Abstract
The family C G protein-coupled receptor (GPCR) T1R2 and T1R3 heterodimer functions as a broadly acting sweet taste receptor. Perception of sweet taste is a species-dependent physiological process. It has been widely reported that New World monkeys and rodents can not perceive some of the artificial sweeteners and sweet-tasting proteins that can be perceived by humans, apes, and Old World monkeys. Until now, only the sweet receptors of humans, mice and rats have been functionally characterized. Here we report characterization of the sweet taste receptor (T1R2/T1R3) from a species of New World squirrel monkey. Our results show that the heterodimeric receptor of squirrel monkey does not respond to artificial sweeteners aspartame, neotame, cyclamate, saccharin and sweet-tasting protein monellin, but surprisingly, it does respond to thaumatin at high concentrations (>18 μM). This is the first report that New World monkey species can perceive some specific sweet-tasting proteins. Furthermore, the receptor responses to the sweeteners cannot be inhibited by the sweet inhibitor lactisole. We compared the response differences of the squirrel monkey and human receptors and found that the residues in T1R2 determine species-dependent sweet taste toward saccharin, while the residues in either T1R2 or T1R3 are responsible for the sweet taste difference between humans and squirrel monkeys toward monellin. Molecular models indicated that electrostatic properties of the receptors probably mediate the species-dependent response to sweet-tasting proteins.
Keywords: New World monkeys, squirrel monkey, sweet taste receptors, sweet-tasting proteins, G protein-coupled receptor, molecular modeling
1. Introduction
The taste qualities for humans and other mammals can be categorized as sweet, bitter, sour, salty and umami [1]. The heterodimer of T1R2 and T1R3 was identified as a broadly acting sweet taste receptor. In addition to sucrose, the human T1R2/T1R3 receptor responds to all other sweet-taste stimuli tested: natural sugars, sweet amino acids, sweet-tasting proteins, and artificial sweeteners [2-5]. Previous molecular biology experiments using sweet taste receptor chimeras and mutants and molecular modeling studies showed that there are at least five potential binding sites of the sweeteners in the heterodimeric receptor [6-16]. Receptor activity induced by the artificial sweeteners aspartame and neotame implicate residues in the Venus Flytrap Module (VFTM) of human T1R2 [6,10,15,16], while natural sugars bind to the VFTMs of both T1R2 and T1R3 [12]. In contrast, the sweeteners cyclamate and neohesperidine dihydrochalcone (NHDC) [8,13], and the sweet-taste inhibitor lactisole acts on the Transmembrane Domain (TMD) of human T1R3 [9,14], and the sweetener SWT819 acts on the TMD of human T1R2 [17]. Furthermore, receptor activity toward the sweet protein brazzein depends on the cysteine rich domain (CRD) of human T1R3 [7].
Many physiological and molecular biological studies have shown that some sweeteners, such as the small molecule sweeteners aspartame, neotame and cyclamate, and sweet-tasting proteins can be perceived by humans, apes and Old World monkeys, but not by New World monkeys and rodents [18-21]. Thus, an intriguing question is what the molecular basis of species-dependent sweet taste toward these sweeteners as well as the activation mechanism is. In this regard, it is necessary to investigate the properties of sweet taste receptors from different species. Previously, only the sweet receptors of humans, mice and rats had been well studied [6-9,11]. However, the structure and function of sweet taste receptors from New World monkeys, which share nearly 90% sequence identity with the receptors of humans, have not been well studied. Recently, we demonstrated the molecular basis of species-dependence of sweet taste receptor toward artificial sweeteners aspartame and neotame by using human/squirrel monkey chimera receptors, mutagenesis and molecular modeling [15]. In this study, we further characterized the newly cloned sweet taste receptors from squirrel monkeys (Saimiri sciureus), which belong to the genus Samiri of New World monkeys. We used heterologous expression and calcium mobilization assay to assess the function of the heteromeric receptors (T1R2 and T1R3) from squirrel monkeys (named smT1R2 and smT1R3, respectively). By comparing the functional properties of the sweet taste receptors with those of humans and mice and by using human/squirrel monkey chimeric T1R2/ T1R3, we demonstrated that the residues in T1R2 determine species-dependent sweet taste toward saccharin, while the residues in either T1R2 or T1R3 mediate the sweet taste difference between humans and squirrel monkeys toward monellin. Molecular models indicated that electrostatic properties of the receptors probably mediate the species-dependent response to sweet-tasting proteins.
