Abstract
Activation of sphingosine kinase/sphingosine 1-phosphate (SK/S1P) –mediated signaling has emerged as a critical cardioprotective pathway in response to acute ischemia/reperfusion injury. S1P is released in both ischemic pre- and post-conditioning. Application of exogenous S1P to cultured cardiac myocytes subjected to hypoxia or treatment of isolated hearts either before ischemia or at the onset of reperfusion exerts prosurvival effects. Synthetic congeners of S1P such as FTY720 mimic these responses. Gene targeted mice null for the SK1 isoform whose hearts are subjected to ischemia/reperfusion injury exhibit increased infarct size and respond poorly either to ischemic pre- or postconditioning. Measurements of cardiac SK activity and S1P parallel these observations. Experiments in SK2 knockout mice have revealed that this isoform is necessary for survival in the heart. High density lipoprotein (HDL) is a major carrier of S1P, and studies of hearts in which selected S1P receptors have been inhibited implicate the S1P cargo of HDL in cardioprotection. Inhibition of S1P lyase, an endogenous enzyme that degrades S1P, also leads to cardioprotection. These observations have considerable relevance for future therapeutic approaches to acute and chronic myocardial injury.
1. Introduction
Sphingosine 1-phosphate (S1P) is a lipid signaling molecule formed when either one of two isoforms of the enzyme sphingosine kinase (SK) catalyzes the addition of a phosphate group to sphingosine, most of which is derived from the ubiquitous membrane lipid sphingomyelin. Among other functions, signals generated by this pathway are critical for cell motility, cytoskeletal organization, vasculogenesis, cell growth, lymphoid trafficking and immune function. Activation or inhibition of this pathway can determine cell fate by altering the ceremide/S1P “rheostat”, i.e., the balance between ceramide, which is pro-apoptotic, and S1P, which stimulates prosurvival signaling [1].
With regard to the cardiovascular system, little was known until recently about the functional importance of the SK/S1P pathway in cardiac myocytes or in the heart. Moreover, there were no data regarding responses under conditions of oxidative stress such as acute or chronic ischemia or ischemia/reperfusion injury. However, during the last decade, it has become abundantly clear that SK and S1P are crucial mediators of cardioprotection. Moreover, it has become increasingly recognized that these molecules or their synthetic analogues have therapeutic potential as modulators of cardiac responses to both acute and chronic myocardial injury. In addition, a major carrier of S1P in the serum is high-density lipoprotein (HDL), which is known to be cardioprotective, and the role of S1P in the remodelling process after severe cardiac injury is only now being recognized.
2. Sphingosine Kinase(s)
The synthesis of S1P is catalyzed by sphingosine kinase which is responsible for linking a phosphate group to sphingosine. There are two isoforms of SK designated as SK1 and SK2. Each of these isoforms harbors several splice variants [2]. Mouse and human SK1 exhibit substantial homology and SK2 is highly homologous to SK1 except for ~240 additional amino acids located at the N terminus and in the center of the enzyme, thereby accounting for its larger molecular mass [3, 4]. The genes encoding these isoforms are localized on different chromosomes [5]. Genetic deletion of both isoforms results in fetal death from severe bleeding and inadequate vasculogenesis [6]. In contrast, mice null for either the SK1 or the SK2 isoform exhibit normal development and are otherwise unremarkable in the basal state [7, 8]. These individual deletions have provided highly informative experimental models that allow investigators to probe the function of each of these isoforms.
The regulation of SK activity is complex and responds to stimulation by G-protein coupled receptor agonists (muscarinic agonists, S1P itself, histamine, lysophosphatidic acid, angiotensin II), agonists at receptor tyrosine kinases (PDGF, EGF, VEGF, TGF-α, TGF-β), immunoglobulin receptor crosslinking, interleukins, estrogen, and activators of PKCε [5, 9]. Both TNFα and phorbol ester, which stimulates PKC, phosphorylate and thus activate SK1 at serine225 mediated by ERK1/2 [10]. The TNF-α response requires binding by TNF receptor-associated factor -2 (TRAF2) [11]. Other interacting proteins that stimulate SK include delta-catenin/neutral plakophilin-related armadillo repeat protein [12], aminocyclase 1 [13], and eukaryotic elongation factor 1A [14]. Reported inhibitory interacting proteins are SKIP (SK1-inteacting protein) [15, 16], PECAM-1 (platelet endothelial adhesion molecule-1) [17], and FHL2/SLIM3, a Lim-only factor [18]. A recent study reported that FHL-2 suppressed VEGF-induced PI-3 kinase/Akt activation via interactions with SK-1 [19]. An interacting factor that is a putative adaptor molecule is RPK118 [20]. Additional regulators include phospholipase D and possibly calcium [21, 22]. In vitro evidence also has identified cellular export of SK, which may account for substantial enzyme activity in both mouse and human blood [23, 24].
Highly pertinent to acute ischemia/reperfusion injury is the observation that reactive oxygen species (ROS) generated by the action of monoamine oxidase A on serotonin contained in platelets is responsible for the degradation of SK1 [25]. Conversely, in glioma cells hypoxia increases SK1 mRNA, protein levels, and activity that is dependent on hypoxia inducing factor 2α (HIF-2α) [26]. Thus, the balance of hypoxia and ROS levels (and other factors as yet unidentified) could serve to regulate SK1 activity during oxidative stress in the heart (see below).
Based on numerous in vitro studies, it is generally accepted that SK1 promotes cell survival. An early report involving the heart showed that in cultured rat cardiac fibroblasts the monoganglioside GM-1, which activates SK via PKC, protected against apoptosis induced by the PKC inhibitor staurosporine and by C2-ceramide [9]. It was also reported that GM1 induced the synthesis of S1P, an effect that was partially blocked by the SK inhibitor N,N-dimethylsphingosine [9]. The latter has recently been shown to be predominantly an inhibitor of SK1 [27]. Knockdown of SK1 by small interfering RNA caused cell cycle arrest in MCF-7 cancer cells and induced apoptosis [28]. Endogenous SK1 also is an important regulator of intracellular ceramide levels. Downregulation of SK1 results in enhanced ceramide synthesis via the de novo pathway and its accumulation in mitochondria, which may be key in initiating mitochondrial events leading to cell death [28]. Underlying the anti-apoptotic effects of SK1 is its function, noted above, as the terminal step in the intracellular synthesis of S1P. There is considerable evidence that intracellular S1P can be exported to activate prosurvival signaling pathways in an autocrine and/or paracrine manner, and this phenomenon, termed “inside-out signaling” [5], has been described in cardiac myocytes [29].
In contrast, based largely on in vitro evidence, SK2 has been shown to have opposing actions to SK1 [30]. In vitro, SK2 inhibits cell growth and enhances apoptosis, in part by regulating ceramide levels [30]. We have reported that both the synthetic sphingosine analogue FTY720 and the putative SK inhibitor dimethylsphingosine inhibit primarily the SK1 form, but can activate the SK2 form at low substrate concentrations in rat heart [27]. However, the latter effect has not been verified for the purified enzyme and might be an indirect effect of the cytosolic preparation used in the reported experiments. SK2 is also necessary to phosphorylate and thus activate FTY720, which is being used worldwide to prevent relapses of multiple sclerosis [31]. Contrary to the view that SK2 is pro-apoptotic, recent experimental data obtained in isolated murine hearts from SK2 null mice subjected to ex vivo ischemia/reperfusion injury have documented that the presence of SK2 is necessary for successful ischemic pre- and post-conditioning [32, 33]. These observations have been confirmed by Gomez et al. in an in vivo mouse model [34]. In fact, recent evidence indicates that in the heart both SK2 and S1P are associated with mitochondria and may regulate the assembly of complex IV and respiration via mitochondrial prohibitin- 2 [35]. Mitochondrial dysregulation in the absence of SK2 results in increased susceptibility to permeability transition and hence to an inability to respond favorably to preconditioning [34].
Other direct intracellular effects of S1P generated by SK2 have also been reported. Hait et al. found that SK2 was associated with histone H3 and produced S1P that regulates intracellular histone acetylation [36]. It was noted that S1P specifically binds to and inhibits the enzyme activity of the histone deacetylases HDAC1 and HDAC2 thereby preventing the removal of acetyl groups from lysine residues within histone tails. SK2 is associated with these HDACS in repressor complexes and is selectively enriched in the promoters encoding the cyclin-dependent kinase inhibitor p21 and the transcription regulator c-fos, where it enhances local histone acetylation and transcription. Thus, HDACs are direct intracellular targets of S1P via SK2, thereby providing a link between intracellular SK2, nuclear S1P and epigenetic regulation of gene expression.
