0.05) increase in footpad swelling at days 2 and 4 post-challenge compared to syngeneic confirming that GFP-NPCs were antigenic within the context of allogeneic injection whereas syngeneic injection elicits no immune response (data not shown).
Figure 5. Allogeneic GFP-NPCs elicit T cell response.
(A) Representative flow cytometric analysis revealing >99% purity of FACS sorted CD4+ and CD8+ T cells from spleens of mice transplanted with allogeneic NPCs at day 21 p.t. (B) Purified T cells from (A) were co-cultured with GFP-NPCs and splenocytes treated with either medium or IFN-γ (100U/ml) and proliferation determined by EF670 dye expression. Statistical significance was determined by comparing the number of dividing T cells from Balb/c mice at day 21 p.t. with the number of dividing T cells from naive non-transplanted Balb/c mice (* p<0.05, # p<0.01). (C) IFN-γ production from T cells isolated from spleens of mice receiving either allogeneic NPCs or naïve mice was determined by ELISPOT following exposure to GFP-NPCs or C57BL/6 splenocytes. (D) Footpad swelling was determined at 24 and 48 h post GFP-NPC footpad injection (d21 p.t.) in naive Balb/c mice and Balb/c mice that received intraspinal transplant of GFP-NPCs.
T cell depletion increases survival of allogeneic NPCs
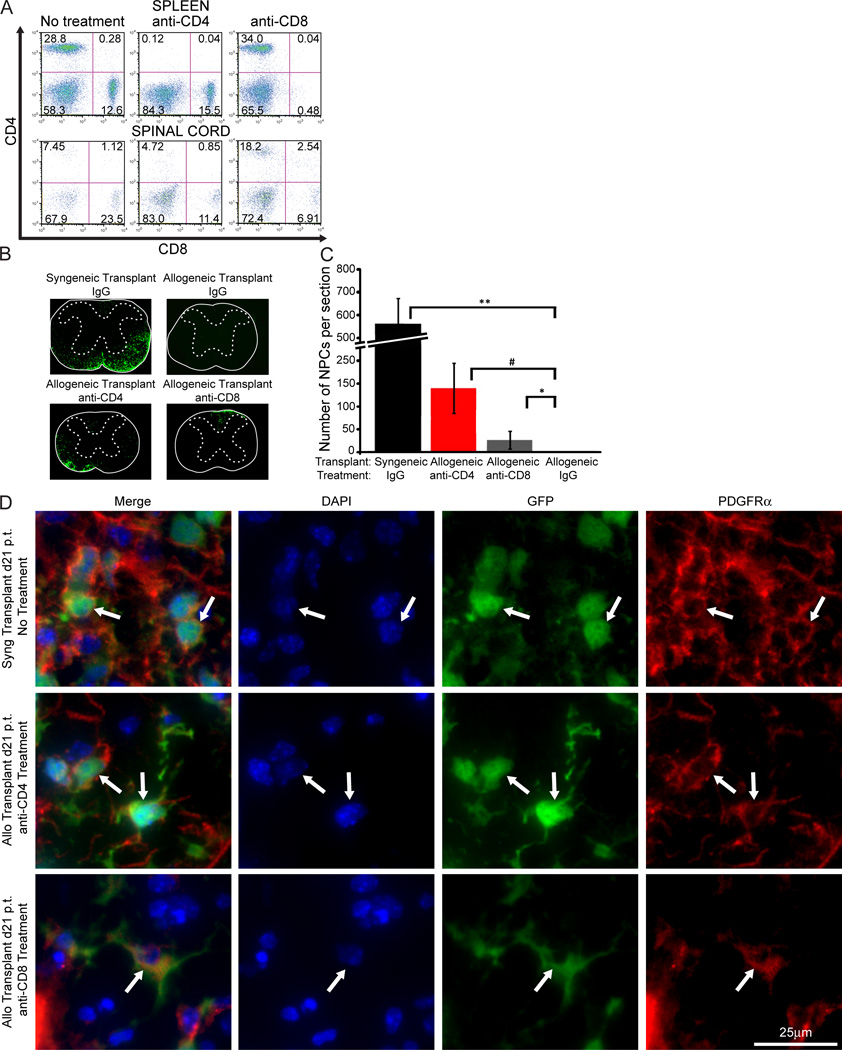
As an additional test to confirm the importance of T cells in contributing to rejection of MHC-mismatched NPCs, JHMV-infected Balb/c mice were transplanted with GFP-NPCs, treated with depleting monoclonal antibodies specific for either CD4+ or CD8+ T lymphocytes, and allogeneic cell survival determined at 3 weeks p.t. Treatment with antibodies specific for either T cell subset resulted in >98% depletion of both CD4+ and CD8+ T cells within the periphery (Figure 6A, top panel). However, within the spinal cord, antibody treatment only resulted in ~45% reduction in CD4+ and CD8+ T cell levels (Figure 6A, bottom panel). This most likely reflects limited penetration of monoclonal antibodies into the CNS and/or muted efficacy in depleting T cells within the CNS. Nonetheless, T cell depletion resulted in a significant increase in numbers of allogeneic NPCs when compared to allogeneic recipients treated with control antibody (Figure 6C). Depletion of CD4+ T