Abstract
Cigarette smoke is a complex mixture of chemicals including multiple genotoxic lung carcinogens. The classic mechanisms of carcinogen metabolic activation to DNA adducts, leading to miscoding and mutations in critical growth control genes, applies to this mixture but some aspects are difficult to establish because of the complexity of the exposure. This paper discusses certain features of this mechanism including the role of nicotine and its receptors; lung carcinogens, co-carcinogens and related substances in cigarette smoke; structurally characterized DNA adducts in the lungs of smokers; the mutational consequences of DNA adduct formation in smokers’ lungs; and biomarkers of nicotine and carcinogen uptake as related to lung cancer. While there are still uncertainties which may never be fully resolved, the general mechanisms by which cigarette smoking causes lung cancer are well understood and provide insights relevant to prevention of lung cancer, the number one cancer killer in the world, causing 1.37 million deaths per year.
Keywords: tobacco smoke, carcinogens, DNA adducts, nicotine
Introduction
The new century has witnessed a continuing decrease in age-adjusted lung cancer mortality in the United States, from 55.4 per 100,000 persons in 1999 to 50.7 in 2007, the most recent year for which data are available (http://wonder.cdc.gov/). This encouraging positive trend, which has resulted almost entirely from decreased cigarette smoking, illustrates the power of cancer prevention. However, the facts about lung cancer are still undeniably ugly. It is estimated that 156,940 lung cancer deaths will have occurred in the U.S. in 2011.1 Lung cancer accounted for 1.37 million deaths in the world in 2008 (http://www.who.int/mediacentre/factsheets/fs297/en/index.html). Ninety percent of this unimaginable death toll was caused by cigarette smoking in populations with prolonged use.2 An estimated 1 billion men and 250 million women in the world are smokers. Male smoking prevalence is particularly high in parts of eastern Europe and Asia, while female smoking prevalence is highest in some parts of eastern Europe.3 Apparently, the now common knowledge of the relationship between smoking and lung cancer cannot fully counteract the marketing prowess of the tobacco industry and the addictive power of nicotine. Still, there are some hopeful signs on the horizon with the passing of the Framework Convention on Tobacco Control by the World Health Organization and the Family Smoking Prevention and Tobacco Control Act by the U.S. government, the latter giving the U.S. Food and Drug Administration the power to regulate tobacco products.
A better understanding of mechanisms of lung carcinogenesis by cigarette smoke is likely to provide new insights on cancer prevention. This paper will present a mechanistic framework for understanding how cigarette smoking causes lung cancer, focusing on genotoxic caricnogens and their DNA addition products (commonly called adducts). Specific aspects of this mechanism will be discussed, with attention to some more recent developments or to gaps in the literature. The pathways by which tobacco products cause lung cancer essentially recapitulate established mechanisms of carcinogenesis by individual compounds, which were elucidated in classic studies during the late 20th century. It is the complexity of tobacco carcinogenesis due to the presence of multiple carcinogens and toxicants however which continues to challenge investigators to identify specific mechanisms that fully explain the ways in which smoking causes lung cancer.
Overall mechanism of lung carcinogenesis by tobacco smoke
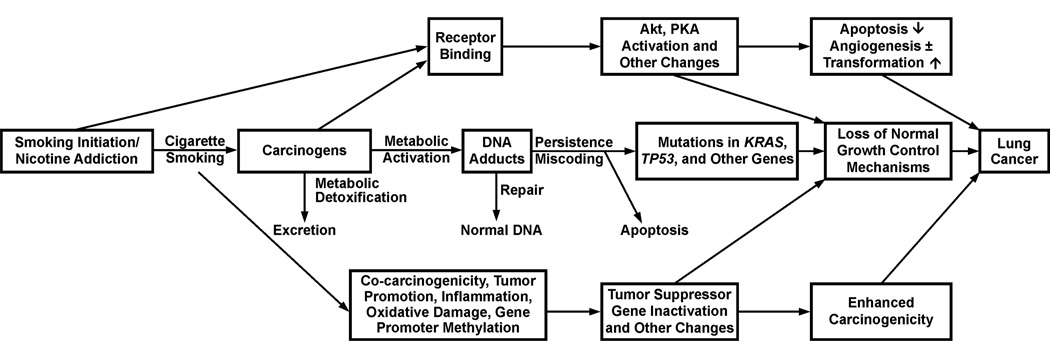
Figure 1 presents the current version of a mechanistic framework for understanding how cigarette smoking causes lung cancer. This figure has evolved somewhat since its first presentation which featured only its current central track,4 but the central track is still the main player. Each box and horizontal arrow in the central track represents a significant event or combination of events that drive the process towards cancer, while vertical arrows are protective. Details of these pathways have been discussed in the 2010 U.S. Surgeon General Report entitled “How Tobacco Smoke Causes Disease” http://www.surgeongeneral.gov/library/tobaccosmoke/report/index.html and an overview is presented here.
Figure 1.
Mechanistic framework for understanding how cigarette smoking causes lung cancer. All events can occur chronically since a smoker typically uses multiple cigarettes per day for many years.
