Abstract
The fact that reproductive factors have significant influence on the risk of breast cancer is well known. Early age of first full-term birth is highly protective against late-onset breast cancers, but each pregnancy, including the first one, increases the risk of early-onset breast cancer. Estradiol and progesterone induce receptor activator of NF-kappa B ligand (RANKL) in estrogen receptor (ER)- and progesterone receptor (PgR)-positive luminal cells. RANKL then acts in a paracrine fashion on the membranous RANK of ER/PgR-negative epithelial stem cells of the breast. This reaction cascade is triggered by chorionic gonadotropin during the first trimester of pregnancy and results in the morphological and functional development of breast tissue. On the other hand, the administration of non-steroidal anti-inflammatory drugs in the early steps of weaning protects against tumor growth through reduction of the acute inflammatory reaction of post lactation remodeling of breast tissue. This is experimental evidence that may explain the short-term tumor-promoting effect of pregnancy. The protective effect of prolonged breast feeding may also be explained, at least in a part, by a reduced inflammatory reaction due to gradual weaning. Delay of first birth together with low parity and short duration of breast feeding are increasing social trends in developed countries. Therefore, breast cancer risk as a result of reproductive factors will not decrease in these countries in the foreseeable future. In this review, the significance of reproductive history with regard to the risk of breast cancers will be discussed, focusing on the age of first full-term birth and post lactation involution of the breast.
Keywords: Breast cancer risk, Reproductive history, First full-term birth, Post lactation involution, Breast feeding
Introduction
The classification of breast cancers according to the molecular profile of gene expression patterns has improved the fundamental understanding and the rational strategy of research and treatment of patients with breast cancer [1]. Breast cancers have been classified into 5 subtypes: luminal-A, luminal-B, Her2-enriched, basal-like, and claudin-low [2]. The alternative but practical classification using immunohistochemical staining of estrogen receptor (ER), progesterone receptor (PgR), and Her2 protein has been used worldwide [3]. Hormone dependency, per se, is a well-known important characteristic of breast cancers [4]. Therefore, the actual treatment of patients with breast cancer has often been based on the cancer subtype classification [5].
On the other hand, individual patient differences, especially regarding their hormonal environment due to age, menopausal status, pregnancy, and/or lactation, have also been considered mainly from the viewpoint of diagnosis and treatment [6]. However, the fundamental and clinical importance of postpartum involution of breast tissues as a tumor-promoting risk factor has recently received a great deal of attention [7, 8]. Expression of inflammation-related genes, such as COX2 [9], Stat3 [10], and tenascin C [11, 12], has been investigated, and the suppression of the resulting inflammatory reactions with non-steroidal anti-inflammatory drugs (NSAIDs) was reported to protect against tumor invasion and metastasis [13]. Thus, postpartum involution, together with the molecular subtypes may have a significant impact on the prognosis of patients, especially those who are premenopausal.
It is well known that pregnancy and breast feeding have dual effects on breast cancer development [14, 15]. Although early age of first full-term birth is highly protective against late occurrence of hormone-dependent breast cancers [16–19], each successive pregnancy of multiparous women has a progressive effect on breast cancers irrespective of hormone dependency [20]. This review will discuss the significance of pregnancy and lactation as risk factors for breast cancer.
Age of first full-term birth
In a worldwide case–control study, MacMahon [14] was the first to report the protective effect of early age of first full-term birth against breast cancer. Compared to nulliparous women, mothers with their first full-term birth before 20 years of age had a 50 % reduced risk of breast cancer. On the other hand, those who had their first baby after age 35 had a 22 % increased risk. These relative risks were comparable across countries. The protective effect of early age of first full-term birth in parous women was similarly observed in other series from the USA [21] and Japan [22]. Except for one study from Japan [23], many reports observed a protective effect of early age of first full-term birth on hormone receptor-positive cancers [16, 18, 19, 24]. A meta-analysis of 9 cohort or case–control studies also revealed a reduced risk among patients with hormone receptor-positive cancers [17]. This protective effect was also observed in lobular carcinomas that are often positive for hormone receptors [25]. In this context, the relative protective effect of young age of first full-term birth has been observed mainly for postmenopausal women [17, 23, 24, 26].