cells had the greatest effect on allogeneic NPC survival when compared to animals treated with anti-CD8+ T cells (Figure 6C). In recipients of allogeneic NPCs treated with anti-CD4, surviving NPCs accumulated within white matter tracts in which demyelinating lesions are present whereas anti-CD8 treatment restricted migration of cells which retained primarily the dorsal funiculus (Figure 6B). These observations implicate T cells as important in contributing to rejection of allogeneic NPCs. Finally, surviving allogeneic GFP-NPCs from transplanted mice treated with either anti-CD4 or anti-CD8 were capable of differentiating into PDGFRα+ oligodendrocytes, similar GFP-NPCs in a syngeneic transplant without treatment. This demonstrates that the differentiation fate of allogeneically transplanted NPCs are not affected by the antibody treatment (Figure 6D).
Figure 6. Depleting T cells increases survival of allogeneic GFP-NPCs within the spinal cords of JHMV-infected mice.
JHMV infected mice transplanted with either syngeneic and allogeneic GFP-NPCs were treated with anti-CD4 (GK1.5, n=5), anti-CD8 (Ly2.2, n=3), or isogenic IgG control (n=4) at d -1, 1, 3, 5, 12, and 19 p.t. and mice sacrificed at d21 p.t. Presence of NPCs was determined by immunofluorescence imaging of GFP expression. (A) Representative flow analysis of the spleen (top panel) and spinal cord (bottom panel) reveals the presence of CD4+ and CD8+ T cells following anti-CD4 or anti-CD8 treatment. (B) Representative spinal cord sections from transplanted mice treated with either CD4 or CD8 depleting antibodies showing the presence of GFP-NPCs. (C) Quantification of GFP-NPCs in transplanted mice treated with T cell depleting antibodies and numbers of GFP-NPCs determined along the length of the spinal cord both rostral and caudal to implantation site. Depletion of either CD4+ or CD8+ T cell subsets increases survival (* p<0.05, # p<0.01, ** p<0.001) in mice transplanted with allogeneic NPCs compared to mice transplanted with syngeneic NPCs. (D) Representative immunofluorescence 40× images showing PDGFRα+ (red) GFP-NPCs (green), with DAPI stained nuclei (blue), at day 21 p.t. of syngeneically transplanted mice without antibody treatment or allogeneically transplanted mice with anti-CD4 or anti-CD8 treatment.