People start smoking at a relatively young age, generally as teen-agers, before they fully appreciate the addictive power of cigarettes and general issues of mortality. They become addicted to nicotine and cannot break the cigarette habit, leading to years of smoking in spite of their best intentions. Nicotine, perhaps in concert with other tobacco smoke constituents, is highly addictive. Nicotine is not a carcinogen. But each puff of each cigarette results in the delivery of a mixture of carcinogens and toxicants along with nicotine. Over 5,000 compounds have been identified in cigarette smoke, including 73 compounds which are considered carcinogenic to either laboratory animals or humans by the International Agency for Research on Cancer.5,6
Most cigarette smoke carcinogens are substrates for drug metabolizing enzymes such as the cytochromes P450, glutathione S-transferases, and UDP-glucuronosyl transferases which catalyze their conversion to more water soluble forms that are detoxified and can be readily excreted. But during this process, reactive intermediates such as carbocations or epoxides are produced and these electrophilic compounds can react with nucleophilic sites in DNA such as the nitrogen or oxygen atoms of deoxyguanosine and other DNA bases. The result is the formation of DNA adducts which are critical in the carcinogenic process. We know that DNA adduction is important because evolution has dictated the development of DNA repair enzymes that can fix damaged DNA. People with rare syndromes in which DNA repair is deficient, such as Xeroderma pigmentosum, are highly prone to cancer development. If the DNA adducts persist unrepaired, they can cause miscoding during DNA replication as bypass polymerases catalyze the insertion of the wrong base opposite the adduct. The result is a permanent mutation. If this mutation occurs in a critical region of an oncogene such as KRAS or a tumor suppressor gene such as TP53, the result is undeniably loss of normal cellular growth control mechanisms and development of cancer. Multiple recent studies using next generation sequencing methods demonstrate the presence of thousands of mutations in the lungs of smokers, including in critical growth regulatory genes, most frequently KRAS and TP53, but others as well.7–10
Some tobacco smoke constituents such as nicotine and tobacco-specific nitrosamines bind directly to cellular receptors without a metabolic activation process. This can lead to activation of Akt, PKA and other pathways which can contribute to the carcinogenic process.11 Furthermore, cigarette smoke contains compounds that can induce inflammation resulting in enhanced pneumocyte proliferation,12 as well as co-carcinogens, tumor promoters, inducers of oxidative damage and gene promoter methylation, all processes which undoubtedly contribute to lung cancer development. In the following, some specific aspects of this general mechanism are discussed in more detail.
Nicotine is not a carcinogen
Nicotine (Figure 1, first box, central track) is the reason that people cannot stop smoking, but it is not the cause of lung cancer. There has been a steady stream of recent studies demonstrating effects of nicotine in in vitro systems (reviewed in 13 and 14). The results of these studies have shown among other effects increased cell proliferation, inhibition of apoptosis, stimulation of cancer cell growth, and enhancement or inhibition of angiogenesis. Nicotine was also reported to promote tumor growth and metastasis in cancer xenograft models. Some lung tumors were induced in hamsters treated with nicotine under hypoxic conditions. Collectively, these results have conveyed a message: nicotine may be a carcinogen, tumor promoter, or co-carcinogen, and it has been suggested to be the “estrogen of lung cancer.” This is in spite of numerous negative carcinogenicity studies of nicotine in laboratory animals. The question of nicotine’s possible carcinogenicity is highly relevant to mechanisms of carcinogenesis by cigarette smoking and to nicotine replacement therapy, a smoking cessation aid used by millions of former smokers.
Two recent studies have squarely addressed this issue. In one, Murphy et al treated A/J mice with nicotine in the drinking water (0.44 µmol/ml) resulting in a daily dose to the mouse on a mg/kg basis which was much higher than that experienced by a smoker.13 The A/J mouse is extremely susceptible to lung tumor induction by carcinogens. Many compounds, including some which were ultimately shown to be fairly weak carcinogens in other models, elicited a response in the A/J mouse by increasing the number of lung tumors per mouse compared to controls.15 In contrast, nicotine had no effect whatsoever when provided in the drinking water to A/J mice for 46 weeks.13 The number of lung tumors per mouse, 0.32 ± 0.1 was statistically indistinguishable from that in controls, 0.53 ± 0.1. Furthermore, this study demonstrated that nicotine had no effect on lung tumor induction by the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Lung tumor multiplicity in NNK-treated mice was 18.4 ± 4.5 and was not affected by nicotine given before, after, or concurrently with NNK. The second study used A/J mice crossed with C57BL/6 mice.14 These mice were treated with NNK followed by nicotine (0.62 µmol/ml). Nicotine treatment did not alter tumor multiplicity or size. Similar results were obtained in a mutant Kras-mediated lung tumor model and in syngeneic murine lung cancer cell lines. Nicotine had no effect.
Collectively, these results are consistent with most previous studies in laboratory animals and demonstrate conclusively that nicotine is not a carcinogen, co-carcinogen, tumor promoter, or inhibitor of NNK carcinogenesis. The lack of carcinogenicity of nicotine is completely consistent with studies that demonstrate that nicotine is not metabolically activated to DNA-binding intermediates.
Variants in nicotinic acetylcholine receptor subunit genes CHRNA3 and CHRNA5 and lung cancer
The addictive properties of nicotine result from its binding to nicotinic acetylcholine receptors. An association between common variants in the CHRNA5-CHRNA3-CHRNB4 nicotinic acetylcholine receptor subunit gene cluster on chromosome 15q25 and lung cancer risk was originally reported in three whole-genome association studies.16–18 These genes had been shown previously to be strongly associated with self-assessed nicotine dependence.19 Multiple studies have confirmed and extended the association of variants in these and related genes with lung cancer.20–25
We demonstrated that this association was likely due to smoking behavior resulting in increased uptake of nicotine and the lung carcinogen NNK in carriers of these variants, as determined by quantitation of nicotine metabolites and the NNK metabolite total NNAL in urine.26 The association between increased levels of NNK uptake and higher risk for lung cancer is completely plausible based on the powerful genotoxic carcinogenicity of NNK for the lung that has been observed in studies with mice, rats, hamsters, and ferrets treated with NNK, including some studies which used very low doses.27 The increased uptake of nicotine, which was confirmed by measurement of its metabolite cotinine in a similar study based in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, is an effective surrogate for uptake of other carcinogens and toxicants in cigarette smoke.23,28,29 Thus, carriers of these variants smoke more intensely and are consequently exposed to higher levels of NNK and other carcinogens in cigarette smoke, thereby increasing their risk for lung cancer. Their increased risk is not due to increased uptake of nicotine itself because nicotine is not a carcinogen.
