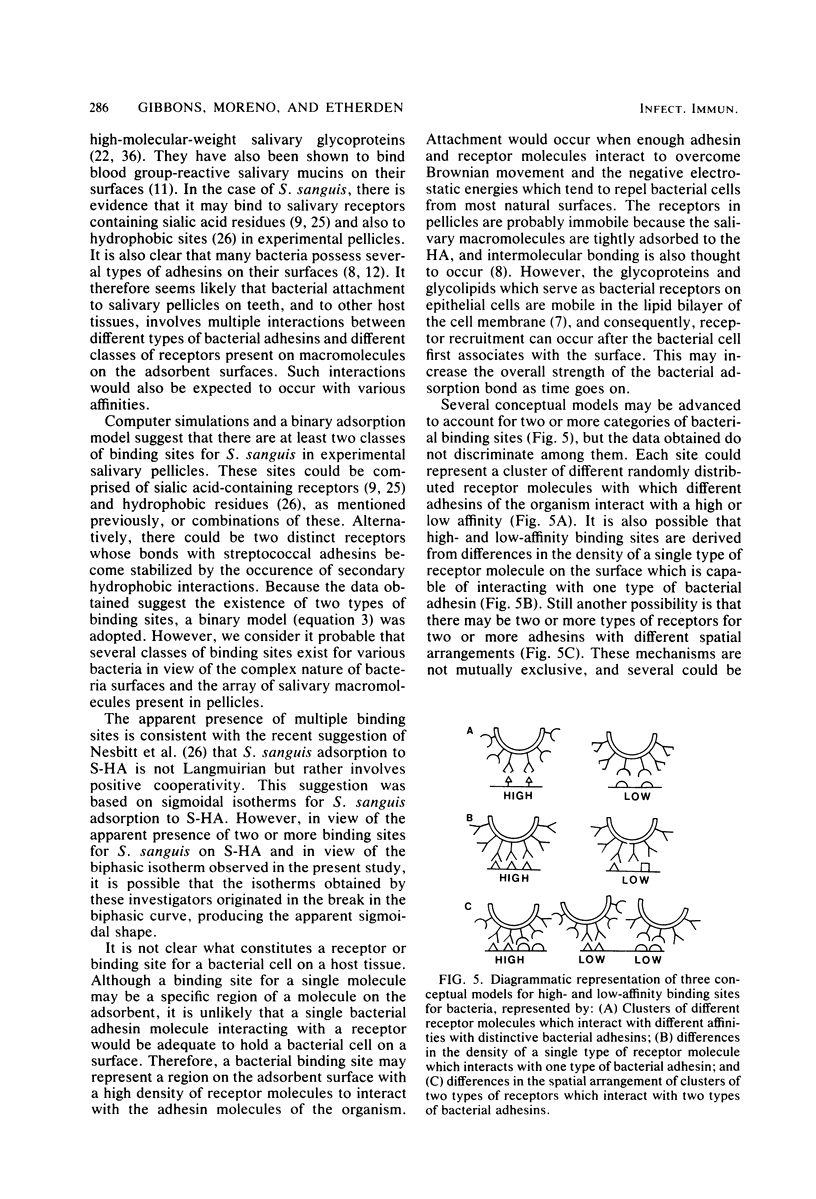
Abstract
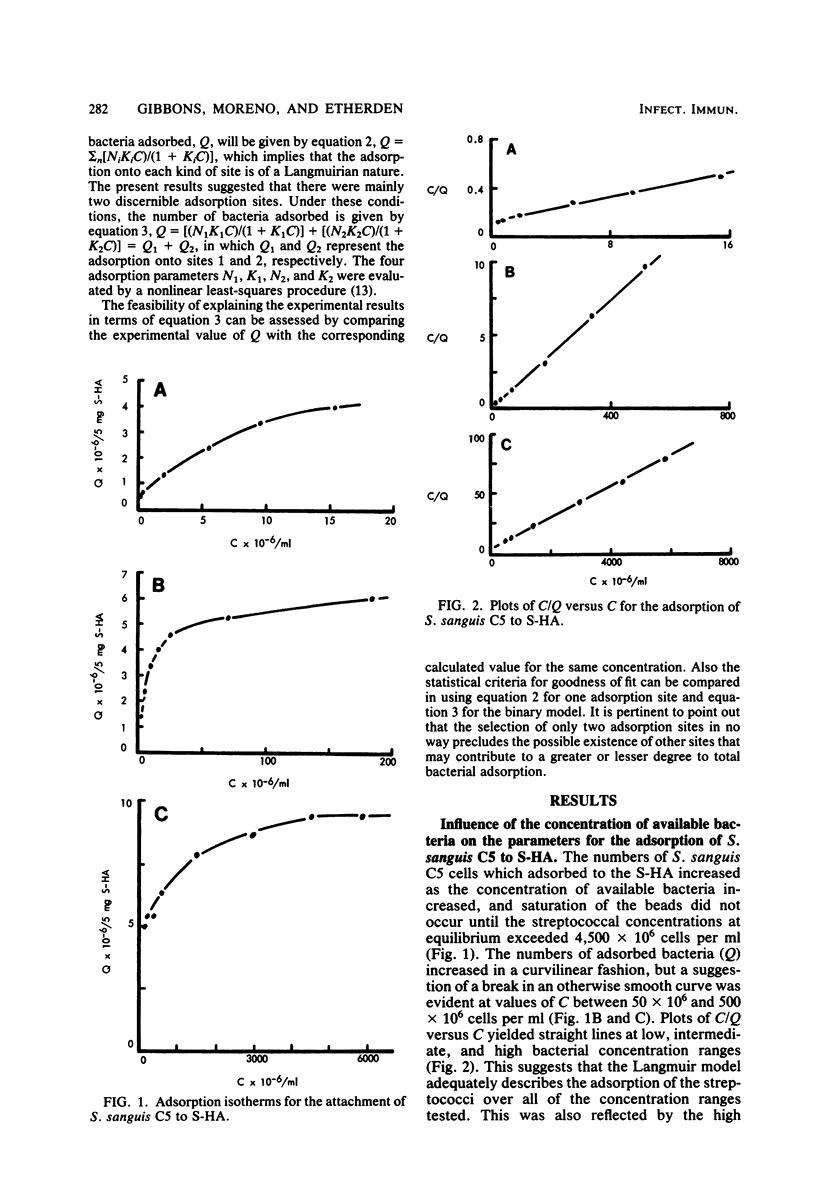
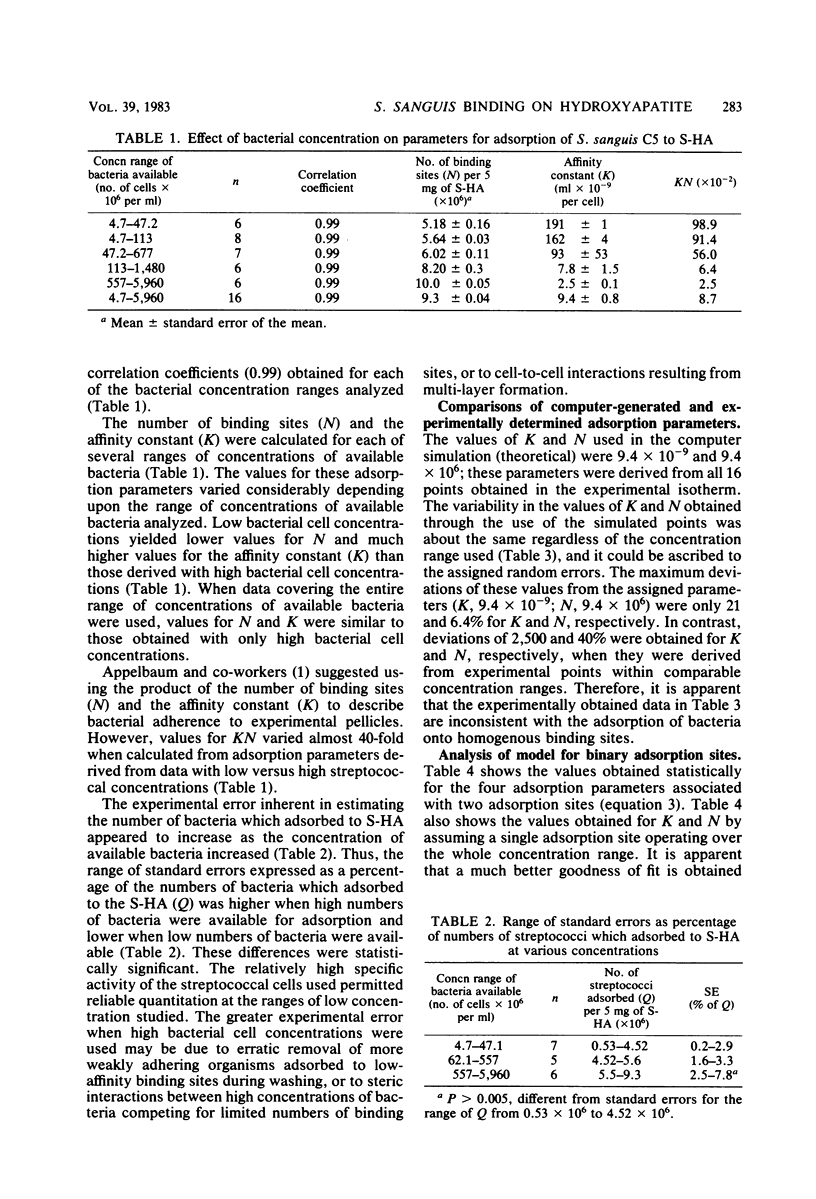
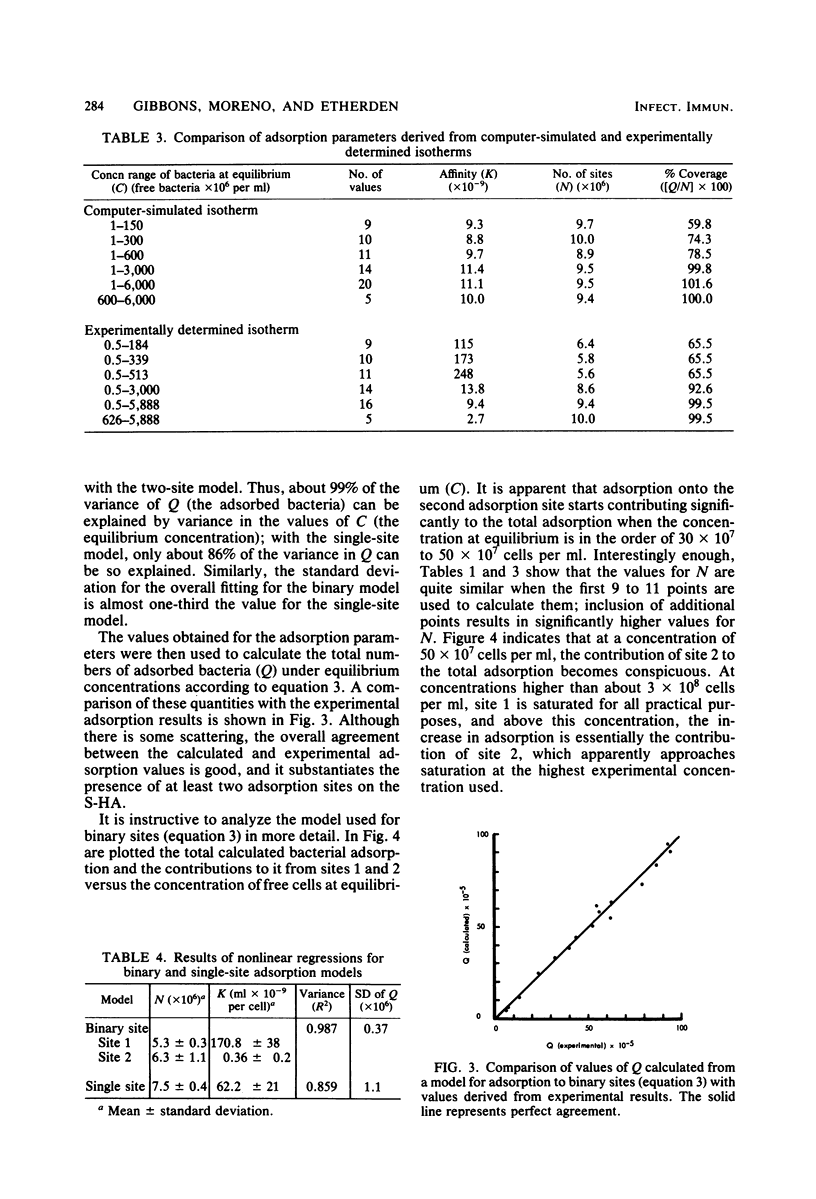
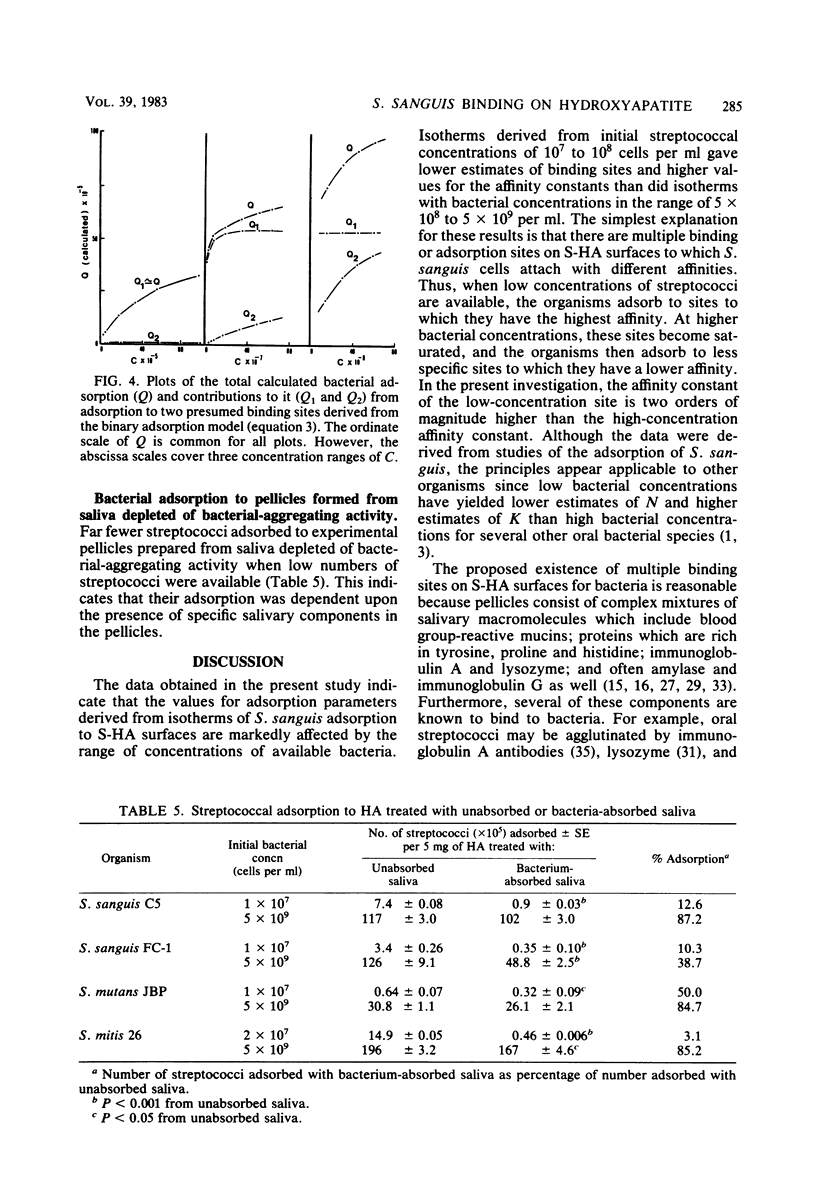
The influence of bacterial cell concentration on estimates of the number of binding sites and the affinity for the adsorption of a strain of Streptococcus sanguis to saliva-treated hydroxyapatite was determined, and the possible presence of multiple binding sites for this organism was tested. The range of concentrations of available bacteria varied from 4.7 × 106 to 5,960 × 106 cells per ml. The numbers of adsorbed bacteria increased over the entire range tested, but a suggestion of a break in an otherwise smooth adsorption isotherm was evident. Values for the number of binding sites and the affinity varied considerably depending upon the range of available bacterial concentrations used to estimate them; high correlation coefficients were obtained in all cases. The use of low bacterial cell concentrations yielded lower values for the number of sites and much higher values for the affinity constant than did the use of high bacterial cell concentrations. When data covering the entire range of bacterial concentrations were employed, values