2. Materials and methods
2.1. Materials
Aspartame, saccharin, cyclamate, sucrose, D-tryptophan, NHDC, lactisole, monellin, and thaumatin were obtained from Sigma-Aldrich. Sucralose was obtained from Splendex. Neotame was obtained from American Health Foods & Ingredients. Stevioside was obtained from Nusci Institute & Corp. Unless noted, the concentration of the compounds used were: aspartame (2.5 mM), neotame (0.25 mM), saccharin (1 mM), cyclamate (10 mM), sucrose (150 mM), sucralose (1 mM), D-tryptophan (5 mM), stevioside (1 mM), neohesperidin dihydrochalcone (0.25 mM), monellin (37 μM), thaumatin (18 μM), and lactisole (1.25mM) based on the previous studies [7,22].
2.2. Constructs and calcium mobilization functional assay
The coding nucleotide acid sequence and deduced amino acid sequence of squirrel monkey T1R2 and T1R3 were as described previously [23,24]. To generate the smT1R2 expression construct, BamHI and NotI sites were introduced at the 5’ and 3’ ends of the full-length coding sequence, respectively, double digested, and then ligated into the expression vector pcDNA3.1. To obtain the smT1R3 construct, the full protein-coding region of smT1R3 was synthesized from the GenScript Biology CRO. An EcoRI site was then introduced at the 5’ end before the start codon, and a NotI site was introduced at the 3’ end after the stop codon. The smT1R3 gene was double restriction digested with EcoRI and NotI and then ligated into the expression vector pcDNA3.1. Human T1R2 (hT1R2) and T1R3 (hT1R3) expression constructs were generated in pcDNA3.1 vectors. The Gα16-gust44 clone was as described previously [25]. All the constructs were confirmed by DNA sequencing. Calcium mobilization functional assay was as described previously [15].
2.3. Molecular modeling
We constructed homology models of the VFTM-CRD of T1Rs from the human and squirrel monkey based on metabotropic glutamate receptor (mGluR) crystal structure templates using the MODELLER program [26]. The mGluR is the only family C GPCR with crystal structures of the VFTM-CRD available, which shares ~26% sequence identity with the VFTM-CRD of human T1Rs [27]. The sequence alignment between the template and the models was generated by the ClustalW program. We generated 40 homology models for each species based on the glutamate/gadolinium bound form (closed-closed/A, PDB: 1ISR) of the mGluRI-VFTM [28], and glutamate bound form (closed-closed/R, PDB: 2E4U) of the mGluRII-VFTM-CRD crystal structures [29]. The models were evaluated by the Verify 3D server to identify regions of improper folding. After further manual modifications (merging of fragments with best scoring models and minimizing energy by using SYBYL graphic software package (Tripos Inc.)), the models with the best Verify 3D scores were selected. The stereochemical quality of the models was evaluated by PROCHECK program [30], which showed reasonable ϕ and ψ distributions in the Ramachandran plot. All these assessments indicated that the model structures are reasonable.
3. Results
3.1 Sequence analysis of the squirrel monkey sweet taste receptors
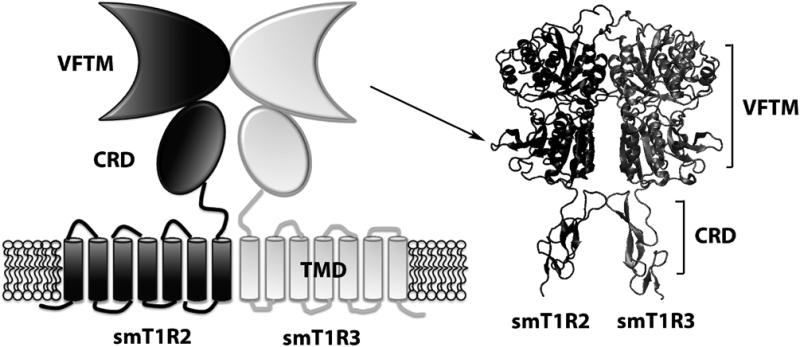
It is well known that the sweet taste receptor is a heterodimer of T1R2 and T1R3 (Fig. 1) [2]. The squirrel monkey (Saimiri sciureus) T1R2 gene consists of a 2520bp coding sequence and encodes an 839 amino acid protein (Genbank accession no: A3QP08), while the T1R3 gene consists of 2559bp and encodes an 852 amino acid protein (ABD14701). Although sequence identity between these two monomers is low (30%), smT1R2 has high overall identity (blastp, NCBI) with the sweet taste receptors from primates Callithrix pygmaea (A3QP09, 93%), Papio hamadryas (A3QP07, 89%), Macaca mulatta (A3QP01, 89%) and Pongo pygmaeus (A3QP01, 89%). Notably, it shows 88% and 69% sequence identity to T1R2s of humans (Q8TE23) and mice (Q925I4), respectively. For the squirrel monkey (Saimiri sciureus) T1R3, the high sequence identity T1R3 genes are from Pan troglodytes (Q717C2, 85%), Pongo pygmaeus (ABD14700, 84%) and Gorilla gorilla (AAQ11897, 84%), with 84% and 72% identity to humans (Q7RTX0) and mice (Q925D8), respectively. The sequence alignments of squirrel monkey T1R2 and T1R3 with human and mouse receptors were provided in the supplementary data (Fig. S1). Conserved domains prediction (NCBI, http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) showed that squirrel monkey T1R2 and T1R3 receptors appear to have a large ligand binding domain (VFTM) followed by a CRD and a TMD and an intracellular region, suggesting that the squirrel monkey sweet taste receptor shares a similar tertiary structural arrangement and ligand-receptor interaction mechanism with the receptors from other mammalian species, such as, humans and rodents.