While not the focus of this review, there is a significant body of evidence demonstrating that polymorphisms in CYP2A6, the major gene for catalysis of nicotine metabolism, have a significant effect on smoking behavior and possibly on lung cancer risk.30,31
Lung carcinogens in cigarette smoke
Carcinogens (Figure 1, second box, central track) form the link between nicotine addiction and lung cancer. Considering the chemical complexity of tobacco smoke, with more than 5,000 identified constituents,5 and its biological variety typified by the presence of carcinogens, toxicants, irritants, tumor promoters, co-carcinogens, and inflammatory agents, any attempt to identify individual compounds which cause lung cancer in smokers is perilous. Among the 73 compounds in cigarette smoke which have been classified by IARC as having sufficient evidence for carcinogenicity in either laboratory animals or humans, there are more than 20 which are lung carcinogens. These include polycyclic aromatic hydrocarbons (PAH), NNK, volatiles such as 1,3-butadiene and ethylene oxide, metals such as cadmium, and the radioactive compound 210Po.4,32 Evidence and unresolved issues pertaining to the involvement of these carcinogens in lung cancer have been discussed recently.32 The strongest evidence for a role of individual carcinogens as causes of lung cancer is for the PAH and NNK, which reproducibly and robustly induce tumors of the lung in laboratory animals.27,33,34 PAH are all genotoxic carcinogens, requiring enzymatic activation to DNA-reactive intermediates such as bay region diol epoxides which are mutagenic and carcinogenic and react easily with DNA forming adducts with well characterized miscoding consequences.35,36 Depending on PAH structure, animal model system, and route of administration, the lung can be a significant target of PAH carcinogenesis.33 There is solid evidence from animal studies and in vitro studies that metabolically activated PAH bind to DNA and cause mutations in TP53 and KRAS, as indicated in Figure 1. NNK is also a genotoxic carcinogen, converted enzymatically in the body to highly electrophilic DNA-binding diazohydroxides and related intermediates which bind to DNA causing mutations in KRAS, consistent with the general mechanism shown in Figure 1.34,37 The requirement for local metabolic activation of NNK in the mouse lung has been elegantly demonstrated by experiments using mice in which cytochrome P450-reductase, the requisite co-factor for cytochrome P450-catalyzed metabolic activation of NNK, has been knocked out in the lung,38 and a polymorphism in Cyp 2A5 decreased NNK-induced DNA adduct formation and lung tumorigenesis, as seen in parallel epidemiologic studies of lung cancer.39 NNK readily and selectively induces tumors of the lung in all species tested, independent of the route of administration, and frequently at low doses.27 Lung tumor induction by PAH and NNK occurs smoothly in standard laboratory animal models, without the need for unrealistic doses or genetic modification. The total amount of five carcinogenic PAH and NNK in the mainstream smoke of a single cigarette is about 0.6 nmol, or 360 trillion molecules.40
Inhalation studies of cigarette smoke have many difficulties which have been previously summarized, all relating to the fact that laboratory animals do not voluntarily inhale cigarette smoke, but rather try to avoid it.2,41 Nevertheless, a number of relatively recent studies have demonstrated lung tumor induction in both rats and mice exposed to cigarette smoke; in the older literature, tumors of the hamster larynx were produced.41–44 Depending on the study design, both the volatile and particulate phases of cigarette smoke have been implicated in tumor induction. There is little concrete evidence from inhalation studies that can implicate any particular carcinogen or group of carcinogens as the responsible agent. The tumor promoting and inflammatory activities of whole cigarette smoke are also involved, as discussed further below. In summary, it is still difficult to blend the results of carcinogenicity studies of individual tobacco smoke carcinogens with the results of inhalation studies. Some progress in that area was achieved however in older mouse skin studies of cigarette smoke condensate, which demonstrated the combined role of PAH and tumor promoters or co-carcinogens.45
There is still room for considerable further research on analysis of carcinogenic PAH in cigarette smoke. For example, three of these compounds – cyclopenta[c,d]pyrene, dibenzo[a,l]pyrene, and dibenz[a,h]anthracene – have been evaluated by the International Agency for Research on Cancer as “probably carcinogenic to humans” (Group 2A) based on a combination of carcinogenicity studies in laboratory animals and mechanistic data.33 For cyclopenta[c,d]pyrene and dibenzo[a,l]pyrene, there are very limited or no data on their quantities in cigarette smoke, and fairly limilted data have been presented for dibenz[a,h]anthracene.6 There are multiple PAH in cigarette smoke possibly including unidentified carcinogens.46–48
Co-carcinogens, tumor promoters, and inflammatory agents in cigarette smoke
This section pertains to the first box in the lower track of Figure 1. The tumor promoting and co-carcinogenic activities of cigarette smoke condensate were clearly demonstrated in studies starting in the 1960s using mouse skin models and later by inhalation studies in hamsters.43,45 These studies established the presence of tumor promoters in the weakly acidic fraction of the condensate, but the responsible compounds have not been identified. Phenol and catechol, major constituents of these fractions, do not possess significant tumor promoting activity when tested on mouse skin. However, catechol is a potent co-carcinogen with the classic PAH carcinogen benzo[a]pyrene (BaP) as well as its proximate carcinogen BaP-7,8-diol, when tested on mouse skin, and fractions of cigarette smoke condensate enriched in catechol show similar activity.49–51 Co-treatment of catechol with either BaP or BaP-7,8-diol produced larger tumors and caused more inflammatory changes than in the groups not treated with catechol. The multiplicity of BaP-7,8-diol-induced tumors on mouse skin was increased 14-fold by catechol.51 Cellulose and chlorogenic acid in tobacco are good precursors to catechol in cigarette smoke.52 As the levels of catechol in cigarette smoke are considerable, ranging from about 5 – 90 µg (45 – 818 nmol) per cigarette, further studies are required to examine its potentially important role in tobacco carcinogenesis.