Discussion
The use of neural stem/progenitor cells for treatment of human neurologic diseases is recognized as a clinically viable approach for reducing disease severity and promoting recovery. Indeed, human neural stem cells (NSCs) are currently approved for use in clinical trials for improving function in neuronal ceroid lipofuscinosis, a fatal neurodegenerative disorder in children as well as Pelizaeus-Merzbacher Disease (PMD), a fatal myelination disorder (www.stemcellsinc.com). Additionally, use of NPCs has been shown to restore cognition following transplantation into the CNS of mice with neuropathological conditions similar to those observed in patients with Alzheimer’s Disease (AD)38 and ameliorates radiation-induced cognitive dysfunction in rats39,40. NPCs are attractive to use for treating a broad array of human neurologic disease conditions based on evidence that NPCs are capable of differentiating into distinct glial lineages and neurons as well as promoting neurogenesis15,41–43. Further, ongoing clinical trials and experimental models of neurologic disease indicate there is no evidence of tumor or non-neural tissue formation that further strengthens the relevance of using these cells for disease treatment44–46. Therefore, the potential for using NPCs for restoring functional and behavioral deficits arising from loss or damage of host CNS cells holds great promise and clinical interest. A potentially critical aspect in better understanding the biology of NPC transplantation relates to the antigenicity of these cells as well as their ability to modulate the host’s immune response. Highlighting the importance of this area of research are conflicting reports indicating that NPCs are immunologically inert and well-tolerated following transplantation into MHC-mismatched animals16 while other reports demonstrate that both innate and adaptive arms of the immune system are activated and participate in killing allogeneic NPCs20,21.
Our previous studies have convincingly demonstrated that intraspinal transplantation of syngeneic NPCs into mice persistently infected with JHMV results in migration from the site of transplant with selective colonization of areas of white matter damage, enhanced differentiation into oligodendroglia accompanied by axonal sparing and remyelination14,15,36. Based upon this body of work, it is clear that transplanted cells were critical in contributing to the improved histopathological outcome14,47. Therefore, understanding whether allogeneic NPCs survive following transplantation into JHMV-infected mice is important with regards to improved clinical and histologic outcome within the context of this model but also with regards to other models in which NPC transplantation has been shown to be beneficial. Our findings clearly demonstrate that MHC mistmatched NPCs are not immunopriviledged but rather antigenic following transplantation into JHMV-infected mice with established neuroinflammatory-mediated demyelination. Evidence is provided that supports an important role for T cells in contributing to rejection as i) NPCs evoke a DTH response following footpad injection into transplanted mice, ii) co-culture of NPCs with T cells isolated from transplanted mice results in T cell proliferation and IFN-γ secretion, and iii) depletion of T cell subsets increases survival of allogeneic NPCs.
Our data demonstrating that treatment of cultured NPCs with IFN-γ increases expression of both MHC class I and II is consistent with earlier studies indicating cultured NPCs are capable of expressing these molecules in response to IFN-γ treatment16,20,48. Similarly, we show that cultured NPCs do not constitutively express either MHC I and II but detectable levels are present only in response to treatment with IFN-γ. Further, exposure of cultured NPCs with CM obtained following stimulation of virus-specific T cells not only increased the overall frequency of both MHC class I and II on NPCs but also enhanced expression on a per cell basis. These findings reflect that NPCs are sensitive to inflammatory cytokines that subsequently increase surface expression of MHC antigens.. Lack of constitutive MHC expression on NPCs is consistent with other studies reporting that both embryonic stem cells (mouse and human) and induced pluripotent stem cells do not express MHC antigens49,50. Muted expression of MHC antigens on stem/progenitor cells has been suggested as one potential mechanism contributing to prolonged survival following transplantation into an MHC incompatible host as it would limit recognition by inflammatory T cells. We also report that co-stimulatory molecules CD80 and CD86 are constitutively expressed on NPCs and expression is not modulated following exposure to IFN-γ in-vitro. This is in contrast to previous reports that show that although mouse NPCs express CD80 and CD86, these co-stimulatory molecules are upregulated following IFN-γ treatment or under pathological conditions51,52. Collectively, these findings highlight that the environmental inflammatory cytokine milieu may be critical with regards to influencing expression of surface antigens on allogeneic NPCs that allow for detection by activated immune cells.
Our findings are in contrast with earlier studies16,21 indicating that NPCs possess inherent immune privilege and are capable of prolonged survival in an unsensitized host. Indeed, our findings are more consistent with Chen et al20 indicating that allogeneic NPCs exhibit limited survival in response to transplantation into the CNS although innate, rather than adaptive, immune responses were responsible for NPC killing in their model system. However, caution must exercised when considering these studies collectively. Both Hori et al16 and Chen et al20 used normal recipient mice in which no inflammatory disease conditions were present prior to transplantation.