Fig. 1.
Schematic representation of the heterodimer of smT1R2 and smT1R3 receptor. The VFTM-CRD of smT1R2/smT1R3 homology model is represented as newcartoon.
3.2. Functional properties of the squirrel monkey sweet taste receptors
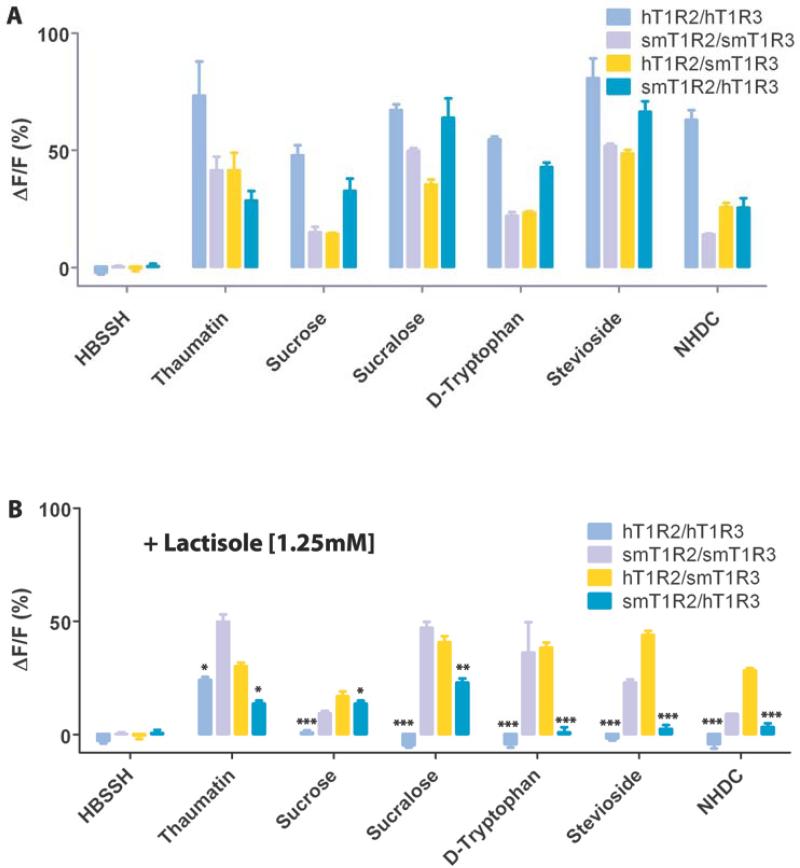
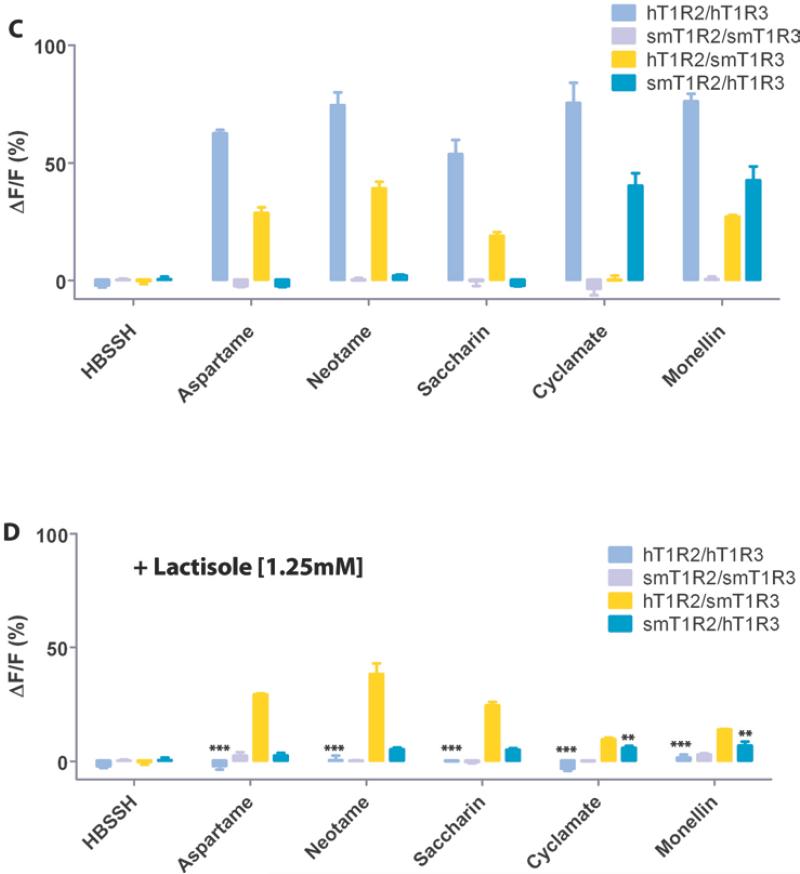
To characterize the newly cloned T1R2/T1R3 of the squirrel monkey, we examined the responses of HEK293E cells heterologously expressing smT1R2 and smT1R3 receptors, and Gα16-gust44 to a group of structurally diverse sweeteners: aspartame, neotame, saccharin, cyclamate, sucrose, sucralose, D-tryptophan, stevioside, NHDC, and sweet-tasting proteins monellin and thaumatin. Compared with the hT1R2/hT1R3 receptor, which showed strong responses to all of the sweeteners and sweet-tasting proteins tested, the smT1R2/smT1R3 receptor responded to most of these sweeteners except aspartame, neotame, cyclamate, saccharin, and monellin (Fig. 2A and 2C). These results are consistent with reported behavior test responses, which indicate that squirrel monkey cannot perceive aspartame, neotame, and sweet-tasting proteins as sweeteners [20,24,31]. In contrast to human and mouse sweet taste receptors, squirrel monkey receptor does not respond to saccharin. Interestingly, the sweet receptor of squirrel monkey is able to respond to the sweet-tasting protein thaumatin but not to monellin.
Fig. 2.
Human and squirrel monkey T1R2/T1R3 selectively respond to sweet taste stimuli with/without lactisole present. (A) The responses of the cells to HBSSH (buffer solution), thaumatin, sucrose, sucralose, D-tryptophan, stevioside, and NHDC were assayed by calcium mobilization. (B) Same as (A) with lactisole (1.25mM) present. (C) The responses of the cells to HBSSH, aspartame, neotame, saccharin, cyclamate and monellin. (D) Same as (C) with lactisole (1.25mM) present. F is the base-line level of fluorescence, and ΔF is the change in fluorescence from the base-line level (peak-base line). Data are expressed as the mean ± S.E. of the ΔF/F values from three independent experiments. Lactisole reduced the overall responses of the receptors. For comparison, the signals in panels (B) and (D) were amplified by a factor of 1.31 based on the smT1R2/smT1R3 response to sucralose with (39.6%) and without (52%) lactisole present. The asterisks indicate significant differences tested by unpaired Student's test (*: p<0.05; **: p<0.01; ***: p<0.001) compared with the receptor responses without lactisole.