Pro-inflammatory changes have been observed in the lungs of smokers and inflammation is closely associated with tumor promotion and activation of NF-κB.53–55 Anti-inflammatory agents are effective chemopreventive agents against tobacco carcinogen-induced lung tumorigenesis in mouse models.56 Chronic obstructive pulmonary disease, particularly emphysema, is an independent risk factor for lung cancer in smokers, further implicating inflammation.57 One recent study examined the tumor promoting activity of cigarette smoke in mouse models. Smoke exposure subsequent to treatment of A/J mice with NNK increased lung tumor multiplicity, and similar results were obtained in K-rasLA2 mice with a mutation in K-ras codon 12 identical to that produced by NNK. These results demonstrated the tumor promoting activity of cigarette smoke in a mouse lung tumor model. Further experiments demonstrated that IkB kinase β (IKKβ), required for NF-κβ activation, played a critical role in tumor promotion, most likely through the induction of inflammation and associated changes.12 The authors claimed to have provided the first evidence for tumor promotion by tobacco smoke, but in reality this was established in studies with Syrian golden hamsters nearly 40 years ago.58 Collectively, these data demonstrate the need for further investigation of inflammatory agents in cigarette smoke. Prime candidates would include catechol and acrolein.
DNA adducts in smokers’ lungs
This section pertains to the critical DNA adducts box in the central track of Figure 1. We know from multiple studies carried out in the second half of the 20th century that genotoxic carcinogens, either directly or after metabolic activation, react with DNA to form covalently bound DNA adducts that are absolutely central in the carcinogenic process because they can cause miscoding events in critical genes. In many experimental systems, cancer induction increases in tandem with increases in specific promutagenic DNA adducts and decreases when adduct formation is blocked.
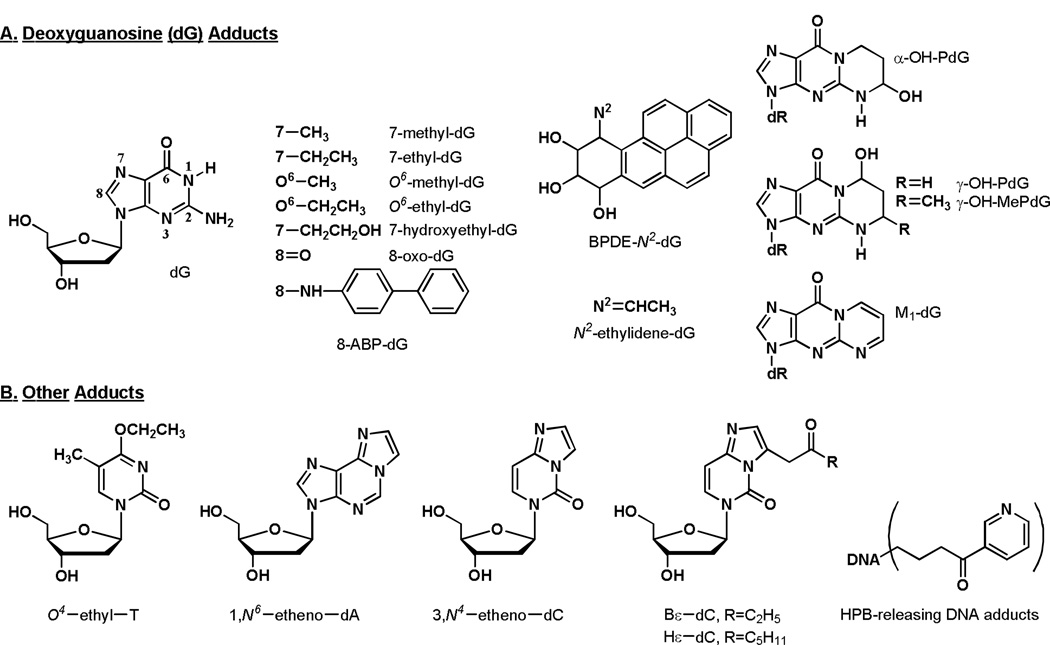
As summarized in the chapter by Phillips et al in this issue, there is convincing evidence for the presence of multiple DNA adducts in the lungs of smokers, with levels that are higher than those in non-smokers in many studies. Most of this evidence results from the use of non-specific DNA adduct detection techniques such as 32P-postlabelling and immunoassay. While these studies are convincing in aggregate, none of them provide information on DNA adduct structures, thus preventing one from working backwards to the offending carcinogen or toxicant. Only 20 structurally characterized DNA adducts in the lungs of smokers have been reported to date.59–63 This is remarkable in view of the fact that there are 73 established carcinogens in cigarette smoke and each can form multiple DNA adducts. The structures of the identified adducts are illustrated in Figure 2. Clearly, this is an area where further research is required.
Figure 2.
Structures of DNA adducts identified in human lung. Abbreviations: dG, deoxyguanosine; dR, 2′-deoxyribose; T, thymidine; dA, deoxyadenosine; dC, deoxycytidine; HPB, 4-hydroxy-1-(3-pyridyl)-1-butanone.
The DNA adducts in Figure 2 have multiple potential sources in cigarette smoke.59–65 7-Methyl-dG, 7-ethyl-dG, O6-methyl-dG and O6-ethyl-dG could be formed by direct acting methylating or ethylating agents in cigarette smoke (mostly unidentified to date) or from the metabolic activation of nitrosamines such as N-nitrosodimethylamine or N-nitrosodiethylamine. 7-Hydroxyethyl-dG could originate from reaction of ethylene oxide with DNA, while 8-oxo-dG is a well established product of oxidative damage. 8-ABP-dG is a product of metabolic activation of 4-aminobiphenyl. BPDE-N2-dG results from reaction of BaP-7,8-diol-9,10-epoxide with DNA. N2-Ethylidene-dG is formed by the reaction of acetaldehyde with DNA. α-OH-PdG and γ-OH-PdG are products of the reaction of acrolein with DNA while γ-OH-MePdG originates from reaction of crotonaldehyde with dG. M1-dG results from reaction of the lipid peroxidation product malondialdehyde with dG. O4-Ethyl-T is formed upon reaction of an ethylating agent with thymidine, while 1,N6-etheno-dA, 3,N4-etheno-dC, Bε-dC and Hε-dC are mainly products of lipid peroxidation. The HPB-releasing adducts are the only adducts in this figure that are reasonably specific to tobacco smoke exposure, as they arise mainly from tobacco-specific nitrosamines, or possibly other HPB-transferring compounds in tobacco smoke. Most studies of these specific DNA adducts have analyzed relatively few lung DNA samples and there is a paucity of quantitative data available. This prevents generalizations about levels of these specific adducts in lung tissue of smokers vs. non-smokers. Further quantitative studies of specific DNA adducts are needed to advance our understanding of the role of individual tobacco smoke components in the etiology of lung cancer.