The presence of inflammatory cytokines, such as IFN-γ, within diseased tissue may be a relevant factor in contributing to allogeneic NPC survival. Exposure of allogeneic NPCs to proinflammatory cytokines upon transplant could presumably increase MHC antigen expression leading to recognition by infiltrating T cells. Consistent with this possibility is our data demonstrating that allogeneic NPCs are quickly rejected following transplantation into spinal cords of JHMV-demyelinated spinal cord, an environment where activated T cells secreting IFN-γ are readily present. Moreover, we demonstrate that NPCs are sensitive to exposure to conditioned media obtained from JHMV-specific T cells stimulated with defined viral antigens and this increases expression of MHC class I and II antigens.
Based on our results, both CD4+ and CD8+ T cells contribute to rejection as depletion of either subset increased survival of engrafted cells. However, greater numbers of inflammatory CD4+ T cells were present within the spinal cords of allogeneic NPC recipients when compared to infiltrating CD8+ T cells. Further, treatment with anti-CD4+ T cell subset depleting antibodies resulted in enhanced survival of allogeneic NPCs when compared to animals treated with anti-CD8+ T cell subset antibodies. These data support a recent report demonstrating a more important role for CD4+ T cells in immune-mediated rejection of hESC xenografts50. Nevertheless, T cells are clearly sensitized to allogeneic NPCs following injection into MHC mismatched JHMV-infected mice as demonstrated by increased proliferation, detectable DTH responses, and IFN-γ secretion by T cells. It is important to emphasize that administration of T cell specific depleting antibodies did not completely eliminate T cells within the CNS although these cells were efficiently depleted from the periphery.
Mechanisms by which T cells participate in killing NPCs include direct recognition of surface-bound antigen(s) that are either constitutively expressed or induced upon transplantation. Therefore, increased expression of MHC antigens on transplanted allogeneic NPCs would represent a potential target for activated T cells present within the spinal cords of persistently-infected mice. In addition, indirect recognition (e.g. phagocytic engulfment of dead/dying NPCs) and subsequent presentation of antigens represents an additional scenario by which T cells become sensitized to alloantigens. This is not an unreasonable scenario as many NPCs will die during injection into the spinal cord and these cells are presumably removed by activated inflammatory macrophages that could then migrate to secondary lymphatic tissue and present novel antigens to naïve lymphocytes. Alternatively, alloantigens could be shed following transplantation and presented by local APCs following phagocytic uptake. At this time, we have not distinguished whether direct and/or indirect recognition of NPC antigens is occurring in response to NPC transplantation into JHMV-infected mice. However, indirect recognition may favor a more important role for CD4+ T cells in contributing to rejection and this is consistent with our findings. Interestingly, we also demonstrate NPCs that survive following depletion of T cell subsets exhibit normal differentiation into oligodendrocyte progenitor cells (OPCs) similar to syngeneic NPC recipients. These findings argue that the local microenvironmental niche remains capable of providing necessary growth factors required for fate commitment to the oligodendroglia lineage.
Although T cells are clearly important in killing allogeneic NPC transplants, it does not rule out the possibility that cellular components of the innate immune response are also contributing to rejection. For example, NK cells have been shown to be involved in numerous models of allograft rejection including bone marrow, skin, and cornea allografts53–55. The observed result that not every allogeneic NPC transplanted mouse yielded DTH swelling or IFN-γ production in response to allogeneic NPC or splenocyte stimulators could be due to NK cell-mediated rejection, thus limiting memory T cell formation. As recently illustrated by Palmer and colleagues20, transplantation of allogeneic NPCs into the hippocampus of normal mice resulted in a dramatic reduction in survival and this was the result of innate immune responses directed to transplanted cells. Induction of immune suppression through treatment with cyclosporine A did not increase allogeneic NPC survival yet treatment with nonsteroidal anti-inflammatory drugs protected NPCs from destruction20. These findings clearly illustrate the importance of innate immune responses in participating in allograft destruction and further highlight the importance of the local microenvironment in allogeneic NPC transplantation as the lingering effects of inflammation at the transplant site are likely to result in killing of allografts through both innate and adaptive immune responses.
