Abstract
In the field of tissue engineering and regenerative medicine there is significant unmet need for critically-sized, fully degradable biomaterial scaffold systems with tunable properties for optimizing tissue formation in vitro and tissue regeneration in vivo. To address this need, we have developed a silk-based scaffold platform that has tunable material properties, including localized and bioactive functionalization, degradation rate, and mechanical properties and that provides arrays of linear hollow channels for delivery of oxygen and nutrients throughout the scaffold bulk. The scaffolds can be assembled with dimensions that range from millimeters to centimeters, addressing the need for a critically-sized platform for tissue formation. We demonstrate that the hollow channel arrays support localized and confluent endothelialization. This new platform offers a unique and versatile tool for engineering `tailored' scaffolds for a range of tissue engineering and regenerative medicine needs.
1. Introduction
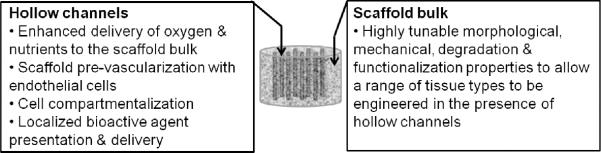
A prominent debilitating issue in the field of regenerative medicine and tissue engineering is the lack of comprehensive scaffold platforms for the development of fully vascularized tissue-engineered constructs that integrate successfully with host tissue. We describe a silk scaffolding platform, which addresses this need. The platform possesses versatile physical and mechanical properties and incorporates tunable hollow channels to enhance nutrient delivery to cells, enables scaffold pre-vascularization and supports cell compartmentalization.
The general approach for engineering tissue equivalents is to coordinately combine relevant cell types with biophysical/chemical cues on an appropriate scaffolding material. The scaffolding material plays a significant role in modulating cell behavior and tissue formation. To achieve desired cell and tissue function, scaffold properties, including stiffness, biodegradation rate, porosity, and surface chemistry must be optimized[1, 2]. Silk fibroin is an exemplary scaffolding material because its material properties can be highly tuned while exhibiting excellent cell compatibility with no adverse immune responses in vivo. Silk has robust mechanical properties and slow degradation rates in vivo (weeks to years to completely resorb) both of which can be tuned via silk processing[3–7]. In contrast to many synthetic polymers which can release inflammatory degradation products, the degradation products of silk fibroin are amino acids[8]. Silk scaffolds can take on a variety of porosities and pores sizes (nanometer scale up to several hundred micrometers) depending on the scaffold assembly method[9–12]. The biological properties of silk scaffolds can be easily augmented through bulk loading, surface decoration or construction of composite materials[13]. Silk processing and scaffold assembly are performed in aqueous solutions thereby enabling the addition of bioactive components without loss of function. Furthermore, silk has been shown to stabilize bioactive agents, such as enzymes and therapeutics, thereby prolonging their activity under physiologic conditions[14–16]. In contrast to other biologically-derived polymers, silk is abundantly available as a raw material. Other biomaterials currently reported in the literature do not offer the same suite of advantages as silk or the same range of control over physical, mechanical and biological properties. Thus, silk-based scaffolding systems can serve as a comprehensive platform for the regeneration of a wide range of tissues.
The primary obstacle in engineering tissue equivalents is the diffusion limit of oxygen and nutrients. Constructs that exceed critical dimensions (i.e. several hundred micrometers depending on the tissue type) are prone to necrosis at the core of the construct and ultimately fail to integrate with host tissue due to lack of blood perfusion[17, 18]. Vascularization within a critically-sized tissue construct does not occur within a sufficient time frame to supply the entire construct with the necessary oxygen and nutrients. Furthermore, necrosis can stimulate undesirable inflammatory responses in vivo. Therefore, there is a significant demand for efficient and reproducible oxygen and nutrient delivery throughout the bulk of critically-sized engineered tissues. To address this need, research efforts are focused on developing strategies for pre-vascularizing engineered tissues to enable immediate perfusion upon implantation[19]. One active area of research employs microfabrication techniques to build microfluidic channels into scaffold platforms that resemble physiologic microvasculature. While microfabrication is efficient and reproducible, microfabricated platforms undergo extensive handling in order to stack the scaffolds to the desired construct dimensions and therefore do not provide a rapid means of generating critically-sized scaffolds. Further, most materials used in such systems are either non-degradable (e.g. polydimethylsiloxane) or remodel too quickly to maintain adequate mass transport (e.g. collagen)[20, 21]. An alternate approach for incorporating nutrient delivery conduits into tissue constructs employs methods for building linear channels that extend through the construct bulk, including laser piercing[22], sacrificial fibers[23, 24], and removable wire arrays[25, 26]. While these methods have successfully introduced linear channels into polymer scaffolds, they demonstrate limited versatility in terms of channel diameter, spacing and functionalization and bulk morphological, mechanical, and biodegradation properties.