Despite the fact that SK1 and SK2 catalyze the same reaction, they appear to influence different biologic processes, as described above. One reason for this is location that causes differential site-specific targeting of SK. This results in highly localized release of S1P within the cell. In the absence of stimulation, SK1 is a predominantly cytosolic enzyme, but upon activation it is transported to membranes such as endoplasmic reticulum and plasma membrane [37, 38]. Phosphorylation at Ser225 is essential for translocation of the enzyme to the plasma membrane [39], including lipid rafts [40]. Recently, Jarman et al. reported an essential role for the calciuimmyristoyl switch protein, calcium and integrin-binding protein 1 (CIB1), in the process of SK1 translocation (41). CIB1 interacts withSK1 in a calciuim-dependent manner at a previously identified calmodulin binding site of SK1 (41). Translocation of SK1 to the cell surface promotes S1P secretion but does not affect the downstream metabolism of S1P [37]. SK1 localization also determines substrate utilization. Thus, SK1 can divert dihydrosphingosine from the ceramide biosynthetic pathway, except when it is translocated to the plasma membrane [37]. In contrast to SK1, SK2 is localized predominantly to the cytoplasm and the nucleus. As described above, SK2 generates nuclear S1P that influences gene transcription [36]. Also, as described above, it now appears to play an important role in cardiac mitochondria [34, 35]. Agonist regulation of SK2 is less well studied. Thus, the localization and function of intracellular S1P and its cellular export is determined largely by which SK isoform is responsible for its synthesis.
3. Sphingosine 1-Phosphate
As indicated above, sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid that regulates many important cellular processes [29, 42–46]. Most cells are able to synthesize and export S1P. In plasma and serum S1P concentrations range between ~200 – ~900 nM [47–49], but these likely vary under pathophysiologic conditions [see below]. Sources of S1P in plasma include erythrocytes [46], platelets [50], and endothelial cells [51]. Pertinent to cardioprotection is the observation that a major carrier of S1P in blood is high density lipoprotein (HDL) [47, 48]. S1P is subject to hydrolysis by lipid phosphatases and by a lyase enzyme [44]. Chronic inhibition of the latter can lead to persistently increased S1P levels and to lymphocyte sequestration and disruption of S1P gradients [52].
In the heart, the presence of S1P receptors was first reported by Bünemann et al. [53] in 1995. It is now accepted that many S1P actions are mediated through subtypes of S1P G protein-coupled receptors, which comprise S1P1–5 [54–56]. Recent evidence supports the notion that S1P binding to S1P1, 2 or 3 receptors in the heart activates downstream signaling pathways that promote myocyte survival. S1P1 interacts with Gαi, whereas S1P2 and S1P3 couple with Gαq and Gα13, as well as Gαi, in a ligand-dependent manner [57]. Subsequent studies have shown that the S1P1 receptor exhibits the most prominent expression pattern in cardiomyocytes [58, 59]. The S1P1 receptor has been linked to proliferative/survival and migratory signaling in many different cell types [60–62]. The S1P2 and 3 receptors are also important for regulation of vascular tone [63, 64]. Signaling responses to S1P during oxidative stress are described below.
Diverse mechanisms for S1P1 receptor downregulation and trafficking have been described. These include translocation to perinuclear vesicles [65], plasmalemmal caveolae [66], N-glycosylation [67], and ubiquitination [68]. These studies have been performed in cell lines requiring transfection of the S1P1 receptor. In adult mouse cardiomyocytes we have recently found that S1P-mediated activation of ERK 1/2 is desensitized after 1 hour but responses are normal 24 hours later, suggesting receptor recycling [45]. The immune modulator and S1P analogue FTY720 induced internalization and degradation of S1P1 receptors [50, 76]. The timing and extent of S1P receptor internalization are highly dependent on FTY720 concentration [50].
In experimental settings acute effects of S1P include variable blood pressure and heart rate responses [69], calcium dysregulation and myocyte hypertrophy [70, 71], coronary artery vasoconstriction via the S1P2 receptor [72], and bradycardia via the S1P3 receptor [73]. Other reported effects of the synthetic S1P1 agonist SEW 2871 potentially pertinent to cardioprotection include a negative inotropic response in isolated adult mouse ventricular myocytes [74], and exacerbation of reperfusion arrhythmias in an isolated rat heart preparation [75]. Of note is that S1P1 agonist also prevented allograft rejection in a rat heart transplantation model [76] and promoted in vivo angiogenesis in ischemic hindlimbs of mice [77]. Among other important functions of S1P in the cardiovascular system are regulation of cytoskeletal dynamics [78] and early heart development in conjunction with SK [79]. Thus, the S1P1 receptor has been reported to mediate S1P action during cardiac development [80]. Another function of S1P is regulation of stem cell development [81]. Recently it was found that S1P promotes the differential of human umbilical cord mesenchymal stem cells into cardiomyocytes [82].
Serum S1P may also be a predictive marker for the presence and severity of obstructive coronary artery disease in humans [49]. In patients with stable coronary artery disease, Sattler et al. observed that plasma S1P levels normalized for HDL, a major circulating carrier of S1P, were higher than controls [83]. These levels were even higher in patients with acute myocardial infarction [83]. In contrast to these findings, Knapp et al. reported a reduction in the plasma concentration of S1P in a small cohort of patients with acute myocardial infarction compared with controls [84]. These observations are consistent with reduced S1P levels we observed in isolated murine hearts undergoing acute ischemia/reperfusion injury [85]. In another study it was reported that the S1P, dihydro-S1P and c24:1-ceramide levels in HDL correlate inversely with the presence of ischemic heart disease [86]. These findings would seem to be at odds with the results of Sattler et al. [83], but may reflect differences in methodology and patient selection.
4. The Sphingosine Kinase/S1P Pathway in Cardioprotection
4.1 Studies of S1P in cardiomyocyte cell culture
The cardioprotective effect of S1P was first reported in 2001. In neonatal rat cardiac myocytes we found that exogenously applied S1P enhanced cardiac myocyte survival during hypoxia (87). Subsequent studies employed cultured adult mouse cardiac myocytes for hypoxia experiments that serve as a model for in vivo responses resulting from coronary artery occlusion. This system permitted measurements of S1P effects on myocyte viability during stress and activation of cell signaling from plasma membrane to mitochondria. There were three major findings that advanced understanding of S1P prosurvival effects during hypoxia [88]. First, using a selective S1P1 receptor antibody and VPC23019, a commercially available S1P analog which is predominantly an S1P1 receptor competitive antagonist (pKi = 7.86) that also inhibits but has less affinity for S1P3 receptors (pKi = 5.93) [89], it was found that S1P1 receptors are abundantly expressed by adult mouse cardiac myocytes. These findings were confirmed by quantitative real-time PCR assays. Second, exogenously applied S1P enhanced survival during prolonged in vitro hypoxia through mechanisms that required S1P1 receptor function and Gi-dependent activation of the prosurvival kinase Akt (protein kinase B). Finally, Akt-mediated phosphorylation of myocyte substrates that interact with mitochondria, such as GSK-3 and BAD, contributed to cardioprotection. In these studies the selective S1P1 receptor agonist SEW2871 and the S1P analogue FTY720 were as effective as S1P in preserving myocyte viability during hypoxia [88].
In contrast, Means et al. was unable to demonstrate prosurvival signaling mediated by the S1P1 receptor [90]. The divergent observations surrounding the cardioprotective effects of S1P1 agonism may result from methodologic differences. The data strongly suggest that the S1P1 receptor, which is the most abundant S1P receptor subtype in cardiac myocytes, is at least partially responsible for S1P-mediated prosurvival signaling and for maintaining myocyte viability during hypoxia [88], and during hypoxia/reoxygenation [45].
In another report, it was shown that combined deletion of S1P2 and S1P3 receptors augmented infarct size in mice subjected to ischemia/reperfusion injury [91]. In these hearts activation of Akt was markedly attenuated compared with wildtype mice, but the absence of either receptor subtype alone affected neither infarct size nor Akt activation after ischemia/reperfusion injury. S1P augmented Akt activity in control murine myocytes, but was ineffectual in the double knockout cells. Thus, these observations suggest that the less abundant cardiac myocyte S1P receptors (S1P 2 and 3) may also be necessary for cell survival during ischemia/reperfusion injury. Because targeting of the S1P1 receptor gene is lethal to the embryo [92], studies in a conditional cardiac –specific S1P1 receptor gene knockout [93, 94] will be necessary to further delineate the role of the various receptor subtypes in the heart.