In conclusion, our findings provide evidence that allogeneic NPCs are not immune privileged upon transplantation into an established inflammatory environment. Rejection is rapid and involves T cell responses directed against NPC alloantigens, although CD4+ T cells are a more important mediator of immune-mediated rejection. In consideration of using NPCs for treating chronic neuroinflammatory diseases such as MS, the use of immunomodulatory drugs should be considered in order to increase survival of allografts in order to improve clinical outcome.
Acknowledgements
This work was funded by National Institutes of Health (NIH) Grant R01 NS074987 and a National Multiple Sclerosis Society (NMSS) Collaborative Center Research Award (CA1058-A-8) to T.E.L. C.M.W. is supported by NIH R01AI63419, NMSS RG4288, and JDRF 5-2010-306. J.G.W. is supported by a NMSS postdoctoral fellowship (FG 1960-A-1) and S.M.K. is supported by training grant number is T32 GM08620.
Footnotes
Author Contributions: J.G.W. designed experiments, collected data for analysis and interpretation, and wrote the manuscript; B.M.W., W.C.P. and S.M.K. collected data for analysis and interpretation; C.M.W. provided financial support and reagents; T.E.L. designed experiments, analyzed and interpreted data, provided financial support, and wrote the manuscript.
References
- 1.Weinshenker BG. The natural history of multiple sclerosis: update 1998. Semin Neurol. 1998;18:301–307. doi: 10.1055/s-2008-1040881. [DOI] [PubMed] [Google Scholar]
- 2.Rothman KJ. Epidemilogy, an introduction. New York: Oxford Univeristy Press; 2002. What is causation. [Google Scholar]
- 3.Ebers GC, Sadovnick AD, Risch NJ. A genetic basis for familial aggregation in multiple sclerosis. Canadian Collaborative Study Group. Nature. 1995;377:150–151. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 4.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 7.Goldschmidt T, Antel J, Konig FB, et al. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 8.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 9.Hagemeier K, Bruck W, Kuhlmann T. Multiple sclerosis - remyelination failure as a cause of disease progression. Histol Histopathol. 2012;27:277–287. doi: 10.14670/HH-27.277. [DOI] [PubMed] [Google Scholar]
- 10.Pluchino S, Zanotti L, Brini E, et al. Regeneration and repair in multiple sclerosis: the role of cell transplantation. Neurosci Lett. 2009;456:101–106. doi: 10.1016/j.neulet.2008.03.097. [DOI] [PubMed] [Google Scholar]
- 11.Sher F, Balasubramaniyan V, Boddeke E, et al. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Curr Opin Neurol. 2008;21:607–614. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- 12.Totoiu MO, Nistor GI, Lane TE, et al. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardison JL, Nistor G, Gonzalez R, et al. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp Neurol. 2006;197:420–429. doi: 10.1016/j.expneurol.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbajal KS, Schaumburg C, Strieter R, et al. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Totoiu MO, Nistor GI, Lane TE, et al. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori J, Ng TF, Shatos M, et al. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Baroja ML, Majumdar A, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 18.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 19.Anderson AJ, Haus DL, Hooshmand MJ, et al. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient. Regen Med. 2011;6:367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Phillips LK, Gould E, et al. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One. 2011;6:e14787. doi: 10.1371/journal.pone.0014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Xu CJ, Lu HZ, et al. Long-term fate of allogeneic neural stem cells following transplantation into injured spinal cord. Stem Cell Rev. 2010;6:121–136. doi: 10.1007/s12015-009-9104-y. [DOI] [PubMed] [Google Scholar]
- 22.Walsh KB, Lanier LL, Lane TE. NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis. J Virol. 2008;82:3031–3044. doi: 10.1128/JVI.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogasawara K, Hamerman JA, Hsin H, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 24.Klein D, Bugl B, Gunzburg WH, et al. Accurate estimation of transduction efficiency necessitates a multiplex real-time PCR. Gene Ther. 2000;7:458–463. doi: 10.1038/sj.gt.3301112. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y, Koido S, Xia J, et al. Development of antigen-specific CD8+ CTL in MHC class I-deficient mice through CD4 to CD8 conversion. J Immunol. 2004;172:7848–7858. doi: 10.4049/jimmunol.172.12.7848. [DOI] [PubMed] [Google Scholar]