Parity-related functional and morphological changes of breast tissue have been extensively studied by Russo and co-workers. The gene expression signature and differentiation-related chromatin remodeling in the breast tissues of parous women were distinct from those of nulliparous women [27]. Chorionic gonadotropin is secreted mostly during the first trimester of pregnancy and stimulates the ovarian granulosa-lutein cells to form the corpus luteum of pregnancy. The high levels of progesterone from the corpus luteum are critical to regulate the initial stages of the pregnancy, including breast development to prepare for a fully functional organ. Human chorionic gonadotropin (hCG) administration induced differentiation-related gene expression changes in breast epithelial cells in culture [28], and the profiles were similar in pregnancy and after hCG administration [29, 30]. On the other hand, a prolonged suppressive effect on carcinogen-induced mammary tumor formation was observed with the administration of hCG in rats [31]. A similar protective effect of hCG was observed in humans in a case–control study from Sweden [32]. Women over 50 years of age with higher blood levels of hCG during their first full-term pregnancy showed a 33 % reduced risk of breast cancer. A possible decrease in the number of mammary epithelial stem cells after pregnancy was reported in transplantation experiments [33]. Thus, the high progesterone conditions induced by the hCG seem to be essential to pregnancy-associated breast development, presumably as a result of mammary epithelial stem cell expansion.
Progesterone acts after binding to the PgR, which is in turn induced by estrogen action via ER binding. There had been, however, great controversy about the presence or absence of ER and PgR in mammary epithelial stem cells. Chimeric ER-positive and ER-negative cells were demonstrated using a biostaining technique in ER−/− mouse mammary tissue by the transplantation of cells from wild-type mice [34]. Unexpectedly, the colony forming ability that was assumed to be one of the stem cell characteristics was distinctly observed not in the ER/PgR-positive luminal cell population, but in the negative basal cell population [35]. In 2010, the detailed mechanisms of hormone action on the development of mammary tissues during pregnancy were clarified by 2 different groups [36, 37] (Fig. 1). Elevated progesterone, which is induced by the increased hCG from the trophoblast after the first trimester of pregnancy, acts on the ER/PgR-positive luminal cells and induces receptor activator of NF-kappa B ligand (RANKL). RANKL acts in a paracrine fashion on the ER/PgR-negative mammary stem cells that express RANK on their cell membrane and promotes expansion through NF-kappa B activation. This relationship of proliferation between ER/PgR-positive and -negative cells was also observed in hormone-dependent mammary carcinogenesis [38, 39] and metastasis [40]. In the case of breast cancer, RANKL may act as an autocrine fashion, or the RANK–RANKL pathway may act without the intervention of progesterone. However, there is fundamental consensus about the significance of this pathway at least in the case of breast expansion associated with pregnancy.
Fig. 1.
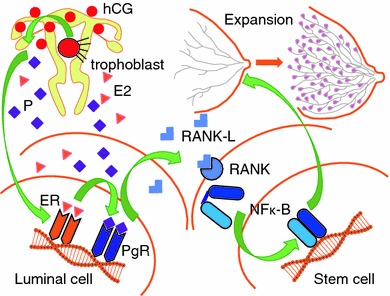
Hormonal induction of stem cell expansion for lactation triggered by hCG from the trophoblast. hCG human chorionic gonadotropin, E2 estradiol, P progesterone, ER estrogen receptor, PgR progesterone receptor, NFk-B nuclear factor kappa B, RANK receptor activator of NFk-B, RANKL RANK ligand
Post lactation involution and risk of breast cancer
Early in 1980, Woods and co-workers reported an important observation about parity and breast cancer. Among 371 consecutive patients with breast cancer, the mean age of diagnosis of parous women (57.9) was significantly younger than that of nulliparous women (63.1). Additionally, the mean age of diagnosis fell with increasing parity and almost 40 % of women with 3 or more children were diagnosed at less than 50 years of age [15]. This observation suggested the protective effect of parity on late-onset (postmenopausal) breast cancers together with a promoting effect on cancers occurring at younger ages (premenopausal). A transient postpartum increase in breast cancer was also reported in women younger than 50 years old [41], and premenopausal women [42]. A meta-analysis of 8 reports from northern European countries revealed that a higher age of first childbirth correlated with a higher risk of breast cancer between the ages of 35 and 54 [43]. The risk of breast cancer was increased until 15 years post delivery and decreased thereafter. The transient risk increase was more prominent in women with their first full-term birth at age 35 or older [44].