Consequences of DNA adduct formation – mutations in critical genes
This section pertains to the 4th box of the central track of Figure 1. The availability of the human genome and modern sequencing technology has made it possible to carry out large scale studies of mutations in human cancers. Based on the presence of multiple genotoxic carcinogens in cigarette smoke along with persuasive evidence for the presence of DNA adducts in the lungs of smokers, even if many of them have yet to be structurally characterized, wouldn’t one expect to find multiple mutations in critical genes that could drive the process toward lung cancer? The answer to this hypothetical question is clearly affirmative, and several recent large scale sequencing studies confirm this expectation, consistent with the central track of the general mechanistic scheme shown in Figure 1.
In one study, Greeenman et al reported on the presence of mutations in the coding exons of more than 500 protein kinase genes in over 200 diverse human cancers including lung cancer. The number of somatic point mutations, including driver mutations, varied widely within and between different types of cancer. Lung cancers were among those with the highest numbers of somatic mutations (4.21 per megabase) and this was attributed to recurrent exogenous mutagen exposure.10
A second study carried out sequencing of 188 primary lung adenocarcinomas. A total of 247 megabases of tumor DNA sequence were analyzed and 1,013 non-synonymous somatic mutations in 163 of the 188 tumors were identified. These included 915 point mutations, 12 dinucleotide mutations, 29 insertions and 57 deletions. A total of 26 significantly mutated genes were identified, including several tumor suppressor genes and oncogenes known to be mutated in lung cancer: TP53, KRAS, CDKN2A, STK11, and others. The greatest number of mutations was found in TP53 and KRAS.7
A third investigation used massively parallel sequencing technology to sequence a small cell lung cancer cell line. A total of 22,190 somatic substitutions were identified including 134 in coding exons. G → T transversions were the most common change observed (34%), followed by G →A transitions (21%) and A →G transitions (19%), which is quite similar to data that have been obtained by analysis of the TP53 gene, discussed below.8
A fourth report focused on a non-small cell lung cancer from a 51-year old who reported smoking an average of 25 cigarettes per day for 15 years prior to excision of the tumor, a poorly differentiated sample with 95% tumor content, most likely an adenocarcinoma. Single nucleotide variants were common, occurring predominantly at G →C base pairs, with the most prevalent change being G →T transversions, as in other studies, statistically distinct from germline mutations. More than 50,000 single nucleotide variants were observed, approximately 17.7 mutations per megabase. The results showed that lung cancers can harbor large numbers of varied mutations. For example, at least 8 genes in the EFGR-RAS-RAF-MEK-ERK pathway were either mutated or amplified.9
The results of these studies amplify and confirm those of earlier investigations which focused on mutations in TP53 and KRAS in lung tumors from smokers (reviewed in 4,59). Mutations in TP53 and KRAS are more common than in other genes.7 Consistently, updates of the TP53 mutation data base (http://www-p53.iarc.fr) demonstrate that, in lung tumors from smokers, G → T transversions are the most prevalent mutations, followed by G → A transitions. There are significantly more G → T transversions in smokers than in non-smokers, while G → A transitions are more common in non-smokers. Hotspots for mutation in the TP53 gene in tobacco smoke associated lung cancers occur at codons 157, 158, 245, 248, 249, and 273.66,67 Multiple studies carried out in a variety of systems using different techniques ranging from the UvrABC nuclease incision method in combination with ligation-mediated PCR to mass spectrometry of specific isotopically labeled TP53 sequences demonstrate that PAH diol epoxide metabolites react preferentially at the TP53 hotspots observed in tobacco smoke associated lung cancers.66–71 These results provide strong support for the involvement of PAH in lung cancer etiology in smokers. However, similar results have been reported in experiments with acrolein, which occurs in cigarette smoke in concentrations up to 10,000 times greater than those of BaP.72 Acrolein-DNA adducts (α-OH-PdG and γ-OH-PdG, Figure 2) have been detected in human lung tissue, but no data are available on differences between smokers and non-smokers. Human leukocyte DNA contains mainly γ-OH-PdG, with similar levels in smokers and non-smokers,73,74 while the acrolein metabolite 3-hydroxypropyl mercapturic acid is consistently significantly elevated in the urine of smokers compared to non-smokers.75 The possible role of acrolein in the generation of TP53 mutations in human lung tumors requires further investigation, but it should be noted that acrolein, while toxic to the lung and other tissues, has not been shown to be a lung carcinogen, in contrast to BaP and other PAH which are potent in this respect.76
The COSMIC data base (http://www.sanger.ac.uk/genetics/CGP/cosmic/) stores and displays somatic mutations in genes associated with cancer. For KRAS, it indicates that the most common mutations are in codon 12, mainly G →T transversions and, to a lesser extent, G →A transitions. G → T at the first position of codon 12 is most common, and this has also been associated with PAH exposure, while G → A transitions may arise from exposure to NNK or related compounds.77 However, caution is advisable in drawing firm conclusions from these data as there are numerous genotoxic carcinogens that mutate codon 12 of KRAS.4
In summary, the available results of late generation sequencing studies as well as the extensive data bases on TP53 and KRAS mutations are completely consistent with the induction of multiple mutations in critical growth control genes by metabolically activated carcinogens. The question of which carcinogen is responsible for each mutation is more complex, and while some data provide convincing support for a role of PAH, there are still uncertainties.