- 27.Castro RF, Evans GD, Jaszewski A, et al. Coronavirus-induced demyelination occurs in the presence of virus-specific cytotoxic T cells. Virology. 1994;200:733–743. doi: 10.1006/viro.1994.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane TE, Liu MT, Chen BP, et al. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiles LN, Hosking MP, Edwards RA, et al. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- 30.Trifilo MJ, Lane TE. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8alpha- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pohlers D, Nissler K, Frey O, et al. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen-induced arthritis: influence on T helper cell activation. Clin Exp Immunol. 2004;135:409–415. doi: 10.1111/j.1365-2249.2003.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 33.Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramakrishna C, Stohlman SA, Atkinson RA, et al. Differential regulation of primary and secondary CD8+ T cells in the central nervous system. J Immunol. 2004;173:6265–6273. doi: 10.4049/jimmunol.173.10.6265. [DOI] [PubMed] [Google Scholar]
- 35.Marten NW, Stohlman SA, Bergmann CC. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J Virol. 2000;74:7903–7910. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardison JL, Nistor G, Gonzalez R, et al. Transplantation of glial-committed progenitor cells into a viral model of multiple sclerosis induces remyelination in the absence of an attenuated inflammatory response. Exp Neurol. 2006;197:420–429. doi: 10.1016/j.expneurol.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbajal KS, Miranda JL, Tsukamoto MR, et al. CXCR4 signaling regulates remyelination by endogenous oligodendrocyte progenitor cells in a viral model of demyelination. Glia. 2011;59:1813–1821. doi: 10.1002/glia.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya MM, Christie LA, Lan ML, et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71:4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acharya MM, Roa DE, Bosch O, et al. Stem cell transplantation strategies for the restoration of cognitive dysfunction caused by cranial radiotherapy. J Vis Exp. 2011 doi: 10.3791/3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gage FH, Coates PW, Palmer TD, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fricker RA, Carpenter MK, Winkler C, et al. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sher F, Balasubramaniyan V, Boddeke E, et al. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Curr Opin Neurol. 2008;21:607–614. doi: 10.1097/WCO.0b013e32830f1e50. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz SC, Wittlinger J, Schober R, et al. Transplantation of human neural precursor cells in the 6-OHDA lesioned rats: effect of immunosuppression with cyclosporine A. Parkinsonism Relat Disord. 2006;12:302–308. doi: 10.1016/j.parkreldis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cloutier F, Siegenthaler MM, Nistor G, et al. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med. 2006;1:469–479. doi: 10.2217/17460751.1.4.469. [DOI] [PubMed] [Google Scholar]
- 47.Whitman LM, Blanc CA, Schaumburg CS, et al. Olig1 function is required for remyelination potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mammolenti M, Gajavelli S, Tsoulfas P, et al. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. [DOI] [PubMed] [Google Scholar]
- 49.Pearl JI, Lee AS, Leveson-Gower DB, et al. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swijnenburg RJ, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergent-Tanguy S, Veziers J, Bonnamain V, et al. Cell surface antigens on rat neural progenitors and characterization of the CD3 (+)/CD3 (−) cell populations. Differentiation. 2006;74:530–541. doi: 10.1111/j.1432-0436.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 52.Laguna Goya R, Busch R, Mathur R, et al. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol Dis. 2011;41:407–414. doi: 10.1016/j.nbd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Ito A, Shimura H, Nitahara A, et al. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int Immunol. 2008;20:1343–1349. doi: 10.1093/intimm/dxn092. [DOI] [PubMed] [Google Scholar]
- 54.Ogasawara K, Benjamin J, Takaki R, et al. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartzkopff J, Schlereth SL, Berger M, et al. NK cell depletion delays corneal allograft rejection in baby rats. Mol Vis. 2010;16:1928–1935. [PMC free article] [PubMed] [Google Scholar]