In SK1 null ventricular myocytes subjected to in vitro hypoxia, cell death and cytochrome c release were greater than in wild-type controls [29]. Exogenous S1P enhanced survival of both wild-type and SK1 null cells. Monoganglioside GM-1 treatment, which activates PKC and subsequently SK to produce S1P, induced cytoprotection in wild-type cardiac myocytes but not in SK1 null cells. These observations indicate that GM-1 activates SK1, presumably via PKCε-mediated phosphorylation (see below). Interestingly, the beneficial effects of GM-1 on wild-type cardiac myocytes were abolished by pretreatment with either an S1P1 receptor antagonist or pertussis toxin, which ADP-ribosylates and thereby inactivates Gi, suggesting that endogenous S1P was transported to the extracellular space for activation of its cognate G-protein coupled receptors [29]. A potential mechanism for extrusion of S1P is via ABC transporters, which have been demonstrated in a variety of cell types [24, 95], as well as in murine and human hearts [96, 97]. Recently, a specific S1P transporter, SPNS2, which also transports the phosphorylated form of FTY720, has also been described [98].
4.2 Studies on Sphingosine Kinase
As noted above, the monoganglioside GM-1 enhanced the survival of cardiac fibroblasts subjected either to PKC inhibition or C2-ceramide treatment [9]. GM-1 also increased S1P levels, an effect abrogated by the SK inhibitor N,N-dimethylsphingosine [9]. Using isolated adult mouse hearts (Langendorff technique), exogenous S1P and GM-1 separately induced substantial resistance to ischemia-reperfusion injury in wild-type mouse hearts as determined by hemodynamic and infarct size measurements [99]. Lecour et al. reported similar results in isolated rat hearts [100]. The importance of the prosurvival kinase PKCε was emphasized by experiments in which GM-1proved to be ineffective in PKCε null hearts. In addition, GM-1, but not exogenous S1P, stimulated translocation of activated PKCε to myocyte particulate fractions. Nevertheless, exogenously administered S1P was effective both in isolated PKCε null hearts subjected to ischemia/reperfusion injury [99] and in isolated cardiac myocytes from these hearts subjected to hypoxia [29]. Thus, S1P acting at cell surface receptors or activation of intracellular SK confers cardioprotection during acute ischemia/reperfusion injury. These experiments also provided evidence for the postulate that PKC is a critical modulator of SK activity and endogenous S1P production in cardiac tissue.
A subsequent series of experiments directly tested the hypothesis that SK activation mediates ischemic preconditioning (IPC) in isolated mouse hearts [101]. It was determined that IPC sufficient to reduce infarct size in wild-type hearts increased SK localization and activity in tissue membrane fractions. Interestingly, IPC triggered SK translocation to tissue membrane fractions in PKCε null hearts but did not enhance enzymatic activity or decrease infarction size after ischemia/reperfusion [101]. As noted above, N,N-dimethylsphingosine (DMS), the endogenous sphingolipid generated by N-methylation of sphingosine, inhibited tissue SK activity. As predicted,10 µM DMS pretreatment abolished IPC-induced cardioprotection in wild-type hearts [101]. The specificity of DMS as an inhibitor of SK activity is further supported by experiments in isolated transfected cells in which SK1 activity was increased by 10-fold and substantially reduced by DMS treatment [102].
Subsequent experiments identified unpredicted effects of low DMS concentrations on SK [103]. In contrast to moderate dose DMS (10 µM), low dose DMS (0.3 to 1.0 µM) enhanced cytosolic SK activity. Low dose DMS also stimulated translocation of activated PKCε to tissue particulate fractions and reduced cardiac ischemia-reperfusion injury. Importantly, low dose DMS effects were abolished in PKCµ null hearts, and SK1 was found to co-immunoprecipitate with activated PKCµ phosphorylated at serine729. In addition, low dose DMS increased activated Akt phosphorylated at serine473.
Another example of the concentration dependence of molecules usually considered to be inhibitory in the SK/S1P pathway is sphingosine, the immediate precursor of S1P. Although there is abundant evidence that sphingosine is toxic to cells, including cardiac myocytes [104, 105], Vessey et al. recently reported that at lower, more physiologic (submicromolar) concentrations [106], sphingosine was cardioprotective in isolated Langendorff perfused rat hearts subjected to ischemia/reperfusion injury. Unlike S1P, sphingosine-induced cardioprotection appears to be mediated by cyclic nucleotide (protein kinases A and G) dependent pathways [106]. At the higher concentrations usually employed (e.g., 5 µM), sphingosine proved to be cardiotoxic.
We then developed a new solvent-extraction-based radioassay for SK that exhibits equal or greater sensitivity than thin-layer chromatography and HPLC methods, but is much more rapid [107]. Thus, it became feasible to perform numerous time point assays that are necessary when nonsaturating substrate concentrations and interfering enzymes are present in microsomal and mitochondrial fractions. In studies employing this assay it was found that fractionation of cytosolic SK activity by gel filtration chromatography yielded two peaks of activity [107]. The early peak eluted as a 96 kDa protein and reacted with an SK2 antibody but not an SK1 antibody. This is larger than the molecular weight of SK2 based on sequence, and suggests association with an accessory protein. The second peak appeared to be heterogeneous with a molecular weight centered on 46 kDa. This is consistent with the known existence and size of multiple forms of SK1 [2]. This fraction reacted with an SK1 antibody but not an SK2 antibody. Thus these clearly separated enzymes were identified as SK2 and SK1, respectively.
When tested with the classic SK inhibitor N,N-dimethylsphingosine (DMS) the activity of SK2 was unaffected by concentrations as high as 20 µM. Consistent with this observation, DMS was only a partial inhibitor of total cytosolic SK activity [27]. The sphingosine analogue FTY&20 is established as a substrate for SK2 but has a low Vmax and therefore should competitively inhibit SK2 [108]. However, Vessey et al. reported that SK2 was not inhibited by FTY720 [27]. As noted earlier, SK1 was efficiently inhibited by both DMS and FTY720 [27]. Consistent with these observations, FTY720 has subsequently been shown to be a substrate for purified SK1 and a competitive inhibitor of sphingosine [102, 109]. Further, when the cytosolic fraction from an SK1 knockout mouse was tested, residual activity due to SK2 was not inhibited by DMS or FTY720. These observations confirmed the specificity of SK1inhibition, and indicated that the lack of inhibition of SK2 was not an artifact of purification. SK2 from rat liver and spleen was also not inhibited by DMS. In contrast, L-sphingosine was an effective inhibitor of both forms [27]. Taken together, along with data obtained in SK1 null hearts (see below), these observations indicate that DMS inhibits only the SK1 form in the heart. Thus, prior experiments in other cells and tissues in which DMS was used as inhibitor of SK may require reinterpretation.
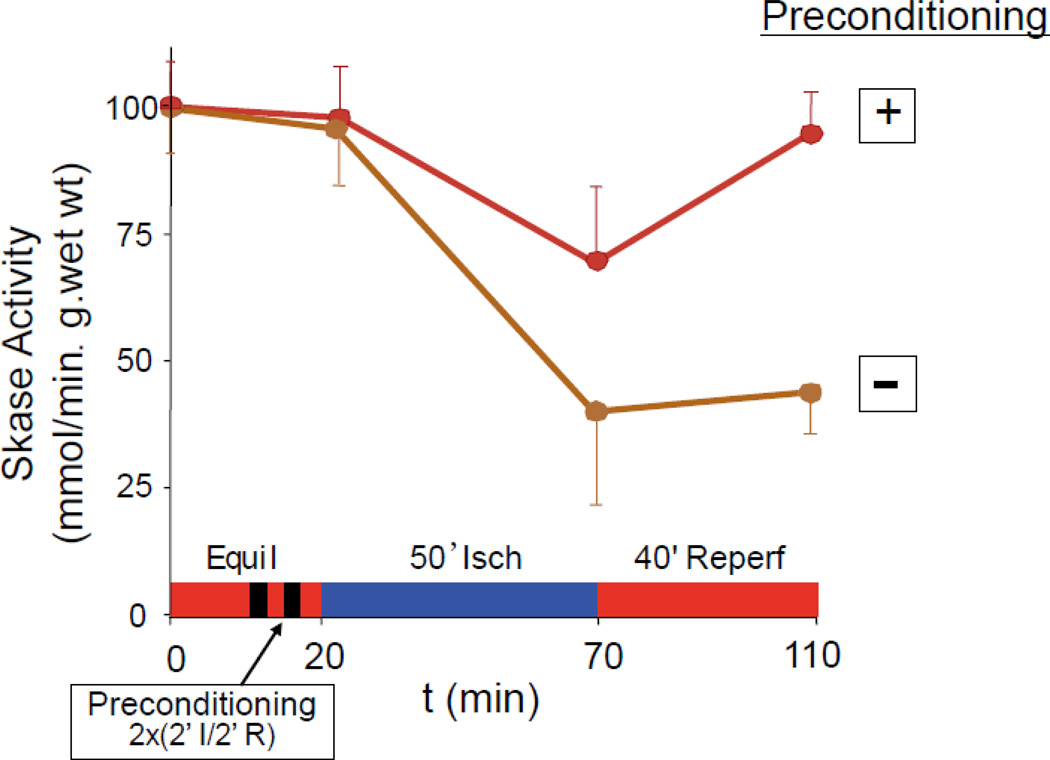
In addition to signal transduction assays for studies of S1P receptor subtype function described above, the time course of SK activity in adult rat hearts subjected to ischemia/reperfusion injury and ischemic preconditioning has been reported [85, Figure 1]. Cytosolic SK activity declined by 61% during ischemia and did not recover upon reperfusion, paralleling effects on left ventricular developed pressure (LVDP). Ischemic preconditioning reduced the decrease in enzyme activity during ischemia by half, and upon reperfusion activity returned to normal. LVDP recovered to 79% of control values and infarct size was reduced. The low baseline specific activity of SK declined by 67% after 45 minutes of ischemia and remained at that level during reperfusion. Ischemic preconditioning restored SK activity almost to normal during reperfusion. Parallel effects were observed in mitochondria from the same hearts [85].