for the number of sites and the affinity were similar to those obtained by using only high bacterial cell concentrations. The simplest explanation for these results is that there are multiple binding sites for S. sanguis on saliva-treated hydroxyapatite surfaces. When present in low concentration, the streptococci evidently attach to more specific high-affinity sites which become saturated when higher bacterial concentrations are employed. The possibility of multiple binding sites was substantiated by comparing estimates of the adsorption parameters from a computer-simulated isotherm with those derived from the experimentally generated isotherm. A mathematical model describing bacterial adsorption to binary binding sites was further evidence for the existence of at least two classes of binding sites for S. sanguis. Far fewer streptococci adsorbed to experimental pellicles prepared from saliva depleted of bacterial aggregating activity when low numbers of streptococci were used, but the magnitude of this difference was considerably less when high streptococcal concentrations were employed. This suggests an association between salivary components which possess bacterial-aggregating activity and bacterial adsorption to high-affinity specific binding sites on saliva-treated hydroxyapatite surfaces.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Dental plaque as a source of salivary streptococci. Odontol Revy. 1967;18(2):173–178. [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Webb E. L., Wheeler T. T., Fischlschweiger W., Birdsell D. C., Mansheim B. J. Role of surface fimbriae (fibrils) in the adsorption of Actinomyces species to saliva-treated hydroxyapatite surfaces. Infect Immun. 1981 Sep;33(3):908–917. doi: 10.1128/iai.33.3.908-917.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T. Salivary glycoproteins. Composition and adsorption to hydroxylapatite in relation to the formation of dental pellicles and calculus. Acta Odontol Scand. 1968 May;26(1):3–21. doi: 10.3109/00016356809004577. [DOI] [PubMed] [Google Scholar]
- Gall W. E., Edelman G. M. Lateral diffusion of surface molecules in animal cells and tissues. Science. 1981 Aug 21;213(4510):903–905. doi: 10.1126/science.7196087. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Enzymatic modification of bacterial receptors on saliva-treated hydroxyapatite surfaces. Infect Immun. 