The worse prognosis of breast cancers diagnosed during pregnancy or lactation had been explained as being due to the difficulty of diagnosis and treatment [6, 20, 45, 46]. However, prognosis of those occurring shortly after delivery seemed to be even worse. Survival rates improved with increasing interval between their last birth and diagnosis [47]. The worse prognosis was more significant in patients younger than 35 years old [48]. The dual effect of pregnancy was also confirmed by a cohort study [49]. Very interestingly, women with higher blood hCG levels during the first trimester of their first full-term pregnancy had a higher risk of breast cancer occurring at a young age (age less than 40; low hCG vs. high hCG, 1.00 vs. 1.78) or shortly after delivery (less than 10 years; low hCG vs. high hCG, 1.00 vs. 4.33) [32].
The terminal duct lobular units (TDLUs) of breast tissue decrease in size and number during age-related involution. Histologically demonstrated spontaneous involution was correlated with a reduced risk of late-onset breast cancers [50]. However, since the breast tissues must provide for a possible next pregnancy after weaning, the post lactation involution of the breast is distinct from that of aging. The process begins with a cell death phase and progresses to the tissue remodeling phase [51]. Gene expression profiling revealed the induction of the Stat3-mediated cell death-related pathway at the initial step, followed by an acute phase inflammatory reaction [7, 52]. The involuting breast tissues accumulated fibrillar collagen and stimulated migration and metastasis of inoculated breast cancer cells [8]. The prognostic significance of tumor-associated macrophages has been reported in patients with breast cancer [53]. Accumulated collagen acts as an extracellular matrix mediator to recruit a tumor-promoting subtype of macrophage in the involuting breast tissues [54]. The macrophage and mast cell infiltration was observed at the late phase of involution with a chronic inflammatory reaction [10] which is controlled by TIMP3 via TNF expression [55].
Tenascin C is an extracellular matrix glycoprotein often upregulated in breast cancers [56]. The downregulation of tenascin C acts as a suppressor of the epithelial to mesenchymal transition, which is important in tumor invasion and metastasis [57]. Tenascin C suppresses Stat5 signaling and stimulates the WNT and NOTCH pathways. It is abundantly expressed at the metastatic front facilitating migration and metastasis of the tumor-initiating cells [12]. Significant accumulation of tenascin C together with collagen fiber in the involuting breast has been observed [11]. The administration of NSAIDs suppresses the progression of breast cancer cells through downregulation of tenascin C expression. These specific characteristics of the post lactation involution of breast tissue that make it a risk factor for cancer were reviewed by Lyons and co-workers who suggested the importance of an inflammatory reaction [9]. COX2 expression was stimulated not only breast tissue itself but also in inoculated breast cancer cells [13]. NSAIDs administration suppressed the COX2 expression and decreased the deposition of fibrillar collagen, resulting in a decline of tumor progression and metastasis.
Kreuzaler and co-workers reported a new concept about the Stat3-mediated cell death-related pathway at the initial step of the post lactation involution [58]. Stat3 induced lysosomal protease independently from the caspase-dependent apoptotic pathway and increased lysosomal membrane permeability. The increased lysosomal proteases digest cell membranes and induce cell death without nuclear fragmentation that is typical in apoptosis. They proposed, therefore, a necrosis type of new programmed cell death pathway in this physiological but special tissue remodeling procedure of post lactation involution.
Breast feeding
Additional important factors relating to pregnancy are breast feeding and number of pregnancies. A longer duration of breast feeding is correlated with a lower risk of breast cancer [16, 59]. Although the risk-reducing effect has been observed only for premenopausal women in reports of small series it was also observed for pre- and postmenopausal women in a large series [16] and in a meta-analysis of 47 reports from all over the world [59]. Periodic influence of estrogen/progesterone on breast tissue can be postponed by prolonged breast feeding or increased number of pregnancies. If this hormonal environment of the post delivery period influences the risk of breast cancer, it should be restricted to ER/PgR-positive cancers. However, the protective effect was observed in both ER/PgR-positive and -negative cancers [16]. Instead, longer duration of breast feeding reduced the risk of triple-negative cancers but not of luminal cancers defined by immunohistochemical staining in premenopausal patients [60]. Since data typically define the length of breast feeding as total lifetime duration, it was correlated with the number of pregnancies, but they were not necessarily identical [59]. The significance of the number of pregnancies as a risk-reducing factor has been controversial [59, 61]. However, the data in multiparous women limited to those with more than 3 children [26] or more than 5 children [62] showed significant risk-reducing effects. Interestingly enough, the duration of breast feeding per child was also inversely related to risk of breast cancer [63, 64]. Although it has not yet been adequately studied why the prolonged breast feeding is protective against breast cancer, these findings suggest a close relationship between slow-paced weaning and mitigation of inflammatory reaction during the involution. O’Brien and co-workers observed the coexistence of 2 kinds of lobules in the breast tissue from gradually weaning mothers, suggesting a stepwise turnover of TDLUs. One type was actively lactating and the other was involuting, as indicated by infiltration of CD45-positive acute reactive immune cells and CD68-positive macrophages [54].