Figure 1.
Schematic representation of the time course of total sphingosine kinase activity in the presence and absence of ischemic preconditioning modified from reference 80. Buffer perfused mouse hearts were equilibrated for 20 min on a Langendorff rig and then subjected to 50 min of ischemia followed by 40 min of reperfusion. SK activities are expressed as pmols of S1P formed per min per g wet weight of heart. SK activity at 75 min and 120 min was significantly different (P<0.05) when preconditioned and non-preconditioned samples were compared by analysis of variance followed by post-hoc testing using the Student Neuman Keuls method (n=5 for each data point).The error bars are the standard deviation. Some hearts received two cycles of ischemic preconditioning in which flow was stopped for 2 min and then resumed after 30 seconds. As can be seen, the preconditioned hearts displayed a smaller decline in enzyme activity which returned to normal by the end of reperfusion, while the non-preconditioned hearts exhibited a much steeper decline in enzyme activity which remained depressed throughout the reperfusion period. Equil = equilibrium; Isch = no flow ischemia; Reperf = reperfusion.
In these experiments [85], total S1P in cardiac tissue was quantified by LC/MS/MS (liquid chromatography followed by tandem mass spectrometry [46]. In non-preconditioned hearts, S1P content declined from baseline following both ischemia and reperfusion. Preconditioned hearts had higher S1P levels after ischemia/reperfusion relative to control hearts [Figure 2]. Treatment of non-preconditioned hearts at reperfusion (pharmacologic postconditioning) with 100 nM S1P improved recovery of LVDP. Thus, maintenance of SK activity resulting in higher S1P levels is critical for recovery from ischemia/reperfusion injury.
Figure 2.
S1P was measured by liquid chromatography and mass spectrometry as detailed in reference 80. Ischemia/reperfusion and ischemic preconditioning were performed as described in the legend to figure 1. As can be seen the S1P level at the end of reperfusion was significantly higher in the hearts that received ischemic preconditioning. This is presumably the result of the higher sphingosine kinase activity at the end of reperfusion as shown in Figure 1. N= 5/group. * = P < 0.01. I/R = ischemia/reperfusion. IPC = ischemic preconditioning.
In mice subjected to permanent left anterior descending coronary artery occlusion, Yeh et al. reported that total SK activity in the remote myocardium steadily declined over weeks [110]. In these studies cardioprotection as evidenced by reduced apoptosis and improved echocardiographic ejection fraction over the first two weeks after infarction was demonstrated for the selective S1P1 receptor agonist SEW2871, which was fed to the mice by oral gavage [110]. These data suggest a potential role for using an oral S1P receptor agonist as S1P “replacement therapy “ for the post-myocardial infarction heart.
Despite strong corroborating evidence that DMS modulates resistance to injury by effects on SK, this agent alters PKC activity [103] and may confound interpretation of experimental data. Accordingly, SK1 knockout mice were employed in a series of subsequent studies [111]. SK2 expression increased in hearts after SK1 gene disruption, resulting in total SK activity half that of wild-type. Although SK1 null hearts exhibited normal hemodynamic performance under baseline conditions, contractile abnormalities and infarction were more severe after ischemia/reperfusion than in wild-type hearts. As predicted, targeted disruption of the SK1 gene abolished IPC-induced cardioprotection [111]. However, exogenous S1P retained the ability to induce cardioprotection in these SK1 null hearts. Despite an increase in SK2 expression in the SK1 null hearts, infusion of DMS did not affect infarct size, confirming prior in vitro experiments, and suggesting that the absence of SK1 rather than the increased presence of SK2 was critical to the loss of cardioprotection in myocardium null for SK1 [111].
In another study, it was reported that prior adenoviral gene transfer of SK1 protected against hemodynamic deterioration, and reduced creatine kinase release and arrhythmias during acute ischemia/reperfusion injury in isolated rat hearts [112]. When gene transfer was performed at the time of acute left anterior descending coronary artery ligation, studies two weeks later revealed improved left ventricular function in the treated mice, reduced infarct size, more neovascularization, and reduced collagen content.
It has been known for some time that the widely used anesthetic agent isoflurane protects against organ damage. Thus, Kim et al. noted that an important mechanism of protection induced by isoflurane is activation of the SK/S1P pathway [113]. Using an ischemia/reperfusion model of renal injury these investigators reported that isoflurane anesthesia reduced the degree of renal failure and necrosis. Mice deficient in SK1 were not protected, and in wild-type mice, protection was abrogated by DMS and the S1P1 antagonist VPC2309. The authors also demonstrated that isoflurane increased SK1 mRNA in HK-2 cells.
Like ischemic preconditioning, ischemic postconditioning is cardioprotective [114], and this observation has recently been extended to patients undergoing percutaneous coronary interventions [115]. To ascertain whether the SK/S1P pathway is a determinant of successful postconditioning, isolated wildtype and SK1 null mouse hearts were subjected to ischemia/reperfusion injury [116]. At the onset of reperfusion hearts selected for treatment underwent three brief cycles of postconditioning (5 sec of ischemia followed by 5 sec of reperfusion). Results were similar to the preconditioning studies cited above: hemodynamics were improved and infarct size was reduced compared to untreated hearts. Phospho-Akt and phospho-ERK were enhanced. In the post-conditioned hearts SK activity at the end of reperfusion was higher compared to hearts that did not receive post-conditioning [Figure 3]. None of these findings were present in SK1 null hearts. Thus SK1 is also critical for successful ischemic postconditioning. In this connection it has recently been found that a ramped ischemic postconditioning protocol combined with low dose sphingosine + S1P given at the time of reperfusion can rescue isolated hearts from as much as 90 min of ischemia [117].
Figure 3.
Schematic representation of the time course of total sphingosine kinase activity in the presence and absence of ischemic post-conditioning based on data in reference 108. Buffer perfused mouse hearts were equilibrated for 20 min on a Langendorff rig and then subjected to 50 min of ischemia followed by 40 min of reperfusion. Some hearts received four cycles of ischemic post-conditioning in which flow was stopped for 5 seconds and then resumed after 5 seconds. As can be seen, the hearts destined to be post-conditioned displayed an identical decline in enzyme activity to the control hearts. In the post-conditioned hearts, enzyme activity returned toward normal by the end of reperfusion, while the hearts that were not post-conditioned exhibited a persistent reduction in enzyme activity. Equil = equilibrium; Isch = no flow ischemia; Reperf = reperfusion.
5. S1P Lyase
S1P lyase (SPL) catalyzes the irreversible degradation of S1P. By reducing available S1P pools, SPL promotes apoptosis under stress conditions, whereas SPL downregulation promotes cells survival. In a series of experiments we demonstrated that SPL was activated by ischemia in murine hearts and that hearts of heterozygous SPL knockout mice exhibited reduced SPL activity, elevated S1P levels, smaller infarct size and increased functional recovery after ischemia/reperfusion injury compared with littermate controls [118]. A small molecule called tetrahydroxybutylimidazole (THI) is an FDA-approved food additive that inhibits SPL. We then tested the ability of THI to modulate ischemia/reperfusion injury in isolated hearts. When given overnight in the drinking water at a dose of 25 mg/l in drinking water, THI raised serum S1P levels by 30–40% and reduced SPL activity, reduced infarct size and enhanced hemodynamic recovery after ischemia/reperfusion in ex vivo hearts [118]. Thus, inhibition of SPL raises S1P modestly in the serum to a level produced by ischemic preconditioning [85]. This level is sufficient to induce cardioprotection, and represents a new target in the metabolism of S1P that can be utilized to guard the heart against ischemic injury.