We report the development and characterization of versatile silk-based scaffolds with highly tunable properties for engineering of a range of critically-sized tissue constructs. These scaffolds can be assembled with relevant dimensions without the need to stack scaffold pieces and contain an array of hollow channels that 1) serve as nutrient and oxygen delivery conduits, 2) allow scaffold prevascularization with endothelial cells, 3) support cell compartmentalization and 4) allow localized bioactive factor presentation and delivery. The hollow channel array is incorporated into the scaffold using linear wire arrays (LWAs) composed of aligned wires for reproducible control over channel properties, including channel diameters, wall-to-wall spacing, wall morphology and loading of bioactive compounds. In addition, the scaffold bulk surrounding the channels can take on a variety of pore sizes, pore morphologies and mechanical properties to support engineering of a range of tissue types. We show that the channels support a confluent layer of endothelial cells and that the LWAs allow for controlled localization of different cell types within the scaffold. Given the high level of control over the scaffold and channel features, including size, morphology, and bioactive functionalization, the approaches described offer a versatile tool for manipulation of 3D scaffolds and bioengineering of complex, vascularized and fully degradable scaffolds for a variety of tissue engineering needs.
2. Materials Methods
2.1 Linear Wire Array (LWA) Fabrication
LWAs were fabricated by cutting an array of circular holes (Ø = 152 μm-787 μm) into acrylic or corrugated cardboard sheets using a laser cutter. The hole wall-to-wall spacing ranged between 500 μm and 1 mm. Polytetrafluorethylene (PTFE)-coated stainless steel wires (McMaster-Carr) were arranged vertically into the holes and secured by casting polydimethylsiloxane (PDMS) around the base.
2.2 Silk Scaffold Assembly
Silk fibroin solutions (5–6% wt/v) were prepared as previously described [27] and poured into molds containing the wire arrays. Scaffolds with randomly aligned pores were isotropically frozen in a standard laboratory freezer at either −20°C or −80°C. Scaffolds with aligned pores were cast in a custom-built PDMS mold consisting of two chambers separated by a metal plate. One chamber contained the silk solution, while the other chamber contained the freezing agent. As the freezing agent was applied to one side of the metal plate, the silk solution was frozen in a single direction (i.e. anisotropic freezing) resulting in aligned pores. Three different freezing agents were utilized in this system to achieve different freezing rates: liquid nitrogen (fast freezing rate, 2.9 mm/min freezing front), absolute ethanol and dry ice bath (medium freezing rate, 0.7 mm/min), and 70% v/v ethanol and dry ice bath (slow freezing rate, 0.4 mm/min). Frozen samples were lyophilized and water annealed for 12 hours at 25°C to induce β-sheet formation, thereby rendering the scaffolds insoluble in aqueous environments [12, 28].
2.3 Silk Tube Assembly
Silk tubes were assembled according to previously reported methods[29]. Porous silk tubes were obtained by incorporating 1.0% wt/wt polyethylene oxide (PEO) (10,000 MW) in the concentrated silk solution. After the scaffold was assembled around the LWA, the PEO was leached for three days. Silk tubes were also functionalized with 75 μg/ml dextran-conjugated fluorescein or rhodamine (Invitrogen), 2.5 μg/ml bovine collagen-I (BD Biosciences), 0.25 μg/ml human laminin (Sigma Aldrich), or 500 μg/ml horseradish peroxidase (HRP) (Sigma Aldrich).
2.4 Scaffold Characterization
Pore and channel morphology was visualized using a Supra55VP (Zeiss) scanning electron microscope (SEM). Scaffold pore size distribution and porosity were determined using mercury intrusion porosimetry (MIP) as previously described[30] using a PoreMaster 33 porosimeter (Quantachrome Instruments). The pore size distribution of scaffolds created using the anisotropic freezing method was further analyzed by confocal laser scanning microscopy (CLSM) using a DMIRE2 CLSM (Leica). The length and width of pore cross-sections were measured (10 pores/image, 5 images/sample) using Image J. Compressive mechanical properties of hydrated samples were obtained using an Instron 3366 testing frame equipped with a 10 N load cell as previously described[31].