3.3. Either T1R2 or T1R3 of squirrel monkey determines species-dependent sweet taste
To determine which T1R component is responsible for the species-dependent sweet taste in the squirrel monkey, we also examined the responses of human (hT1R2/hT1R3), and human + squirrel monkey chimeric receptors to the same group of sweeteners and sweet-tasting proteins. The hT1R2/smT1R3 chimeric receptor showed responses to most of the sweeteners except cyclamate. In contrast, the smT1R2/hT1R3 chimeric receptor showed no responses to aspartame, neotame and saccharin (Fig. 2A and 2C).
These results indicate that some residues in the hT1R2, which are not present in the smT1R2, are required for the receptor's responses to aspartame, neotame and saccharin. On the other hand, human residues in the hT1R3 are required for the receptor's response to cyclamate. The results are consistent with the previous report that the potential binding site of the sweet receptor for aspartame is located in the extracellular VFTM of hT1R2 [10,11,15] and for cyclamate is located in the TMD of hT1R3 [8]. Electrophysiological and behavioral experiments on the perception of sweet taste showed that common marmosets and New World squirrel monkeys do not respond to saccharin [20,32]. Our results show that the smT1R2/smT1R3 receptor does not respond to saccharin, whereas the hT1R2/smT1R3 receptor does, which indicate the potential binding site of the receptor for saccharin is located in the hT1R2 subunit.
Previous studies showed that the extracellular domain of hT1R2 is required for the responses to monellin. However, the role of hT1R3 could not be evaluated based on the human and mouse T1R2/T1R3 chimeric experiments since the mT1R2 (mouse T1R2) /hT1R3 chimeric receptors were not functional [7]. Our present study shows that both hT1R2/smT1R3 and smT1R2/hT1R3 chimeric receptors respond to several sweeteners: sucrose, sucralose, D-tryptophan, stevioside and NHDC (Fig. 2A). Interestingly, both hT1R2/smT1R3 and smT1R2/hT1R3 chimeric receptors are able to respond to monellin, while the smT1R2/smT1R3 cannot, indicate that specific residues in either the hT1R2 or the hT1R3, which are not in the smT1R2/T1R3, are required for the response to monellin.