Tobacco carcinogen and toxicant biomarkers and lung cancer
This section relates to the central track of Figure 1. Although there are still some challenges to quantitation of DNA adducts in human samples which prevent their use in large studies of tobacco smoke carcinogen metabolic activation as it may relate to lung cancer, this is not the case for urinary metabolites. As examples, validated analytical methods exist for urinary metabolites of nicotine, PAH, tobacco-specific nitrosamines such as NNK, and volatile carcinogens and toxicants such as 1,3-butadiene, ethylene oxide, benzene, acrolein, and crotonaldehyde.75 There are differences in the types of cigarettes that smokers habitually use and in the ways that they smoke them. These differences, which cannot be captured by counting numbers of cigarettes per day smoked, will nevertheless have a significant impact on the central track of Figure 1. In the simplest possible terms, greater exposure to genotoxic carcinogens will increase the probability of DNA damage and mutations, thus increasing the probability of lung cancer. The development of a predictive algorithm for identifying which smoker may be susceptible to lung cancer would be one positive outcome of this research.
The basic hypothesis that biomarker levels are related to lung cancer risk has been tested in a number of studies using the techniques of molecular epidemiology. The nicotine metabolite cotinine is a good biomarker for nicotine uptake. While nicotine is not a carcinogen, it is expected that carcinogen delivery will parallel nicotine delivery in a smoker. Several studies have found a relationship between urinary or serum cotinine levels and lung cancer risk in smokers.23,78–80 In one recent study nested in the Shanghai Cohort Study which enrolled over 18,000 men from 1986–1989 and followed them for lung cancer development, we found a significant relationship between urinary total cotinine (cotinine plus its N-glucuronide) and lung cancer development based on analysis of total cotinine in over 900 urine samples, all from smokers, even after taking into account number of years of smoking and numbers of cigarettes per day.28 Total cotinine was also an effective surrogate for levels of urinary mercapturic acids of volatile carcinogens and toxicants quantified in this study.29 Thus, total cotinine is specifically related to lung cancer in smokers, which is fully consistent with the central track of Figure 1.
The relationships of biomarkers of NNK and PAH uptake to lung cancer have also been examined in nested case-control studies. Total NNAL, the sum of the NNK metabolites NNAL plus its glucuronides, is an accepted and widely used biomarker of uptake of the tobacco-specific lung carcinogen NNK.75,81 Levels of total NNAL in serum were quantified in samples from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial and were significantly associated with increased risk for lung cancer, after adjustment for number of cigarettes per day and years of smoking.82 Similar results were obtained in nested case-control studies within the Singapore Chinese Health Study and the Shanghai Cohort Study, the latter with significantly larger numbers of samples.28,80 The association of total NNAL with lung cancer was still significant after adjusting for total cotinine.
Phenanthrene tetraol is a biomarker of uptake plus metabolism of the PAH phenanthrene which is a ubiquitous but non-carcinogenic PAH with many similarities in metabolism to BaP.83,84 Urinary phenanthrene tetraol levels were also significantly associated with lung cancer risk in the Shanghai Cohort Study, after correction for cigarettes per day and number of years of smoking.28 Phenanthrene tetraol, total NNAL, and total cotinine were all significantly associated with lung cancer risk in this study, with smoking adjusted odds ratios ranging from 2 – 4. The significant associations of phenanthrene tetraol and total NNAL with lung cancer after correction for total cotinine most likely are related to the potent lung carcinogenicity of PAH and NNK, which cannot be completely accounted for simply by measurement of total cotinine in urine.
Based on these results, and after further studies, it may be possible to use a combination of tobacco toxicant and carcinogen biomarkers to predict risk for lung cancer in smokers. Studies to date have used various parameters in risk models, but none have yet incorporated biomarkers.85–91
Conclusions
The assault on lung cells of genotoxic carcinogens in cigarette smoke has disastrous mutagenic effects that have been demonstrated in many studies approaching the problem from different perspectives. This clearly stands out as the greatest voluntary human exposure to a mixture of chemical carcinogens, with horrific consequences in terms of lung cancer mortality. Many aspects of the general mechanism of carcinogenesis outlined in Figure 1 are firmly established but there are still some areas that could profit from further investigation. These include structural analysis of DNA adducts in smokers’ lungs, a better understanding of the role of non-genotoxic tobacco smoke constituents in the lung cancer process, the role of polymorphisms in genes involved in nicotine and carcinogen uptake and metabolism, and the identification of smokers at high risk for lung cancer. A better understanding of mechanisms of lung caner induction by cigarette smoke can provide new insights on prevention of lung cancer in smokers, and possibly on cancer prevention generally.
Acknowledgments
Research in the Hecht laboratory on tobacco and cancer is supported by grants CA-81301, CA-92025, and CA-138338 from the U.S. National Cancer Institute. I thank Bob Carlson for editorial assistance.