6. FTY720
FTY720 is derived from myriocin, a component in the Chinese herb Iscaria sinclarii, which has been used as a treatment for asthma (119). FTY720 is a structural homologue of sphingosine, which is the natural sphingolipid present at high nanomolar concentrations in serum (47–49). After endogenous phosphorylation by SK2, FTY720 serves as a potent agonist of S1P1 receptors, and binds less avidly to S1P3 receptors (120, 121). Studies have shown that FTY720 sequesters T and B lymphocytes from the blood into secondary lymphoid organs resulting in profound immunosuppression (122, 123).
As noted above, FTY720 is an FDA approved drug for the oral treatment of patients with multiple sclerosis (31). Prior experimental studies of FTY720 in the heart have been confined to isolated cells and acute preparations. We previously showed that FTY720 enhanced survival in isolated adult murine cardiac myocytes subjected to severe prolonged hypoxia (88). Egom et al. reported that FTY720 prevented ischemia/reperfusion-induced cardiac arrhythmias in an ex vivo rat heart model via activation of p21-activated kinase (Pak1)/AKT signaling (124). The same group subsequently noted that FTY720 protected neonatal rat ventricular cardiomyocytes against CoCl2-induced hypoxic injury and triggered NO release through a pertussis toxin-sensitive PI3K/AKT/eNOS pathway (125). Acting through Pak 1, FTY720 also prevented pressure overload-induced cardiac hypertrophy [126]. In another study if ischemia/reperfusion injury in isolated adult rat hearts, treatment with FTY720 at the time of reperfusion improved recovery of left ventricular developed pressure and reduced left ventricular end-diastolic pressure without affecting infarct size (127). A subsequent in vivo study from the same group using adult rats subjected to left anterior descending coronary occlusion for 45 min followed by 24 hr of reperfusion showed that either pretreatment with FTY720 or pretreatment combined with treatment at the time of reperfusion did not reduce infarct size, had variable effects on ventricular arrhythmias, and reduced granulocyte infiltration and TNFα levels in the border zone of the infarct but not in the serum (128).
In contrast, we have found that chronic oral treatment with FTY720 in adult wildtype C57BL/6J mice subjected to I/R injury enhanced recovery of left ventricular developed pressure and reduced infarct size via persistent stimulation of prosurvival signals [129]. One of the mechanisms by which prolonged in vivo treatment using FTY720 achieves these effects is that S1P1 receptors activated by this agent retain signaling activity for hours in despite quantitative internalization [130]. We have also demonstrated in adult murine cardiac myocytes that exogenous FTY720 induces reversible S1P1-receptor -mediated ERK phosphorylation, indicative of receptor recycling [45]. It is well-recognized that phosphorylation of AKT and ERK is common to both the SAFE and RISK survival pathways described in the heart [131–133].
In contrast to these beneficial responses in animals, it has been recognized that oral administration of FTY720 to humans induces an initial bradycardia. Thus, Kovarik et al. gave FTY720 to 16 normal subjects and recorded a 28% decrease in heart rate for up to 6.5 hours [134]. In mice, Sanna et al. used a non-selective S1P agonist which produced profound bradycardia except in S1P3 −/− animals [73]. As FTY720 actives both S1P1 and S1P3 receptors, it is reasonable to attribute the bradycardia seen in humans to S1P3 receptor agonism. In this connection the US Food and Drug Administration recently issued a Safety Communication that revised recommendations for cardiovascular monitoring in patients receiving FTY720 for treatment of multiple sclerosis [135]. The revised recommendations were prompted by the death of a patient who was monitored the requisite 6 hours after the first dose but died the next day, within 24 hours of receiving the drug. The cause of death was not identified. The patient had extensive brainstem MS lesions and was taking a beta blocker, which also depresses heart rate. Re-evaluation of clinical trial data revealed a biphasic heart rate lowering effect, with a second decrease 12–20 hours after the initial dose. Extended overnight monitoring has now been recommended for patients with a heart rate of < 45 bpm in the first 6 hours after the initial dose or in those who had their lowest heart rate at 6 hours post-dose. Extended monitoring is also recommended for those patients with certain pre-existing conditions, such as QT interval prolongation and in patients receiving drugs that slow the heart rate or depress atrioventricular conduction. FDA advises against the use of this drug in patients with heart conditions or stroke within the previous 6 months, or in those taking anti-arrhythmic drugs. i.e., FTY720 is contraindicated in such patients. These precautionary recommendations were issued despite the lack of clear evidence that the drug played any role in the patient’s death, and in the absence of any data indicating the cardiovascular death rates are higher in patients taking FTY720 than those patients with MS not taking the drug [136].
7. Cardiac fibroblasts and sphingosine kinase
Cardiac fibroblasts are critical for the maintenance of extracellular matrix deposition and turnover in the normal heart and are key mediators of inflammatory and fibrotic myocardial remodeling in the injured and failing heart. As noted above, SK activation is a well-recognized determinant of cell fate in cardiac myocytes and other cells, but SK responses have not previously been studied in cardiac fibroblasts except for the earlier study by Cavallini et al. cited above [9]. Initially it was found that total SK activity is over 10-fold higher in cardiac fibroblasts than in adult mouse cardiac myocytes [136]. In cardiac fibroblasts isolated from SK1 knockout mice, total SK activity was greatly reduced indicating that SK1 is the major isoform expressed in these cells.
To determine whether SK regulates cell proliferation and the proinflammatory protein inducible nitric oxide synthase (iNOS), cultured cardiac fibroblasts were incubated with the cytokine interleukin-1β (IL-1β) in the presence or absence of hypoxia. Hypoxia did not alter fibroblast SK activity, while IL-1β enhanced enzyme activity. In wildtype cardiac fibroblasts, hypoxia induced proliferation, but in SK1 null fibroblasts this response was blunted even in the presence of serum. In contrast, iNOS expression and NO production were enhanced in SK1 null fibroblasts during hypoxia. In wildtype fibroblasts, IL-1β was only a weak inducer of iNOS and of NO accumulation and hypoxia alone had no significant effect on iNOS activation. However, IL-1β in combination with hypoxia stimulated both iNOS and NO production, and this stimulation was enhanced in SK-1 null fibroblasts. Thus, activation of endogenous SK1 serves a dual regulatory function: it is required for optimal cardiac fibroblast proliferation but is a negative modulator of proinflammatory responses during hypoxia [136].
Following myocardial infarction, fibroblasts transform into myofibroblasts and produce extracellular matrix leading to cardiac fibrosis. A recent report indicated that TGF-β-stimulated collagen production in cardiac fibroblasts involves “inside-out” signaling whereby S1P produced intracellularly by SK1 is released and acts in an autocrine/paracrine fashion to activate the S1P2 receptor which results in increased collagen production [137]. Conversely, when SK1 is inhibited, e.g., by the adipose-derived factor, apelin, cardiac fibroblast activation and collagen production are prevented in vitro [138]. In vivo studies using a mouse aortic banding model revealed that apelin inhibited cardiac remodelling by prevention of myocyte hypertrophy, cardiac fibrosis, and left ventricular dysfunction [138].
8. S1P, High Density Lipoprotein (HDL) and Cardioprotection
As noted above, a major carrier of S1P in serum is HDL [47, 48]. HDL is well-recognized for its role in preventing atherogenesis. One mechanism of this effect is the ability of S1P carried by HDL to preserve endothelium and inhibit proinflammatory responses in endothelial and vascular smooth muscle cells [139–141]. When the synthetic sphingosine analogue FTY720 is phosphorylated by SK2, it acts as an agonist at S1P1 and 3 receptors, and has been shown to reduce atherosclerosis in both low-density lipoprotein receptor-deficient mice [142] and in apolipoprotein E-deficient mice [143]. These responses would be expected to confer chronic cardioprotection and might also constitute part of a therapeutic prevention strategy. In this connection, statins have been reported to induce S1P1 receptors and enhance endothelial NO production in response to HDL [144].