Perspectives
Breast cancer incidence had been significantly lower in Japan compared to America and European countries and the rates of ER/PgR-positive breast cancers from Japanese patients were also lower, especially in postmenopausal women [65–67]. The average age of first birth has increased continuously during the past 5 decades from 24.7 to 29.7. The age-specific incidence of birth has gradually shifted to a higher value and about 24 % of women who gave birth were older than 35 years of age in 2010 (Vital Statistics Japan: Ministry of Health, Labour and Welfare) (Fig. 2). These trends must have influenced the risk of postmenopausal hormone-dependent tumors later in life as well as the risk of more aggressive tumors found shortly after childbirth. Actually, the nationwide occurrence of these two events in Japan has increased significantly during the past 3 decades in number and rates from 11,000 and 20/100,000 to 54,000 and 80/100,000, respectively [68]. The rates of cancers positive for both receptors have also increased from 50 % to more than 80 % [69].
Fig. 2.
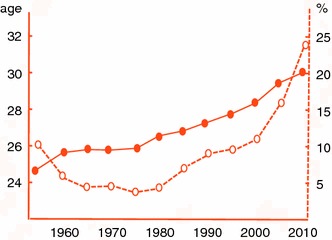
Annual trends of age of first birth (solid line with solid circle) and proportion with delivery after age 35 (dotted line with open circle) in Japan
Russo and co-workers suggested a preventive effect of periodic administration of hCG early in life on late-onset breast cancers [70]. It seems, however, to be extremely difficult to demonstrate efficacy and safety concomitantly while resolving the ethical issues. On the other hand, the administration of NSAIDs from the beginning of weaning to reduce the inflammatory reaction of involution may be applicable to prevent early-onset breast cancers. NSAIDs have been used in a wide variety of conditions and the accompanying side effects and their treatment are well known. Compared to evaluating the use of hCG, evaluation of the use of NSAIDs will require shorter follow-up periods. However, the actual significance of these agents with regard to breast cancer prevention in humans is still theoretical, or at best experimental. Methods other than administration of selective estrogen receptor modulators (SERMs) were not recommended at the present stage of knowledge in a consensus statement about preventive therapy for breast cancer by experts on breast cancer prevention in 2011 [71].
The sequence of events appearing in the lactating breast from pregnancy until weaning affects the risk of breast cancer in two ways (Fig. 3a, b). First, the cell death procedure acts in a suppressive manner. Second, the expansion and tissue remodeling procedure acts in a promotive manner. Regardless of hormone dependency, the inevitable risk of breast cancer as a female mammal become obvious around 40 years of age in humans. Among them the risk of hormone-dependent cancer is influenced largely by a variety of reproductive factors and becomes a major risk at almost 50 years of age and thereafter. On the other hand, since the first full-term birth has the most prominent dual effects on the breast cancer risk, the age when it occurs is particularly important. Early age of menarche increases the risk of ER/PgR-positive cancers [17, 72], and a long interval between menarche and first birth increases the risk by 50 % in ER/PgR-positive cancers [73]. These facts suggest an inevitable accumulation of stem-progenitor cells with unrepaired DNA damage since the initiation of pubertal development. Although these cells would have increased concomitantly during the mammary proliferation and differentiation in the first full-term pregnancy, they will be largely eliminated during the programmed cell death phase of involution after weaning. This can lead to risk reduction of late-onset hormone-dependent tumors. On the other hand, the tumor-promoting environment of the remodeling phase of involution increases the risk of unspecified tumors. The baseline risk of breast cancer is very low during younger ages. The protective effect, therefore, will be emphasized and the progressive effect will be attenuated by early age of first full-term birth. On the other hand, late age of first full-term birth inevitably increases the baseline risk of unspecified breast cancers. Although multiple consecutive pregnancies at a young age protect against late-onset cancers, this pattern in the elderly increases premenopausal cancers [74]. It is, therefore, suggested that the promotive effect is offset by the protective effect in those women with births at older ages.