1982 Apr;36(1):52–58. doi: 10.1128/iai.36.1.52-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I. Fractionation of human parotid salivary proteins and the isolation of an histidine-rich acidic peptide which shows high affinity for hydroxyapatite surfaces. Arch Oral Biol. 1975 Sep;20(9):553–558. doi: 10.1016/0003-9969(75)90073-4. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Moreno E. C. Differential adsorption and chemical affinities of proteins for apatitic surfaces. J Dent Res. 1979 Mar;58(SPEC):930–942. doi: 10.1177/00220345790580024701. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The isolation from human parotid saliva of a tyrosine-rich acidic peptide which exhibits high affinity for hydroxyapatite surfaces. Arch Oral Biol. 1973 Dec;18(12):1531–1541. doi: 10.1016/0003-9969(73)90128-3. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Kondo W., Sato M., Ozawa H. Haemagglutinating activity of Leptotrichia buccalis cells and their adherence to saliva-coated enamel powder. Arch Oral Biol. 1976;21(6):363–369. doi: 10.1016/s0003-9969(76)80004-0. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Competitive binding among oral strptococci to hydroxyapatite. J Dent Res. 1977 Feb;56(2):157–165. doi: 10.1177/00220345770560021001. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W. Amino acid composition of experimental salivary pellicles. J Periodontol. 1977 Feb;48(2):78–91. doi: 10.1902/jop.1977.48.2.78. [DOI] [PubMed] [Google Scholar]
- Mayhall C. W., Butler W. T. The carbohydrate composition of experimental salivary pellicles. J Oral Pathol. 1976 Nov;5(6):358–370. doi: 10.1111/j.1600-0714.1976.tb01787.x. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G., Staat R. H., Arnold R. R. Positive coooperativity in the binding of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Jan;35(1):157–165. doi: 10.1128/iai.35.1.157-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W. The acquired pellicle: immunofluorescent demonstration of specific proteins. J Oral Pathol. 1973;2(1):68–76. doi: 10.1111/j.1600-0714.1973.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D. The in-vitro attachment of an oral Streptococcus sp. to the acquired tooth enamel pellicle. Arch Oral Biol. 1978;23(3):167–173. doi: 10.1016/0003-9969(78)90212-1. [DOI] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönju T., Christensen T. B., Kornstad L., Rölla G. Electron microscopy, carbohydrate analyses and biological activities of the proteins adsorbed in two hours to tooth surfaces in vivo. Caries Res. 1974;8(2):113–122. doi: 10.1159/000260099. [DOI] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