In acute studies HDL is cardioprotective [145]. It has been proposed that at least part of this cardioprotection can be attributed to the S1P content of HDL [145]. A postulated mechanism is S1P-mediated suppression of inflammation that includes inhibition of adhesion molecule expression and impaired recruitment of polymorphonuclear cells to the infarcted area [146]. We have recently shown that HDL directly mediates survival in isolated adult murine cardiomyocytes subjected to hypoxia/reoxygenation [147]. This survival effect was abrogated by the S1P1 and 3 receptor antagonist VPC 23019 and by the S1P3 receptor antagonist CAY10444, ADP-ribosylation of Gi by pertussis toxin, by the MEK inhibitor PD-98059, and by the PI-3 kinase inhibitor wortmannin. Both ERK1/2 and Akt (protein kinase B) were activated by S1P-associated HDL, the former via the S1P1 receptor and the latter via the S1P3 receptor [147]. In contrast, HDL induced phosphorylation of GSK-3β, which is known to inhibit the activity of this molecule and is a cardioprotective intervention. Thus, HDL, via its cargo of S1P, directly protects cardiomyocytes against oxidative injury in the absence of vascular effects. In this study prosurvival signaling was dependent on both S1P1 and S1P3 subtype receptors. In neonatal rat ventricular myocytes, both native and reconstituted HDL were protective against doxorubicin-induced apoptosis due to the S1P component of HDL [148]. In this study, protection was mediated by the S1P2 receptor, ERK1/2 and STAT3.
Both genetic alterations in S1P receptors and responses to HDL have provided additional insight into the role of SK/S1P pathways in cardioprotection. In these studies the focus has been on the role of the S1P3 receptor. Nofer et al. showed that HDL induced NO release in human endothelial cells and caused NO-dependent vasorelaxation via the S1P3 receptor [63]. These effects could be suppressed in mice lacking this receptor. Based on these observations, S1P3 appears to be a major regulator of arterial vasodilation as compared with the S1P2 receptor whose stimulation results in vasoconstriction [64].
Thus, HDL would be expected to improve myocardial perfusion and protect the heart against ischemia/reperfusion injury in vivo via the S1P3 receptor. This indeed may be so, but the evidence is somewhat conflicting. Using a radionuclide technique, Levkau et al. reported that human HDL stimulated murine myocardial perfusion in vivo, but this effect was abolished in S1P3 receptor null mice [149]. Moreover, in this study it was reported that S1P inhibited myocardial perfusion through the S1P3 receptor [149]. However, in a subsequent report Theilmeier et al. demonstrated that HDL and its constituent S1P acutely protected the murine heart against ischemia/reperfusion injury via an S1P3-mediated and NO-dependent pathway [150]. As noted above, Means et al. noted that deletion of neither the S1P2 nor the S1P3 receptor alone affected infarct size or Akt activation [91]. However, in double knockout mice, infarct size following ischemia/reperfusion was increased by 50% and Akt activation was markedly attenuated. As described earlier, other work has emphasized the role of the S1P1 receptor in preserving myocyte viability [88]. Although these studies are not in agreement as to how or which S1P receptor subtypes preserve myocardial viability, regulate the coronary circulation, and influence atherosclerosis, they all point to the importance of S1P in cardioprotection and to a critical role for HDL in transporting S1P, both acutely and chronically, to sites within the coronary vasculature. An additional mechanism facilitating such vasoprotection has recently been elucidated when it was reported that an HDL-associated molecule, apolipoprotein M, is a carrier of S1P [151]. Human apoM HDL+ induced S1P1 receptor internalization, downstream MAPK and Akt activation, endothelial cell migration and formation of endothelial adherens junctions, whereas apoM− HDL did not [151].
9. FTY720 and Atherosclerosis
Previous studies in genetically altered mice have suggested that FTY720 could reduce the atherosclerosis burden in the setting of hyperlipidemia. Keul et al. gave Apoe−/− mice FTY720 at a concentration of 1.25mg/kg/day in water for 4 weeks and then fed these mice a “Western” diet for 20 weeks [152]. In that study atherosclerotic lesion volume, macrophage and collagen content, and monocyte chemoattractant protein were markedly reduced. In another report, Nofer et al. administered FTY720 by the intra-peritoneal route to Ldlr−/− mice at a concentration of 0.4mg/kg 3 times a week, resulting in a steady state concentration of 68ng/ml of FTY720 in the plasma, of which 44% was in HDL [153]. In that study mice were fed a high fat diet for 16 weeks. Lesion size and necrotic core formation were reduced, and there were fewer CD3+ T cells in the aortic root. There was also a decrease in the number of T lymphocytes. More recently, Blom et al. reported that FTY720 stimulates 27-hydroxycholesterol production and confers athero-protective effects in human primary macrophages [154]. As such, FTY720 protects macrophages from free cholesterol - induced toxicity. This protection results from inhibition of cholesterol transport to the endoplasmic reticulum, and directs the cholesterol to ApoA1 via ABCA1 whose synthesis is stimulated by 27-hydroxycholesterol via the liver X receptor. This is a non-S1P receptor mediated effect and indicates that FTY720 has properties beyond S1P receptor agonism.
Not all studies have reported an anti-atherogenic effect of FTY720. Thus, Klingenberg et al. fed Apoe−/− mice a normal chow diet and 3mg/kg body weight oral FTY720, for 12 weeks [155]. These mice developed a 2.4-fold increase in serum cholesterol. Interestingly, this dose of FTY720 also increased plasma S1P by 14%. There were no changes in atherosclerotic lesions in either early or established atherosclerosis. As indicated above, phosphorylated FTY720 is an agonist for S1P3 receptors, albeit at a much higher EC50 value than at S1P1 receptors [121]. In this connection, Keul et al. recently reported that the S1P3 receptor mediates the chemotactic effect of S1P in macrophages in vitro and in vivo and plays a causal role in atherosclerosis by promoting inflammatory monocyte/macrophage recruitment and altering smooth muscle cell behavior [156]. In a recently completed study using severely atherosclerotic ApoeR61h/h/SRB1−/−mice [158], we found placing FTY720 in the drinking water significantly extended the longevity of these animals [129]. After 4 weeks 10/29 control mice died, while only 1/22 mice treated with FTY720 died (P < 0.02).
In summary, during the past few years a plethora of new information identifying the importance of sphingolipid signaling pathways in the cardiovascular system has accumulated. The potential for the development of new therapeutic agents based on this understanding is high, but much work, especially in larger animal models, needs to be performed.
Highlights.
The S1P/sphingosine kinase pathway is a key participant in endogenous cardioprotection.
Both isoforms of sphingosine kinase (SK1 and SK2) are necessary for optimal cardioprotection.
Ischemic pre-conditioning preserves both sphingosine kinase activity and S1P levels.
The immunomodulator FTY720 mimics S1P effects in the heart.