3.4. Sweet responses of smT1R2/T1R3 cannot be inhibited by lactisole
Lactisole is a human specific sweet taste inhibitor, which inhibits the sweet taste of sugars, artificial sweeteners, and sweet-tasting proteins in humans and Old World monkeys but not in rodents [9,14]. We tested lactisole's inhibitory effect on the sweet taste receptors of squirrel monkeys. The results show that lactisole is not able to inhibit the responses of squirrel monkeys T1R2/T1R3 to the sweeteners and sweet-tasting proteins tested (Fig. 2B and 2D), which is similar to the previous findings for the T1R2/T1R3 of mice [9]. We also tested the lactisole inhibitory effect on the human and squirrel monkey T1R2/T1R3 chimeric receptors, smT1R2/hT1R3 and hT1R2/smT1R3. Lactisole inhibits sweet responses of smT1R2/hT1R3 but not of hT1R2/smT1R3 to the sweeteners and sweet-tasting proteins that we tested. These results are consistent with the previous findings, which showed that human specific residue A733 (Fig. S1) in the TMD of T1R3 is responsible for lactisole inhibitory sensitivity [9,14].
3.5. SmT1R2/T1R3 responds to sweet-tasting protein thaumatin but not to monellin
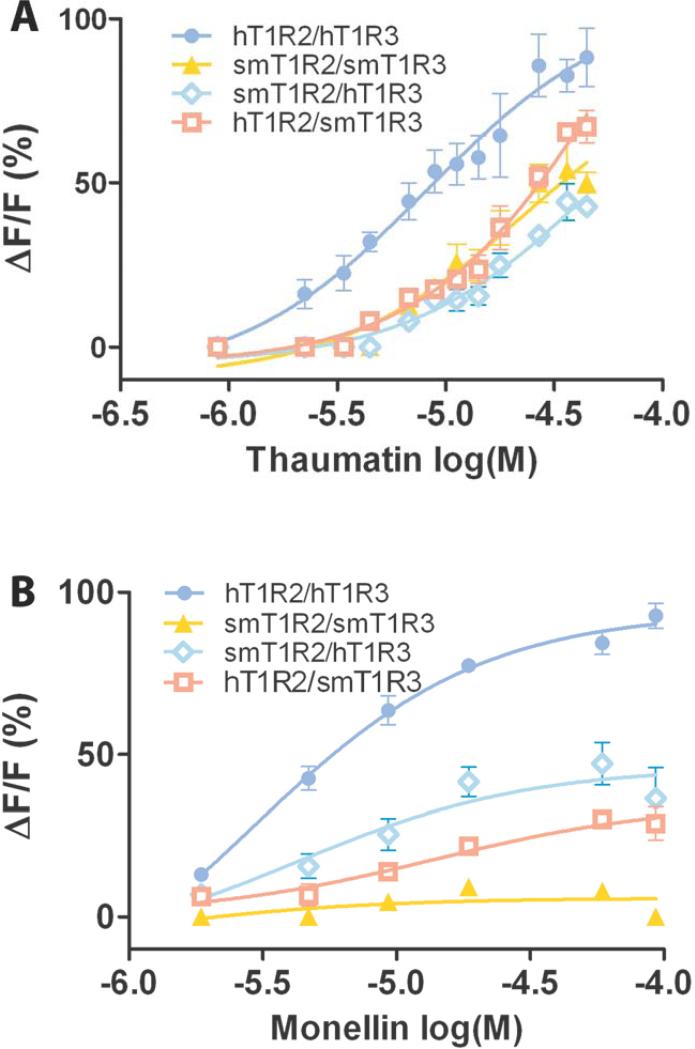
Fig. 2 (A and C) shows that the sweet taste receptor of the squirrel monkey responds to thaumatin but not to monellin. To further investigate the activity of sweet taste receptors from different species, we examined dose-dependent responses of the receptors to sweet-tasting proteins monellin and thaumatin. For comparison, we also included wild type receptors hT1R2/hT1R3 and smT1R2/smT1R3, as well as chimeric receptors hT1R2/smT1R3 and smT1R2/hT1R3. The concentrations of sweet-tasting proteins: monellin ranged from 1.85 μM to 92.5 μM; thaumatin ranged from 0.9 μM to 45 μM. We can see that at lower concentrations of thaumatin (<0.9 μM) all the sweet receptors do not respond to the protein (Fig. 3A). However, as the concentration of thaumatin increases, hT1R2/hT1R3 is the first one to respond to this protein (2.25 μM). As the concentration of thaumatin increases beyond 18 μM, smT1R2/smT1R3 also starts to respond to thaumatin. The order of the efficacy of these receptors’ responses toward thaumatin is hT1R2/hT1R3 > hT1R2/smT1R3 > smT1R2/smT1R3 > smT1R2/hT1R3. However, smT1R2/smT1R3 receptor does not respond to monellin even at high concentrations. Interestingly, both hT1R2/smT1R3 and smT1R2/hT1R3 chimeric receptors do respond to monellin. The dose dependent curve of the responses clearly showed that hT1R2/hT1R3 exhibited the highest response, while smT1R2/hT1R3 showed a stronger response than hT1R2/smT1R3 toward monellin (Fig. 3B). These results indicate that the residues of T1R2/T1R3, which are required to respond to thaumatin, are probably conserved among different species, such as humans and squirrel monkeys, while the differences among T1R2/T1R3 across these species mediate the sensitivity of responses to thaumatin. Our results also indicate that some specific residues on either hT1R2 or hT1R3, which are not on smT1Rs, are required for the sweet taste receptor to respond to monellin.