2.5 Horseradish Peroxidase (HRP) Activity Assay
HRP enzyme was utilized to determine the activity of bioactive agents incorporated into the silk tubes. Silk tubes were functionalized with HRP as described above. 3,3'-diaminobenzidine (DAB) was applied to the scaffolds directly or to scaffold cryosections and the formation of a brown precipitate was monitored.
2.6 Cell Culture
Human mesenchymal stem cells (hMSCs) were isolated from fresh bone marrow aspirate (Lonza) as previously described[32]. Primary human arterial endothelial cells (hAECs) (Lonza) were cultured in Endothelial Cell Growth Media-2, EGM 2-MV supplements and 2% fetal bovine serum (Lonza). Cells were cultured as single cultures or co-cultures of hMSCs and hAECs. Single cultures were performed in an unconfined or confined seeding system.
Unconfined system
hMSCs (P3 – P5) were seeded on silk scaffolds (Ø=12 mm, h=3 mm) containing a 4×4 array of 508 μm diameter silk tubes (+/− collagen-I) at a cell density of 1×105 cells/scaffold in a total volume of 75 μl. Samples were incubated at 37°C in 5% CO2 for 2 hours before media was added. Samples were processed for a DNA content assay 1 day post-seeding (Invitrogen) or stained with 2 μM calcein AM 14 days post-seeding (Invitrogen). For the DNA content assay, to better discern differences in cell numbers in silk tubes, layers of confluent cells on the top and bottom scaffold surfaces were removed prior to quantification using a biopsy punch and a surgical blade.
Confined system
In order to confine cells to the hollow channels, the pores of the surrounding silk scaffold (Ø=12 mm, h=3 mm) were filled with a fibrin gel (10% wt/v human fibrinogen mixed with 5U/mL human thrombin in a 4:1 volume ratio, Sigma Aldrich) prior to LWA removal. hAECs (P4–P7) were then seeded onto the scaffold containing a 4×4 array of 508 μm diameter silk tubes at a cell density of 5×105 cells/scaffold and cultured for seven days. A cross-section through the middle of the scaffold was made and the level of channel/tube endothelialization was assessed by CLSM following cell staining with 2 μM calcein AM. To quantify tube endothelialization, an arbitrary value between 1 and 4 was assigned to 16 tubes per scaffold for a total of 3 scaffolds per treatment (1- no cells or only an occasional cell, 2- sporadic cell coverage, with no areas of contiguous endothelialization, 3- >75% endothelialization with only small areas where the cell layer was not contiguous and 4- complete endothelialization with a completely contiguous layer of cells around the silk tube).
Cell co-culture on silk scaffolds
Cells were compartmentalized by physically confining hMSCs to the scaffold bulk and hAECs to a 5×5 array of 152 μm diameter hollow channels. Prior to cell seeding, hMSCs and hAECs were labeled with 0.01 mM DiD and 0.01 mM DiI, respectively (Vybrant®, Invitrogen). hMSCs were seeded at a density of 4×105 cells/scaffold in the scaffold bulk (Ø=12 mm, h=3mm) prior to LWA removal in the presence or absence of a fibrin hydrogel (10% wt/v human fibrinogen mixed with 5 U/mL human thrombin in a 4:1 volume ratio, Sigma Aldrich). hMSCs were cultured for two days before the LWAs were removed. hAECs were seeded at a cell density of 4×105 cells/scaffold and cultured for two days. A cross-section through the middle of the scaffold was made and imaged by CLSM.
2.7 DNA Content Assay
Cell proliferation was assessed based on DNA content of cells cultured on 3D scaffolds. Scaffolds were minced with microscissors in lysis buffer consisting of 0.2% v/v Triton X-100 and 5 mM magnesium chloride. Scaffold particles were removed by centrifugation at 12,000 rpm for 10 min at 4°C. DNA in the supernatant was quantified using a PicoGreen® assay (Invitrogen) according to manufacturer's protocol.
2.8 Statistical analysis
Data are expressed as mean ± standard deviation. Statistically significant differences were determined by one- or two-way analysis of variance (ANOVA) and Bonferroni post-test. Statistical significance was accepted at p<0.05 and indicated in the figures as *p<0.05, **p<0.01 and ***p<0.001.