Acknowledgments
Grant support: 1P01 HL 68738 and R01 HL 090606 from the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cuvillier O, Pirianov G, Keluser B, Vanek PG, Coso A, Gutkind S, Spiegel SS. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 2.Imamura T, Ohgane J, Ito S, Ogawa T, Hattori N, Tanaka S, Shiota K. CpG island of rat sphingosine kinase-1 gene: tissue-dependent DNA methylation status and multiple alternative first exons. Genomics. 2001;76:117–125. doi: 10.1006/geno.2001.6607. [DOI] [PubMed] [Google Scholar]
- 3.Okada T, Ding G, Sonoda H, Kajimoto T, Hga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Sugiura M, Nava VE, Edsal LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol. Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 5.Alemany R, van Koppen CJ, Dannebeg K, ter Braak M, Meyer zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn-Schmiedeberg’s Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 6.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allende ML, Sasaaki T, Kawai H, Olivera A, Mi Y, Echten-Deckert G, Hajdu R, Dosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 8.Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini L, Venerando R, Miotto G, Alexandre A. Ganglioside GM1 protection from apoptosis of rat heart fibroblasts. Arch Biochem Biophys. 1999;370:156–162. doi: 10.1006/abbi.1999.1378. [DOI] [PubMed] [Google Scholar]
- 10.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK ½-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D’Andreaa RJ, Gamble J JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Okada T, Hayashi S, Hahangeer S, Miwa N, Nakamura S. Delta-catenin/NRRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. Biochem J. 2004;382:717–723. doi: 10.1042/BJ20040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maceyka M, Payne SG, Milstien S, Spiegel S. Aminocyclase 1 is a sphingosine kinase 1-interacting protein. FEBS Lett. 2004;568:30–34. doi: 10.1016/j.febslet.2004.04.093. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq TM, Moretti PA, Vadas MA, Pitson SM. Eukaryotic elongation factor 1 interacts with sphingosine kinase and directly enhances its catalytic activity. J Biol Chem. 2008;283:9606–9614. doi: 10.1074/jbc.M708782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacana E, Maceyka M M, Milstien S, Spiegel S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. J Biol Chem. 2002;277:32947–32953. doi: 10.1074/jbc.M202841200. [DOI] [PubMed] [Google Scholar]
- 16.Kovanich D, van der Heyden MA, Aye TT TT, Van Veen TA, Heck AJ, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type 1 cAMP-dependent protein kinase. Chembiochem. 2010;11:963–971. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda Y, Aoyama Y, Wada A, Igarashi Y. Identification of PECAM-1 association with sphingosine kinase 1 and its regulation by agonist-induced phosphorylation. Biochim Biophys Acta. 2004;1636:12–21. doi: 10.1016/j.bbalip.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Yan G G, Ren A, You B, Liao JK. FHL2/SLIM3 decreases cardiomyocyte survival by inhibitory interaction with sphingosine kinase-1. Circ Res. 2006;99:468–476. doi: 10.1161/01.RES.0000239410.65551.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi H, Hankagami H, Takami Y, Kolriyama H, Mori M, Tamai K, Sun J, Nagao K, Morishita M, Kaneda Y. FHL-2 suppresses VEGF-induced phosphatidylinositol 3-kinase/Akt activation via interaction with sphingosine kinase-1. Arteriosclero Thromb Vasc Biol. 2009;29:909–914. doi: 10.1161/ATVBAHA.108.178541. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi S, Okada T, Igararshi N, Fujita T, Jahngeer S, Nakamura S. Identification and characterization of RPK118, a novel sphingosine kinase-1-binding protein. J Biol Chem. 2002;277:33319–33324. doi: 10.1074/jbc.M201442200. [DOI] [PubMed] [Google Scholar]
- 21.Melendez AJ, Khaw AK AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 22.Meyer zu Heringdorf D. Lysophospholipid receptor-dependent and –independent calcium signaling. J Cell Biochem. 2004;92:937–948. doi: 10.1002/jcb.20107. [DOI] [PubMed] [Google Scholar]
- 23.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 25.Pchejetski D, Kunduzova O, Dayon A, Calise D, Suguelas M-H, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 26.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 27.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Molec Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 28.Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 29.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Maceyka M, Sankkala H, Hait N NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and Sphk2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier D, Hafler D DA. Fingolimod for multiple sclerosis. New Engl J of Medicine. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- 32.Vessey DA, Li L L, Jin Z-Q, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011 doi: 10.1155/2011/961059. 961059. Epub 2011 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vessey DA, Li L, Imhof I, Honbo N, Karliner JS. FTY720 postconditions isolated perfused heart by a mechanism independent of sphingosine kinase 2 and different from S1P or ischemic preconditioning. 2012 doi: 10.12659/MSMBR.883877. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez L, Paillard M, Price M, Chen Q, Teixeira G, Spiegel S, Lesnefsky EJ. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol. 2011;106:1341–1353. doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends in Biochemical Sciences. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Wattenberg BW. Role of sphingosine kinase localization in sphingolipid metabolism. Role of sphingosine kinase localization in sphingolipid signaling. World Journal of Biological Chemistry. 2010;26:362–368. doi: 10.4331/wjbc.v1.i12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitson SM, Morretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hengst JA, Guilford JM, Fox TE, Wang X, Conroy EJ, Yun JK. Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Arch Biochem Biophys. 2009;492:62–73. doi: 10.1016/j.abb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarman KE, Moretti PA, Zebol JR, Pitson SM. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium-and integrin-binding protein 1. J. Biol. Chem. 2020;285:483–492. doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol & Therap. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 44.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 45.Tao R, Hoover HE, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated ERK signaling and desensitization. J Cardiovasc Pharmacol. 2009;53:486–494. doi: 10.1097/FJC.0b013e3181a7b58a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng Y-W, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 47.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ul M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- 48.Okajma F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: Is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 49.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 50.Tani M M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res. 2005;46:2458–2467. doi: 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 53.Bünemann M, Brandts B, Meyer zu Heringdorf D, van Koppen CJ, Jakobs KH, Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 55.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipid-receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 56.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 57.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima N, Cavalli AL, Biral D, Glembotski CC, McDonough PM, Ho PD, Betto R, Sandora D, Palade PT, Dettbarn CA, Klepper RE, Sabbadini RA. Expression and characterization of Edg-1 receptors in rat cardiomyocytes: calcium deregulation in response to sphingosine 1-phosphate. Eur J Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- 59.Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- 60.Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, Shigematsu H, Takuwa Y. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Rasmitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 62.Kon J, Sato K, Watanabe T, Tomura H, Kuwabara A, Kimura T, Tamama K, Ishizuka T, Murata N, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J Biol Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- 63.Nofer JR, Van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Dodecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular duysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–R446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 65.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- 67.Kohno T, Wasa A, Igarashi Y. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 2002;16:983–992. doi: 10.1096/fj.01-0809com. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 69.Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–1103. doi: 10.1002/jcb.20129. [DOI] [PubMed] [Google Scholar]
- 70.Nakajima N, Cavalli AL, Biral D, Glembotski CC, McDonough PM, Ho PD, Eto R, Sanona D, Palade PT, Dettbard CA, Klepper RE, Sabbadini RA. Expression and characterization of Edg-1 receptors in rat cardiomyocytes. Calcium deregulation in response to sphingosine 1-phosphate. Eur J Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- 71.Robert P, Tsui P, Laville MP, Livi GP, Sarau JHM, Bril A, Berrebi-Bertrand I. EDG1 receptor stimulation leads to cardiac hypertophy in rat neonatal myocytes. J Mol Cell Cardiol. 2001;33:1589–1606. doi: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- 72.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 73.Sanna MG, Liao J, Jo E, Alfonso C, Ahn M-Y, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 74.Landeen LK, Dederko DA, Kondo CS, Hu BS, Aroonsakool N, Haga JH, Giles WR. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H736–H749. doi: 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- 75.Tsukada YT, Sanna MG, Rosen H, Gottlieb RA. S1P1-selective agonist SEW2871 exacerbates reperfusion arrhythmias. J Cardiovasc Pharmacol. 2007;50:660–669. doi: 10.1097/FJC.0b013e318157a5fe. [DOI] [PubMed] [Google Scholar]
- 76.Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, Hinterding K, Nuesslein-Hildesheim B, Tuntland T, Lefebvre S, Liu Y, Gao W, Chu A, Brinkmann V, Bruns C, Streiff M, Cannet C, Cooke N, Gray N. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chemistry & Biology. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Oyama O, Sugimoto N, Qi X, Takuwa N, Mizugishi K, Koizumi J, Takuwa Y. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res. 2008;78:301–307. doi: 10.1093/cvr/cvn002. [DOI] [PubMed] [Google Scholar]
- 78.Donati C, Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: Implications in its biological response. Biochim Biophys Acta. 2006;1758:2037–2048. doi: 10.1016/j.bbamem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Wendler CC, Rivkees SA. Sphingosine-1-phosphate inhibits cell migration and endothelial to mesenchymal cell transformation during cardiac development. Develop Biol. 2006;291:264–277. doi: 10.1016/j.ydbio.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Poulsen RR, McClaskey CM, Rivkees SA, Wendler CC. The sphingosine-1-phosphate receptor 1 mediates S1P action during cardiac development. BMC Dev Biol. 2011;11:37. doi: 10.1186/1471-213X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pebay A, Bonder CS, Pitson SM. Stem cell regulation by lysophospholipids. Prostaglandins & other Lipid Mediators. 2007;84:83–97. doi: 10.1016/j.prostaglandins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Z, Chen Z, Zhao X, Pan F, Cai M, Wang T, Zhang H, Lu JR, Lei M. Sphingosine-1-phosphate promotes the differentiation of human umbilical cord mesenchymal stem cells into cardiomyocytes under the designated culturing conditions. J Biomed Sci. 2011;18:37. doi: 10.1186/1423-0127-18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832. doi: 10.1007/s00395-010-0112-5. [DOI] [PubMed] [Google Scholar]
- 84.Knapp M, Baranowski M, Czarnoswski D, Lisowska A, Zabieiski P, Gorski J, Musial W. Plasma sphingosine-1-phosphate concentration is educed in patients with myocardial infarction. Med Sci Monit. 2009;15:CR490–CR493. [PubMed] [Google Scholar]