Reference List
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 33–1187. [PMC free article] [PubMed] [Google Scholar]
- 3.Shafey O, Eriksen MP, Ross H, Mackay J. The Tobacco Atlas. 3rd Edition. Atlanta, GA: American Cancer Society and World Lung Foundation; 2009. p. 19. [Google Scholar]
- 4.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 5.Rodgman A, Perfetti T. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton, FL: CRC Press; 2009. pp. 1483–1784. [Google Scholar]
- 6.Hecht SS. Research opportunities related to establishing standards for tobacco products under the family smoking prevention and tobacco control act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, Lin ML, Beare D, Lau KW, Greenman C, Varela I, Nik-Zainal S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463(7278):184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, Yue P, Zhang Y, Pant KP, Bhatt D, Ha C, Johnson S, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465(7297):473–477. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 10.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O'Meara S, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen RJ, Chang LW, Lin P, Wang YJ. Epigenetic effects and molecular mechanisms of tumorigenesis induced by cigarette smoke: an overview. J Oncol. 2011;2011:654931. doi: 10.1155/2011/654931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy SE, von Weymarn LB, Schutten MM, Kassie F, Modiano JF. Chronic nicotine consumption does not influence 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis. Cancer Prev Res (Phila) 2011;4(11):1752–1760. doi: 10.1158/1940-6207.CAPR-11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier CR, Hollander MC, Hobbs EA, Dogan I, Linnoila RI, Dennis PA. Nicotine does not enhance tumorigenesis in mutant K-ras-driven mouse models of lung cancer. Cancer Prev Res (Phila) 2011;4(11):1743–1751. doi: 10.1158/1940-6207.CAPR-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoner GD, Shimkin MB. Strain A mouse lung tumor bioassay. J Amer Coll Toxicol. 1982;1:145–169. [Google Scholar]
- 16.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 19.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69(17):6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JJ, van den Bree MB, Munafo MR. Association of the CHRNA5-A3-B4 gene cluster with heaviness of smoking: a meta-analysis. Nicotine Tob Res. 2011;13(12):1167–1175. doi: 10.1093/ntr/ntr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timofeeva MN, McKay JD, Smith GD, Johansson M, Byrnes GB, Chabrier A, Relton C, Ueland PM, Vollset SE, Midttun O, Nygard O, Slimani N, et al. Genetic polymorphisms in 15q25 and 19q13 loci, cotinine levels, and risk of lung cancer in EPIC. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2250–2261. doi: 10.1158/1055-9965.EPI-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Broderick P, Matakidou A, Eisen T, Houlston RS. Chromosome 15q25 (CHRNA3-CHRNA5) variation impacts indirectly on lung cancer risk. PLoS One. 2011;6(4):e19085. doi: 10.1371/journal.pone.0019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103(17):1342–1346. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, Tiirikainen M, Wang H. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68(22):9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan JM, Gao YT, Wang R, Chen M, Carmella SG, Hecht SS. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis. 2012 Feb 21; doi: 10.1093/carcin/bgs026. 2012, [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23(3):252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecht SS. Tobacco smoke carcinogens and lung cancer. In: Penning TM, editor. Chemical Carcinogenesis. Springer; 2010. [Google Scholar]
- 33.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 92. Lyon, FR: IARC; 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 421–583. [PMC free article] [PubMed] [Google Scholar]
- 35.Dipple A, Moschel RC, Bigger CAH. Polynuclear aromatic hydrocarbons. In: Searle CE, editor. Chemical Carcinogens, Second Edition, ACS Monograph 182. vol. 1. Washington, D.C.: American Chemical Society; 1984. pp. 41–163. [Google Scholar]
- 36.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbons. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 19–96. [Google Scholar]
- 37.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng Y, Fang C, Turesky RJ, Behr M, Kaminsky LS, Ding X. Determination of the role of target tissue metabolism in lung carcinogenesis using conditional cytochrome P450 reductase-null mice. Cancer Res. 2007;67:7825–7832. doi: 10.1158/0008-5472.CAN-07-1006. [DOI] [PubMed] [Google Scholar]
- 39.Hollander MC, Zhou X, Maier CR, Patterson AD, Ding X, Dennis PA. A Cyp2a polymorphism predicts susceptibility to NNK-induced lung tumorigenesis in mice. Carcinogenesis. 2011;32(8):1279–1284. doi: 10.1093/carcin/bgr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht SS. More than 500 trillion molecules of strong carcinogens per cigarette: use in product labelling? 2011;20(5):387. doi: 10.1136/tc.2011.042853. [DOI] [PubMed] [Google Scholar]
- 41.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–1492. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]
- 42.Stinn W, Arts JH, Buettner A, Duistermaat E, Janssens K, Kuper CF, Haussmann HJ. Murine lung tumor response after exposure to cigarette mainstream smoke or its particulate and gas/vapor phase fractions. Toxicology. 2010;275(1–3):10–20. doi: 10.1016/j.tox.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 43.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 38. Lyon, FR: IARC; 1986. Tobacco Smoking; pp. 37–375. [Google Scholar]
- 44.Gordon T, Bosland M. Strain-dependent differences in susceptibility to lung cancer in inbred mice exposed to mainstream cigarette smoke. Cancer Lett. 2009;275(2):213–220. doi: 10.1016/j.canlet.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann D, Schmeltz I, Hecht SS, Wynder EL. Tobacco carcinogenesis. In: Gelboin H, Ts'o POP, editors. Polycyclic Hydrocarbons and Cancer. New York: Academic Press; 1978. pp. 85–117. [Google Scholar]
- 46.Snook ME, Severson RF, Arrendale RF, Higman HC, Chortyk OT. Multi-alkyated polynuclear aromatic hydrocarbons of tobacco smoke: separation and identification. 1978;9:222–247. [Google Scholar]
- 47.Snook ME, Severson RF, Arrendale RF, Higman HC, Chortyk OT. The identification of high molecular weight polynuclear aromatic hydrocarbons in a biologically active fraction of cigarette smoke condensate. Beiträge zur Tabakforschung. 1977;9:79–101. [Google Scholar]
- 48.Rodgman A, Perfetti TA. The composition of cigarette smoke: a catalogue of the polycyclic aromatic hydrocarbons. Beitr Tabakforschung Intl. 2006;22:13–69. [Google Scholar]
- 49.Van Duuren BL, Goldschmidt BM. Cocarcinogenic and tumor-promoting agents in tobacco carcinogenesis. J Natl Cancer Inst. 1976;56:1237–1242. doi: 10.1093/jnci/56.6.1237. [DOI] [PubMed] [Google Scholar]