3. Results
3.1 Silk scaffolds with hollow channels fabricated using linear wire arrays (LWAs)
Silk scaffolds with hollow channels were prepared using a sequential six step process involving fabrication and placement of the LWA, optional wire functionalization with bioactive agents, application of the silk solution, lyophilization and β-sheet induction (Fig. 1a). Upon completion of this process, the LWA was removed, leaving a 3D, porous scaffold with hollow channels spanning the length of the scaffold. Scaffold size and shape are controlled by the size and shape of the freezing mold, therefore a wide range of scaffold dimensions can be obtained (Fig. S1). Each step of this process allows for the properties of the final scaffold to be tuned for a particular tissue engineering purpose (Fig. 1b).
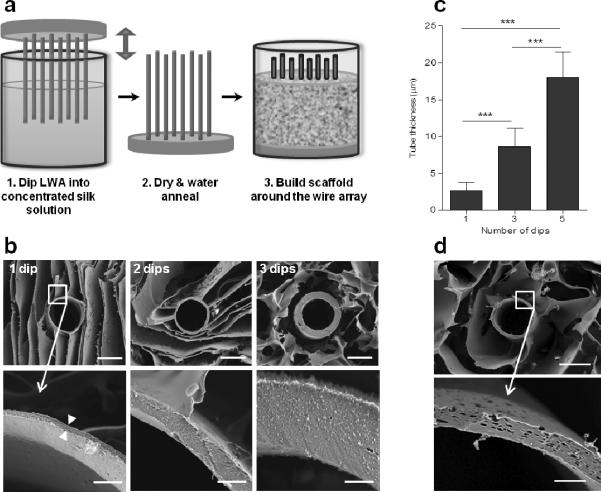
Figure 1. Silk scaffolds with hollow channels.
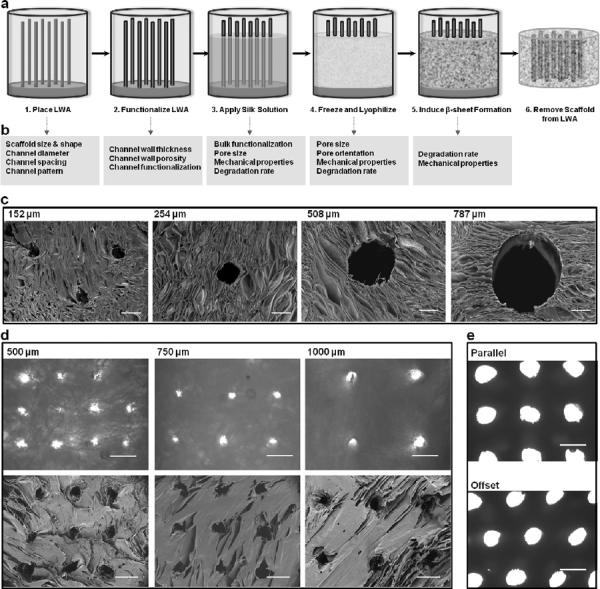
(a) Schematic of the fabrication process for building silk-based porous scaffolds containing hollow channels using a linear wire array (LWA). (b) At each step in the process there are avenues for tuning the scaffold bulk properties (e.g. porosity, mechanical stiffness, and degradation rate) and the hollow channel properties (e.g. diameter, spacing, bioactive agent functionalization and wall morphology). (c) SEM micrographs of channel diameters ranging from 152 μm to 787 μm in anisotropically frozen scaffolds. (d) Channel wall-to-wall spacing ranging between 500 μm and 1000 μm in isotropically frozen scaffolds. Top row shows brightfield light microscopy images, while the bottom row shows corresponding SEM images. (e) Channel patterning is shown in the parallel and offset configuration. Scale bars are (c) 200 μm, (d) 500 μm, and (e) 1 mm.
A number of channel configurations were engineered into silk scaffolds using different LWAs with a range of wire diameters, wire wall-to-wall spacing and wire arrangements. Channels ranging from 152 μm to 787 μm in diameter were introduced into silk scaffolds (Fig. 1c). Each channel was hollow and the channel periphery displayed open porosity similar to that of the scaffold bulk. Channels were positioned with 500 μm, 750 μm or 1000 μm wall-to-wall spacing in either parallel or offset arrangement (Fig. 1d–e).