Fig. 3.
Dose-response of human, squirrel monkey, and chimeric sweet taste receptors toward sweet proteins thaumatin (A) and monellin (B). The calcium response signals were normalized to the maximum response of hT1R2/hT1R3 receptor to thaumatin (45 μM) and monellin (92.5 μM) respectively. Data were fitted with sigmoid dose-response curves using Graphpad Prism software. Values represent the mean ± S.E. of three independent experiments.
3.6. Electrostatic potentials probably mediate the species dependent responses to sweet-tasting proteins
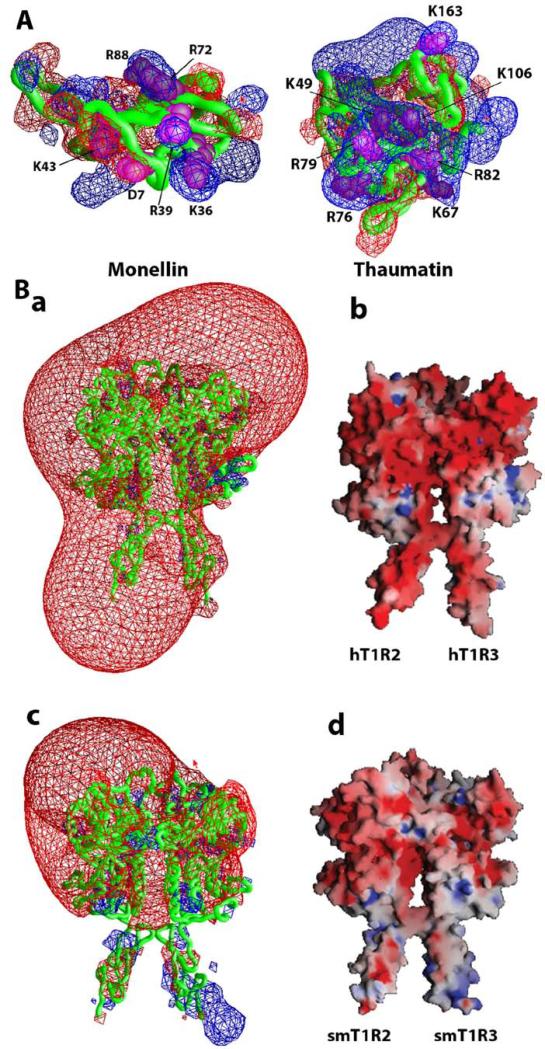
The critical residues for sweetness of sweet-tasting proteins, such as brazzein, monellin, thaumatin and lysozyme, are usually positively charged. For example, site-directed mutagenesis on monellin suggested that the charged residues D7, K36, R39, K43, R72 and R88 are critical for tasting the sweetness of monellin [33]. Similarly, positively charged residues K49, K67, K106, K163, R76, R79 and R82 are critical for tasting the sweetness of thaumatin [34]. Fig. 4A shows the electrostatic properties of sweet-tasting monellin and thaumatin. Interestingly, these critical residues are placed on one side of the proteins, which could form interactive binding surfaces with sweet receptors.
Fig. 4.
Electrostatic properties of sweet-tasting proteins and human/squirrel monkey T1R2 and T1R3. (A) Electrostatic potentials contour maps represent isopotential surfaces of sweet-tasting proteins with +/- 2kT/e for positive (blue) and negative (red) potentials, respectively. Critical residues of the proteins for their sweetness are labeled. (B) Electrostatic potentials for the VFTM-CRDs of human (a and b) and squirrel monkey (c and d). The contour maps (a and c) represent isopotential surfaces of the receptors with +/- 4kT/e for positive (blue) and negative (red) potentials, respectively. The receptors are represented as molecular surfaces color coded by electrostatic potential (+/- 10kT/e).