Fig. 3.
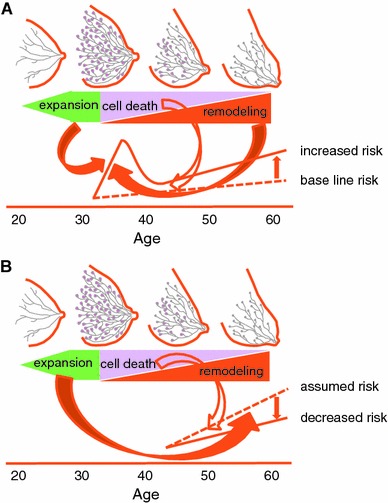
Dual effect of birth and lactation on breast cancer risk. a Effects on the early-onset unspecified cancer, b effects on the late-onset hormone-dependent cancer. Solid arrows promotive, open arrows suppressive effects on the risks
Factors affecting the risk of breast cancer are diverse and are not limited to reproductive history [75]. However, because of the aging of the entire population and low fertility rates, women will be indispensable to the workforce in developed countries including Japan. Such a working environment inevitably favors the delay of the first birth together with low parity and a short duration of breast feeding. Therefore, breast cancer risk due to the increasing effects of reproductive factors will not decrease in those countries in the foreseeable future. It is thus particularly important to take into account the patient’s specific reproductive history in the diagnosis and treatment of breast cancers.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita H, Yando Y, Nishio M, Zhang Z, Hamaguchi M, Mita K, et al. Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer. 2006;13(1):74–83. doi: 10.2325/jbcs.13.74. [DOI] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27(1):45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6(2):R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168(2):608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14(2):87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol. 2012;227(1):106–17. [DOI] [PMC free article] [PubMed]
- 11.O’Brien J, Hansen K, Barkan D, Green J, Schedin P. Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland. Int J Dev Biol. 2011;55(7–8–9):745–55. [DOI] [PubMed]
- 12.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17(7):867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17(9):1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–221. [PMC free article] [PubMed] [Google Scholar]
- 15.Woods KL, Smith SR, Morrison JM. Parity and breast cancer: evidence of a dual effect. Br Med J. 1980;281(6237):419–421. doi: 10.1136/bmj.281.6237.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ursin G, Bernstein L, Lord SJ, Karim R, Deapen D, Press MF, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93(3):364–371. doi: 10.1038/sj.bjc.6602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA, et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case–control study. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1723–1730. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bladstrom A, Anderson H, Olsson H. Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clin Breast Cancer. 2003;4(4):280–285. doi: 10.3816/CBC.2003.n.033. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Akuete K, Fulton J, Chelmow D, Chung MA, Cady B. An increased risk of breast cancer after delayed first parity. Am J Surg. 2003;186(4):409–412. doi: 10.1016/S0002-9610(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 22.Nagata C, Hu YH, Shimizu H. Effects of menstrual and reproductive factors on the risk of breast cancer: meta-analysis of the case–control studies in Japan. Jpn J Cancer Res. 1995;86(10):910–915. doi: 10.1111/j.1349-7006.1995.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki M, Otani T, Inoue M, Sasazuki S, Tsugane S, Japan Public Health Center-based Prospective Study Group Role and impact of menstrual and reproductive factors on breast cancer risk in Japan. Eur J Cancer Prev. 2007;16(2):116–123. doi: 10.1097/01.cej.0000228410.14095.2d. [DOI] [PubMed] [Google Scholar]
- 24.Li CI, Littman AJ, White E. Relationship between age maximum height is attained, age at menarche, and age at first full-term birth and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2144–2149. doi: 10.1158/1055-9965.EPI-07-0242. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb PA, Trentham-Dietz A, Hampton JM, Egan KM, Titus-Ernstoff L, Warren Andersen S, et al. Late age at first full term birth is strongly associated with lobular breast cancer. Cancer. 2011;117(9):1946–1956. doi: 10.1002/cncr.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamakoshi K, Yatsuya H, Wakai K, Suzuki S, Nishio K, Lin Y, et al. Impact of menstrual and reproductive factors on breast cancer risk in Japan: results of the JACC study. Cancer Sci. 2005;96(1):57–62. doi: 10.1111/j.1349-7006.2005.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3(3):301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 28.Guo S, Russo IH, Lareef MH, Russo J. Effect of human chorionic gonadotropin in the gene expression profile of MCF-7 cells. Int J Oncol. 2004;24(2):399–407. [PubMed] [Google Scholar]