- 85.Vessey DA, Kelley M, Li l, Huang Y, Zhou H-Z, Zhu B-Q, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Mont. 2006;12:318–324. [PubMed] [Google Scholar]
- 86.Argraves KM, Sethi AA, Gazzolo PJ, Wilerson BA, Remaley AT, Tybjaerg-Hansen A, Nordestgaard GB, Yeatts SD, Nicholas KS, Barth JL, Argraves WS. S1P, dihydro-S1P and c24:1 ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011;10:70. doi: 10.1186/1476-511X-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-posphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Honbo N, Goetzl EJ, Chatterjee K K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Amer J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 89.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 90.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954–11963. doi: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Means CK, Xiao C-Y, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown J JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 92.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 93.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 94.Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetoa G, Borowski C, Bendelac A, Proia RL. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. 2008;22:307–315. doi: 10.1096/fj.07-9087com. [DOI] [PubMed] [Google Scholar]
- 95.Lee Y-M, Venkatgaraman K, Hwang S-I, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins & other Lipid Mediators. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mungrue IN, Zhao P, Yao Y, Meng H, Rau C, Havel JV, Gorgels TG, Bergen AA, MacLellan WR, Drake TA, Costrom KI, Lusis AJ. ABcc6 deficiency causes increased infarct size and apoptosis in a mouse cardiac ischemia-reperfusion model. Arterioscler Thromb Vasc Biol. 2011;31:2806–2812. doi: 10.1161/ATVBAHA.111.237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solbach TF, Paulus B, Weyand M, Eschenhagen T, Zolk O, Fromm MF. ATP-binding cassette transporters in human heart failure. Naunyn Schmiedebergs Arch Phaarmaol. 2008;377:231–243. doi: 10.1007/s00210-008-0279-6. [DOI] [PubMed] [Google Scholar]
- 98.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin Z-Q, Zhou H-Z, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 100.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNFα and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 101.Jin Z-Q, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 102.Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin Z-Q, Karliner JS. Low dose N,N-dimethylsphingosine is cardioprotective and activates cytosolic sphingosine kinase by a PKCε dependent mechanism. Cardiovasc Res. 2006;71:725–734. doi: 10.1016/j.cardiores.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 104.McDonough PM, Yasui K, Betto R, Salviati G, Glemostski CC, Palade PT, Sabbadini RA. Control of cardiac Ca2+ levels. Inhibitor actions of sphingosine on Ca2+ transients and L-type Ca2+ channel conductance. Circ Res. 1994;75:981–989. doi: 10.1161/01.res.75.6.981. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki E, Handa K, Toledo MS, Hakomori S S. Sphingosine-dependent apoptosis: A unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci USA. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre-and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Molec Toxicol. 2008;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 107.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Analytical Biochem. 2005;337:136–142. doi: 10.1016/j.ab.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 108.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 109.Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, Pyne NJ. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem. 2011;286:18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeh CC, Malhotra D, Huang MC, Zhu B-Q, Goetzl EJ, Vessey DA, Karliner JS, Mann MJ. Sphingolipid signaling and treatment during remodeling of the uninfarcted ventricular wall after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1193–H1199. doi: 10.1152/ajpheart.01032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin Z-Q, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 112.Duan H-F, Wang H, Yi J, Liu H-J, Zhang Q-W, Li L-G, Zhang T, Lu Y, Wu C-T, Wang L-S. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion –induced injury and attenuates its post-ischemic failure. Human Gen Therap. 2007;18:1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 113.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Amer J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 114.Zhao ZQ, Corvera JS, Jalos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 115.Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Gouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 116.Jin Z-Q, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/rperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 117.Vessey DA, Li L, Kelley M, Karliner JS JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, Borowsky AD, Dillard L, Karliner JS, Saba JD. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300:H1753–H1761. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. Fungal metabolites: Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 120.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 121.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76(8) Suppl 3:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- 122.Brinkmann V, Chen S, Feng L, Pinschewer D, Nikolova Z, Hof R. FTY720 alters lymphocyte homing and protects allografts without inducing general immunosuppression. Transplant Proc. 2001;33:530–531. doi: 10.1016/s0041-1345(00)02126-6. [DOI] [PubMed] [Google Scholar]
- 123.Brinkmann V, Pinschewer D, Chiba K, Feng L. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol Sci. 2000;21:49–52. doi: 10.1016/s0165-6147(99)01419-4. [DOI] [PubMed] [Google Scholar]
- 124.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/AKT signaling. J Mol Cell Cardiol. 2010;48:406–414. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, Stringer SE, Ke Y, Shaheen M, Wang T, Chacko S, Wang X, Solaro RJ, Fath-Ordoubadi F, Cartwright EJ, Lei M. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–H1495. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, Neyses L, Solaro RJ, Ke Y, Cartwright EJ, Lei EM, Wang X. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia/reperfusion. Cardiovascular Research. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 128.Hofmann U, Hu K, Walter F, Burkard N, Ertl G, Bauersachs J, Ritter O, Frantz S, Bonz A. Pharmacological pre- and post-conditioning with the sphingosine-1-phosphate receptor modulator FTY720 after myocardial ischaemia/reperfusion. Br J Pharmacol. 2010;160:1243–1251. doi: 10.1111/j.1476-5381.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang G, Kim RY, Imhof I, Honbo N, Li K, Kumar N, Luk FS, Zhu B-Q, Karliner JS, Raffai RL. Immunosuppression by oral FTY720 is cardioprotective and extends longevity in a mouse model of severe coronary atherosclerosis. 2012 Submitted. [Google Scholar]
- 130.Mullershausen F, Zecri F F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 131.Hausenloy D, Yellon D. New directions for protecting the heart against ischemia/reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 132.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 133.Somers SJ, Frias M, Lacerda L, Opie LH, Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012 Mar 6; doi: 10.1007/s10557-012-6376-2. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 134.Kovarik JM, Riviere GJ, Neddermann D, Maton S, Hunt TL, Schmouder RL. A mechanistic study to assess whether isoproterenol can reverse the negative chronotropic effect of fingolimod. J Clin Pharmacol. 2008;48:303–310. doi: 10.1177/0091270007312903. [DOI] [PubMed] [Google Scholar]
- 135.FDA Drug Safety Communication: Revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolomid) Issued 05-14-2012. http://www.fda.gov/Drugs/Drug Safety/ucm303192.htm.
- 136.Kacimi R, Vessey DA, Honbo N, Karliner JS JS. Adult cardiac fibroblasts null for sphingosine kinase-1 exhibit growth dysregulation and an enhanced proinflammatory response. J Mol Cell Cardiol. 2007;43:85–91. doi: 10.1016/j.yjmcc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 137.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc Res. 2009;82:303–312. doi: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pchejetski D, Foussai C, Alfarano C, Lairez O, Caliise D, Guilbeaau-Frugier C, Schaak S, Seguelas MH, Wanecq E, Valet P, Parini A, Kunduzova O. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J. 2011 Oct 25; doi: 10.1093/eurheartj/ehr389. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 139.Nofer J-R, Levkau B, Wolinska I, Junder R, Fobker M, von Ecardstein A, Seedorf U, Assmann G. Suppression of endothelial ell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276:34480–34485. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 140.Kimura T, Sato K, Malchinkhuu E, Tomura T, Tamma K, Kuabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arteriorscler Thromb Vasc Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- 141.Tolle M, Levkau B, Kleuser B, van der Giet M. Sphingosine-1-phosphate and FTY720 as anti-atherosclerotic lipid compounds. Europ J Clin Invest. 2007;37:171–179. doi: 10.1111/j.1365-2362.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- 142.Nofer J-R, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 143.Keul P, Tolle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 144.Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosak H. Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br J Pharmacol. 2007;150:470–479. doi: 10.1038/sj.bjp.0707114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Keul P, Sattler K, Levkau B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail Rev. 2007;12:301–306. doi: 10.1007/s10741-007-9038-x. [DOI] [PubMed] [Google Scholar]
- 146.Argraves KM, Argraves WS. HDL serves as an S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–2333. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 147.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, Raffai R. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010;298:1022–1028. doi: 10.1152/ajpheart.00902.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frias MA, Lang U, Gerber-Wicht C, James RW. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc Res. 2010;85:118–126. doi: 10.1093/cvr/cvp289. [DOI] [PubMed] [Google Scholar]
- 149.Levkau B, Hermann S, Theilmeier G, van der Diet M, Chun J, Schober O, Schafers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004;110:3355–3359. doi: 10.1161/01.CIR.0000147827.43912.AE. [DOI] [PubMed] [Google Scholar]
- 150.Theilmeier G, Schmidt C, Hermann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermman S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbe R, Chun J, Levkau B. High density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 151.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlbäck B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci USA. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keul P, Tölle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet m, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 153.Nofer J-R, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 154.Blom T, Bäck N, Mutka AL, Bittman R, Li Z, de Lera A, Kovanen PT, Diczfalusy U, Ikonen E. FTY720 stimulates 27-hydroxycholesterol production and confers atheroprotective effects in human primary macrophages. Circ Res. 2010;106:720–729. doi: 10.1161/CIRCRESAHA.109.204396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klingenberg R, Nofer J-R, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-phosphate analogue FTY720 causes lymphocyte redistribution and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2392–2399. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 156.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, Heusch G, Levkau B B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 157.Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M. Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation. 2005;111:3457–3464. doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]