To investigate the electrostatic property of (h/sm)T1R2/T1R3, we constructed homology models of the extracellular region of hT1R2/hT1R3 and smT1R2/smT1R3 (closed and active states) based on mGluRs crystal structure templates [29,35]. We calculated the electrostatic potentials for these homology models using GRASP software [36] as shown in Fig. 4B. We can see that the electrostatic potential profile for each receptor is different. Human hT1R2/hT1R3 receptor has 22 negative charges, compared to 14 in the smT1R2/smT1R3 receptor. Interestingly, the main differences between the electrostatic potentials are located on the CRD regions, which were previously found as potential binding sites of hT1R2/hT1R3 for brazzein [7]. The electrostatic property of the h/smT1R2/T1R3 could affect the association and interaction between sweet-tasting proteins and the receptors.
4. Discussion
In this study, we characterized the T1R2 and T1R3 sweet taste receptors from a species of New World squirrel monkeys. The sweet taste receptor of the squirrel monkey does not respond to the artificial sweeteners: aspartame, neotame, cyclamate, saccharin, and sweet-tasting protein monellin, but it does respond to thaumatin at high concentrations. Lactisole cannot inhibit the squirrel monkey sweet taste receptor's responses. Using human and squirrel monkey chimeric receptors, we found that the residues in T1R2 determine species-dependent sweet taste toward saccharin, while the residues in either T1R2 or T1R3 determine the sweet taste difference between the human and squirrel monkey toward monellin. To our knowledge, this is the first characterized sweet taste receptor of New World monkey species in a heterologous expression system. Previously, the role of hT1R3 toward sweet-tasting proteins could not be evaluated based on the human and mouse T1R2/T1R3 chimeric experiments since the mT1R2 / hT1R3 chimeric receptor was not functional [7]. Our functionally expressed smT1R2/ hT1R3 receptor assay overcomes this hindrance, which could be a useful tool to map the binding sites of sweet taste receptors in future studies, for example, the binding site in the receptor for saccharin.
Many physiological and molecular biology studies have indicated that sweet-tasting proteins can be perceived by humans, apes and Old World monkeys, but not by New World monkeys and rodents [18-20,24]. Interestingly, our data shows that sweet taste receptor from New World squirrel monkey responds to the sweet-tasting protein, thaumatin but do not respond to monellin. However, the efficacy of the sweet response of squirrel monkey receptor is lower than that of human receptor toward thaumatin. Although no physiological evidence suggests that New World monkeys or rodents are able to perceive thaumatin at relatively low concentrations, a high concentration of thaumatin (0.2%, 90 μM) was able to increase the chorda tympani nerve activity in a rat [37]. In addition, a study showed that the perceived sucrose sweetness in the rat was able to be enhanced by thaumatin at the concentration of 0.02% (9 μM ) [38]. These results suggest that thaumatin may interact with the sweet taste receptor in rats using a different binding site from sucrose. Our molecular modeling results and comparison of human and squirrel monkey T1R2/T1R3 suggest that electrostatic force could be important for the association and interaction between the sweet-tasting protein and sweet taste receptor. The characterized squirrel monkey heterodimeric sweet receptors T1R2 and T1R3 in the present study provide us a valuable platform for further investigation of the molecular mechanism sweet taste receptor activation by sweeteners and sweet proteins.
Supplementary Material
Acknowledgements
This work was supported by the National Institute Health Grant DC008996 (M.C.) and S10RR027411 (M.C.) and the National Natural Science Foundation of China (31271118, B.L.). The modeling was supported by the Center for High Performance Computing (CHiPC) and Institute of Structural Biology and Drug Discovery, at Virginia Commonwealth University (VCU).