- 29.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11(2 Pt 2):931s–936s. [PubMed] [Google Scholar]
- 30.Misra Y, Bentley PA, Bond JP, Tighe S, Hunter T, Zhao FQ. Mammary gland morphological and gene expression changes underlying pregnancy protection of breast cancer tumorigenesis. Physiol Genomics 2012;44(1):76–88 [DOI] [PMC free article] [PubMed]
- 31.Russo IH, Koszalka M, Russo J. Comparative study of the influence of pregnancy and hormonal treatment on mammary carcinogenesis. Br J Cancer. 1991;64(3):481–484. doi: 10.1038/bjc.1991.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toniolo P, Grankvist K, Wulff M, Chen T, Johansson R, Schock H, et al. Human chorionic gonadotropin in pregnancy and maternal risk of breast cancer. Cancer Res. 2010;70(17):6779–6786. doi: 10.1158/0008-5472.CAN-09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siwko SK, Dong J, Lewis MT, Liu H, Hilsenbeck SG, Li Y. Evidence that an early pregnancy causes a persistent decrease in the number of functional mammary epithelial stem cells—implications for pregnancy-induced protection against breast cancer. Stem Cells. 2008;26(12):3205–3209. doi: 10.1634/stemcells.2008-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103(7):2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176(1):19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 37.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 38.Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 40.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruzzi P, Negri E, La Vecchia C, Decarli A, Palli D, Parazzini F, et al. Short term increase in risk of breast cancer after full term pregnancy. BMJ. 1988;297(6656):1096–1098. doi: 10.1136/bmj.297.6656.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layde PM, Webster LA, Baughman AL, Wingo PA, Rubin GL, Ory HW. The independent associations of parity, age at first full term pregnancy, and duration of breastfeeding with the risk of breast cancer. Cancer and Steroid Hormone Study Group. J Clin Epidemiol. 1989;42(10):963–973. doi: 10.1016/0895-4356(89)90161-3. [DOI] [PubMed] [Google Scholar]
- 43.Ewertz M, Duffy SW, Adami HO, Kvale G, Lund E, Meirik O, et al. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46(4):597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 44.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 45.Albrektsen G, Heuch I, Thoresen S, Kvale G. Clinical stage of breast cancer by parity, age at birth, and time since birth: a progressive effect of pregnancy hormones? Cancer Epidemiol Biomarkers Prev. 2006;15(1):65–69. doi: 10.1158/1055-9965.EPI-05-0634. [DOI] [PubMed] [Google Scholar]
- 46.Mathelin C, Annane K, Treisser A, Chenard MP, Tomasetto C, Bellocq JP, et al. Pregnancy and post-partum breast cancer: a prospective study. Anticancer Res. 2008;28(4C):2447–52. [PubMed]
- 47.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Reproductive history and mortality after breast cancer diagnosis. Obstet Gynecol. 2004;104(1):146–154. doi: 10.1097/01.AOG.0000128173.01611.ff. [DOI] [PubMed] [Google Scholar]
- 48.Largent JA, Ziogas A, Anton-Culver H. Effect of reproductive factors on stage, grade and hormone receptor status in early-onset breast cancer. Breast Cancer Res. 2005;7(4):R541–R554. doi: 10.1186/bcr1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 51.Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8(2):203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6(2):R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–3289. [PubMed] [Google Scholar]