- 50.Hecht SS, Carmella S, Mori H, Hoffmann D. Role of catechol as a major cocarcinogen in the weakly acidic fraction of smoke condensate. J Natl Cancer Inst. 1981;66:163–169. [PubMed] [Google Scholar]
- 51.Melikian AA, Jordan KG, Braley J, Rigotty J, Meschter CL, Hecht SS, Hoffmann D. Effects of catechol on the induction of tumors in mouse skin by 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrenes. Carcinogenesis. 1989;10:1897–1900. doi: 10.1093/carcin/10.10.1897. [DOI] [PubMed] [Google Scholar]
- 52.Carmella SG, Hecht SS, Tso TC, Hoffmann D. Roles of tobacco cellulose, sugars, and chlorogenic acid as precursors to catechol in cigarette smoke. J Agric Food Chem. 1984;32:267–273. [Google Scholar]
- 53.Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18(9):667–677. doi: 10.1080/08958370600742821. [DOI] [PubMed] [Google Scholar]
- 54.Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008;66(3):208–217. doi: 10.1016/j.critrevonc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp Lung Res. 2005;31(1):57–82. doi: 10.1080/01902140490495020. [DOI] [PubMed] [Google Scholar]
- 56.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9(7):476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 58.Dontenwill W, Chevalier HJ, Harke HP, Lafrenz U, Reckzeh G, Schneider B. Investigations on the effects of chronic cigarette-smoke inhalation in Syrian golden hamsters. J Natl Cancer Inst. 1973;51(6):1781–1832. doi: 10.1093/jnci/51.6.1781. [DOI] [PubMed] [Google Scholar]
- 59.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 60.United States Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Ch. 5. Washington, D.C.: U.S. Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 61.Munnia A, Bonassi S, Verna A, Quaglia R, Pelucco D, Ceppi M, Neri M, Buratti M, Taioli E, Garte S, Peluso M. Bronchial malondialdehyde DNA adducts, tobacco smoking, and lung cancer. Free Radic Biol Med. 2006;41(9):1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Anna L, Kovacs K, Gyorffy E, Schoket B, Nair J. Smoking-related O4-ethylthymidine formation in human lung tissue and comparisons with bulky DNA adducts. Mutagenesis. 2011;26(4):523–527. doi: 10.1093/mutage/ger011. [DOI] [PubMed] [Google Scholar]
- 63.Chou PH, Kageyama S, Matsuda S, Kanemoto K, Sasada Y, Oka M, Shinmura K, Mori H, Kawai K, Kasai H, Sugimura H, Matsuda T. Detection of lipid peroxidation-induced DNA adducts caused by 4-oxo-2(E)-nonenal and 4-oxo-2(E)-hexenal in human autopsy tissues. Chem Res Toxicol. 2010;23(9):1442–1448. doi: 10.1021/tx100047d. [DOI] [PubMed] [Google Scholar]
- 64.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 65.Rojas M, Alexandrov K, Cascorbi I, Brockmoller J, Likhachev A, Pozharisski K, Bouvier G, Auburtin G, Mayer L, Koop-Schneider A, Roots I, Bartsch H. High benzo[a]pyrene diol-epoxide DNA adduct levels in lung and blood cells from individuals with combined CYP1A1 MspI/MspI-GSTM1*0/*0 genotypes. Pharmacogenetics. 1998;8:109–118. [PubMed] [Google Scholar]
- 66.Pfeifer GP, Besaratinia A. Mutational spectra of human cancer. Hum Genet. 2009;125(5–6):493–506. doi: 10.1007/s00439-009-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kucab JE, Phillips DH, Arlt VM. Linking environmental carcinogen exposure to TP53 mutations in human tumours using the human TP53 knock-in (Hupki) mouse model. FEBS J. 2010;277(12):2567–2583. doi: 10.1111/j.1742-464X.2010.07676.x. [DOI] [PubMed] [Google Scholar]
- 68.Smith LE, Denissenko MF, Bennett WP, Li H, Amin S, Tang M, Pfeifer GP. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 2000;92:803–811. doi: 10.1093/jnci/92.10.803. [DOI] [PubMed] [Google Scholar]
- 69.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hot spots in P53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 70.Tretyakova NT, Matter B, Jones R, Shallop A. Formation of benzo[a]pyrene diol epoxide-DNA adducts at specific guanines within K-ras and p53 gene sequences: stable isotope-labeling mass spectrometry approach. Biochemistry. 2002;41:9535–9544. doi: 10.1021/bi025540i. [DOI] [PubMed] [Google Scholar]
- 71.Matter B, Wang G, Jones R, Tretyakova N. Formation of diastereomeric benzo[a]pyrene diol epoxide-guanine adducts in p53 gene-derived DNA sequences. Chem Res Toxicol. 2004;17(6):731–741. doi: 10.1021/tx049974l. [DOI] [PubMed] [Google Scholar]
- 72.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103(42):15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1 N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S, Balbo S, Wang M, Hecht SS. Analysis of acrolein-derived 1 N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol. 2011;24(1):119–124. doi: 10.1021/tx100321y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 63. Lyon, France: IARC; 1995. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; pp. 393–407. [PMC free article] [PubMed] [Google Scholar]
- 77.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567(2–3):447–474. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 78.de Waard F, Kemmeren JM, van Ginkel LA, Stolker AAM. Urinary cotinine and lung cancer risk in a female cohort. Br J Cancer. 1995;72:784–787. doi: 10.1038/bjc.1995.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1184–1188. doi: 10.1158/1055-9965.EPI-06-0032. [DOI] [PubMed] [Google Scholar]
- 80.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham K, Gao YT, Yu MC, Hecht SS. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 82.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le C, Zhang Y, Benoit AR, Carmella SG, Hecht SS. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol Biomarkers Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 84.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem Res Toxicol. 2010;23(5):900–908. doi: 10.1021/tx9004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spitz MR, Etzel CJ, Dong Q, Amos CI, Wei Q, Wu X, Hong WK. An expanded risk prediction model for lung cancer. Cancer Prev Res. 2008;1:250–254. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, Field JK. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98(2):270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, Hsieh LJ, Begg CB. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 88.Etzel CJ, Kachroo S, Liu M, D'Amelio A, Dong Q, Cote ML, Wenzlaff AS, Hong WK, Greisinger AJ, Schwartz AG, Spitz MR. Development and validation of a lung cancer risk prediction model for African-Americans. Cancer Prev Res (Phila Pa) 2008;1(4):255–265. doi: 10.1158/1940-6207.CAPR-08-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cronin KA, Gail MH, Zou Z, Bach PB, Virtamo J, Albanes D. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst. 2006;98(9):637–640. doi: 10.1093/jnci/djj163. [DOI] [PubMed] [Google Scholar]
- 90.Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, Riley TL, Commins J, Oken MM, Berg CD, Prorok PC. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Etzel CJ, Bach PB. Estimating individual risk for lung cancer. Semin Respir Crit Care Med. 2011;32(1):3–9. doi: 10.1055/s-0031-1272864. [DOI] [PubMed] [Google Scholar]