References
- 1.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 2.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 3.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 4.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat.Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 5.Kim UK, Breslin PA, Reed D, Drayna D. Genetics of human taste perception. J.Dent.Res. 2004;83:448–453. doi: 10.1177/154405910408300603. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc.Natl.Acad.Sci.U.S.A. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J.Biol.Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 8.Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J.Biol.Chem. 2005;280:34296–34305. doi: 10.1074/jbc.M505255200. [DOI] [PubMed] [Google Scholar]
- 9.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J.Biol.Chem. 2005;280:15238–15246. doi: 10.1074/jbc.M414287200. [DOI] [PubMed] [Google Scholar]
- 10.Jiang P, Cui M, Ji Q, Snyder L, Liu Z, Benard L, Margolskee RF, Osman R, Max M. Molecular mechanisms of sweet receptor function. Chem.Senses. 2005;30(Suppl 1):i17–i18. doi: 10.1093/chemse/bjh091. [DOI] [PubMed] [Google Scholar]
- 11.Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr.Pharm.Des. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 12.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr.Biol. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Winnig M, Bufe B, Kratochwil NA, Slack JP, Meyerhof W. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC.Struct.Biol. 2007;7:66. doi: 10.1186/1472-6807-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winnig M, Bufe B, Meyerhof W. Valine 738 and lysine 735 in the fifth transmembrane domain of rTas1r3 mediate insensitivity towards lactisole of the rat sweet taste receptor. BMC.Neurosci. 2005;6:22. doi: 10.1186/1471-2202-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Ha M, Meng XY, Kaur T, Khaleduzzaman M, Zhang Z, Jiang P, Li X, Cui M. Molecular mechanism of species-dependent sweet taste toward artificial sweeteners. J.Neurosci. 2011;31:11070–11076. doi: 10.1523/JNEUROSCI.0791-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda K, Koizumi A, Nakajima K, Tanaka T, Abe K, Misaka T, Ishiguro M. Characterization of the modes of binding between human sweet taste receptor and low-molecular-weight sweet compounds. PLoS.One. 2012;7:e35380. doi: 10.1371/journal.pone.0035380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Klebansky B, Fine RM, Liu H, Xu H, Servant G, Zoller M, Tachdjian C, Li X. Molecular mechanism of the sweet taste enhancers. Proc.Natl.Acad.Sci.U.S.A. 2010;107:4752–4757. doi: 10.1073/pnas.0911660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danilova V, Hellekant G, Tinti JM, Nofre C. Gustatory responses of the hamster Mesocricetus auratus to various compounds considered sweet by humans. J.Neurophysiol. 1998;80:2102–2112. doi: 10.1152/jn.1998.80.4.2102. [DOI] [PubMed] [Google Scholar]
- 19.Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC.Neurosci. 2003;4:5. doi: 10.1186/1471-2202-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danilova V, Hellekant G. Sense of taste in a New World monkey, the common marmoset. II. Link between behavior and nerve activity. J.Neurophysiol. 2004;92:1067–1076. doi: 10.1152/jn.01183.2003. [DOI] [PubMed] [Google Scholar]
- 21.Baev VI, Drukina MA, Valeeva GA, Volkova ZA. [Krebs cycle in tissue of rats subjected to combined effect of hypercapnia, hypoxia and cooling]. Ukr.Biokhim.Zh. 1975;47:352–357. [PubMed] [Google Scholar]
- 22.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc.Natl.Acad.Sci.U.S.A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Glaser D, Li W, Johnson WE, O'Brien SJ, Beauchamp GK, Brand JG. Analyses of sweet receptor gene (Tas1r2) and preference for sweet stimuli in species of Carnivora. J.Hered. 2009;100(Suppl 1):S90–100. doi: 10.1093/jhered/esp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Bachmanov AA, Maehashi K, Li W, Lim R, Brand JG, Beauchamp GK, Reed DR, Thai C, Floriano WB. Sweet Taste Receptor Gene Variation and Aspartame Taste in Primates and Other Species. Chem.Senses. 2011;36:453–475. doi: 10.1093/chemse/bjq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda T, Ugawa S, Yamamura H, Imaizumi Y, Shimada S. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J.Neurosci. 2003;23:7376–7380. doi: 10.1523/JNEUROSCI.23-19-07376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J.Mol.Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 27.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+. Proc.Natl.Acad.Sci.U.S.A. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc.Natl.Acad.Sci.U.S.A. 2007;104:3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AL, MacArthur MW, Hutchinson EG, Thornton JM. Stereochemical quality of protein structure coordinates. Proteins. 1992;12:345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- 31.Hellekant G, Danilova V. Species differences toward sweeteners. Food Chemistry. 1996;56:323–328. [Google Scholar]
- 32.Fisher GL, Pfaffmann C, Brown E. Dulcin and saccharin taste in squirrel monkeys, rats, and men. Science. 1965;150:506–507. doi: 10.1126/science.150.3695.506. [DOI] [PubMed] [Google Scholar]
- 33.Xue WF, Szczepankiewicz O, Thulin E, Linse S, Carey J. Role of protein surface charge in monellin sweetness. Biochim.Biophys.Acta. 2009;1794:410–420. doi: 10.1016/j.bbapap.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Ohta K, Masuda T, Ide N, Kitabatake N. Critical molecular regions for elicitation of the sweetness of the sweet-tasting protein, thaumatin I. FEBS J. 2008;275:3644–3652. doi: 10.1111/j.1742-4658.2008.06509.x. [DOI] [PubMed] [Google Scholar]
- 35.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls A, Sharp KA, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 37.Brouwer JN, Hellekant G, Kasahara Y, van der Wel H, Zotterman Y. Electrophysiological study of the gustatory effects of the sweet proteins monellin and thaumatin in monkey, guinea pig and rat. Acta Physiol Scand. 1973;89:550–557. doi: 10.1111/j.1748-1716.1973.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 38.Dugas du Villard X, van der Wel H, Brouwer JN. Enhancement of the perceived sucrose sweetness in the rat by thaumatin. Chemical Senses. 1980;5:93–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.