- 54.O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176(3):1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hojilla CV, Jackson HW, Khokha R. TIMP3 regulates mammary epithelial apoptosis with immune cell recruitment through differential TNF dependence. PLoS One. 2011;6(10):e26718. doi: 10.1371/journal.pone.0026718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, et al. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res. 2009;11(2):R24. doi: 10.1186/bcr2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagaharu K, Zhang X, Yoshida T, Katoh D, Hanamura N, Kozuka Y, et al. Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells. Am J Pathol. 2011;178(2):754–763. doi: 10.1016/j.ajpath.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13(3):303–309. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 59.Collaborative Group on Hormonal Factors in Breast C Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360(9328):187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 60.Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alsaker MD, Opdahl S, Asvold BO, Romundstad PR, Vatten LJ. The association of reproductive factors and breastfeeding with long term survival from breast cancer. Breast Cancer Res Treat. 2011;130(1):175–182. doi: 10.1007/s10549-011-1566-3. [DOI] [PubMed] [Google Scholar]
- 62.Warren Andersen S, Newcomb PA, Hampton JM, Titus-Ernstoff L, Egan KM, Trentham-Dietz A. Reproductive factors and histologic subtype in relation to mortality after a breast cancer diagnosis. Breast Cancer Res Treat. 2011;130(3):975–80. [DOI] [PMC free article] [PubMed]
- 63.Chang-Claude J, Eby N, Kiechle M, Bastert G, Becher H. Breastfeeding and breast cancer risk by age 50 among women in Germany. Cancer Causes Control. 2000;11(8):687–695. doi: 10.1023/A:1008907901087. [DOI] [PubMed] [Google Scholar]
- 64.De Silva M, Senarath U, Gunatilake M, Lokuhetty D. Prolonged breastfeeding reduces risk of breast cancer in Sri Lankan women: a case–control study. Cancer Epidemiol. 2010;34(3):267–273. doi: 10.1016/j.canep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Nomura Y, Kobayashi S, Takatani O, Sugano H, Matsumoto K, McGuire WL. Estrogen receptor and endocrine responsiveness in Japanese versus American breast cancer patients. Cancer Res. 1977;37(1):106–110. [PubMed] [Google Scholar]
- 66.Nagai R, Kataoka M, Kobayashi S, Ishihara K, Tobioka N, Nakashima K, et al. Estrogen and progesterone receptors in human breast cancer with concomitant assay of plasma 17beta-estradiol, progesterone, and prolactin levels. Cancer Res. 1979;39(5):1834–1840. [PubMed] [Google Scholar]
- 67.McGuire WL. Steroid receptors in human breast cancer. Cancer Res. 1978;38(11 Pt 2):4289–4291. [PubMed] [Google Scholar]
- 68.Matsuda T, Marugame T, Kamo KI, Katanoda K, Ajiki W, Sobue T, et al. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2012;42(2):139–147. doi: 10.1093/jjco/hyr184. [DOI] [PubMed] [Google Scholar]
- 69.Yamashita H, Iwase H, Toyama T, Takahashi S, Sugiura H, Yoshimoto N, et al. Estrogen receptor-positive breast cancer in Japanese women: trends in incidence, characteristics, and prognosis. Ann Oncol. 2011;22(6):1318–1325. doi: 10.1093/annonc/mdq596. [DOI] [PubMed] [Google Scholar]
- 70.Kocdor H, Kocdor MA, Russo J, Snider KE, Vanegas JE, Russo IH, et al. Human chorionic gonadotropin (hCG) prevents the transformed phenotypes induced by 17 beta-estradiol in human breast epithelial cells. Cell Biol Int. 2009;33(11):1135–1143. doi: 10.1016/j.cellbi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuzick J, DeCensi A, Arun B, Brown PH, Castiglione M, Dunn B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12(5):496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 72.Islam T, Matsuo K, Ito H, Hosono S, Watanabe M, Iwata H, et al. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol. 2012. doi:10.1093/annonc/mdr613 [DOI] [PubMed]
- 73.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, et al. Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol. 2008;167(2):230–239. doi: 10.1093/aje/kwm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kauppila A, Kyyronen P, Lehtinen M, Pukkala E. Dual effect of short interval between first and second birth on ductal breast cancer risk in Finland. Cancer Causes Control. 2012;23(1):187–193. doi: 10.1007/s10552-011-9868-7. [DOI] [PubMed] [Google Scholar]
- 75.Yoshimoto N, Nishiyama T, Toyama T, Takahashi S, Shiraki N, Sugiura H, et al. Genetic and environmental predictors, endogenous hormones and growth factors, and risk of estrogen receptor-positive breast cancer in Japanese women. Cancer Sci. 2011;102(11):2065–2072. doi: 10.1111/j.1349-7006.2011.02047.x. [DOI] [PubMed] [Google Scholar]