3.2 Channel morphology and functionalization
Silk tubes were introduced into the channel periphery by dipping LWAs in a concentrated silk solution (Fig. 2a). Silk formed a thin coating on the wire array that when dried and water annealed formed a continuous silk tube that was insoluble in aqueous environments. By repeating this process, silk tubes of different wall thicknesses were formed (Fig. 2b). One dip resulted in tubes with 2 μm ± 1 μm wall thickness, 3 dips resulted in 9 μm ± 3 μm wall thickness and 5 dips resulted in 18 μm ± 4 μm wall thickness (Fig. 2c). Subsequently, pores ranging from 1.8 ± 0.9 μm to 3.5 ± 1.1 μm were engineered into silk tube walls by incorporating PEO into the concentrated silk solution (Fig. 2d). The PEO acted as a porogen and was leached out of the silk tube following the water annealing step. Pore size and frequency were a function of PEO concentration and can be fine-tuned by adding different amounts of PEO to the concentrated silk solution (Fig. 2e–f).
Figure 2. Hollow channels lined with silk tubes.
(a) The channel wall was lined with a silk tube by dipping the LWA into a concentrated silk solution, which coated the wires and remained in the scaffold when the LWA was removed. Depicted is a schematic of the LWA dipping process and subsequent scaffold fabrication. Step 3 in this process corresponds to steps 3–6 in Fig. 1a. (b–c) Silk tube thickness was determined by the number of times steps 1 and 2 were repeated (i.e. number of dips). (b) SEM micrographs of tubes formed by 1, 3 and 5 dips respectively and (c) the average tube thickness as measured from SEM micrographs (error bars=s.d.;***p<0.001; n=8). (d–f) Porous silk tubes were formed by adding a PEO porogen to the concentrated silk solution. (d) SEM micrographs of tubes formed with varying amounts of PEO (0– 2.0% wt/wt) added to the concentrated silk solution. (e) Average pore diameter (error bars=s.d;***p<0.001; n=30) and (f) number of pores per μm2 as measured from SEM micrographs (error bars=s.d.;***p<0.001; n=13). Pores were often oval in shape, so the diameter was measured in the direction tangent to the tube perimeter. Scale bars are 100 μm in top row images and 10 μm in bottom row images in (b) and 50 μm in top row images and 10 μm in bottom row images in (d).
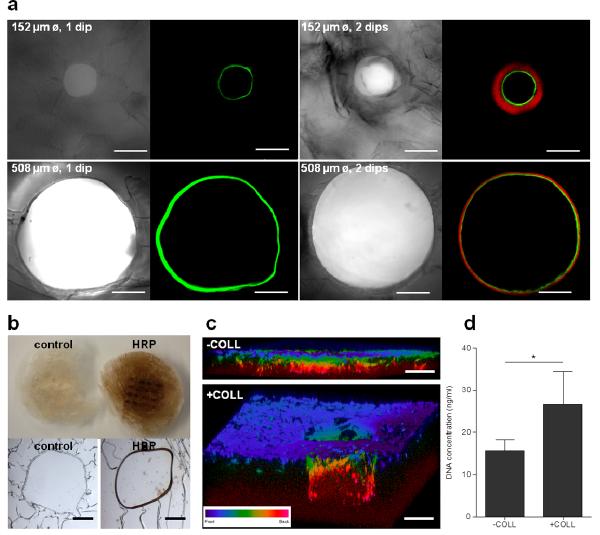
Silk tubes were successfully functionalized with dextran conjugated to fluorescein or rhodamine, HRP enzyme and collagen-I. When the LWA was dipped once in a dextran-fluorescein functionalized silk solution, a contiguous fluorescent tube was observed using both 152 μm and 508 μm diameter wires (Fig. 3a). When this process was repeated twice, with the first dip in dextran-fluorescein functionalized silk and the second dip in dextran-rhodamine functionalized silk, a tube with two distinct bands of florescence was observed. There was no observable mixing of the two dyes, indicating that silk stabilized the dyes within the layer into which they were introduced (Fig. 3a). Functionalization with fluorescent dyes served as proof of principle that compounds could be introduced and localized in the silk tubes.
Figure 3. Silk tube functionalization.
(a) Silk tubes of two different diameters (152 μm and 508 μm) were functionalized with dextran-fluorescein (green) only or with dextran-fluorescein and dextran-rhodamine (red). (b) Silk tubes were functionalized with HRP enzyme and incubated with DAB substrate, which turned dark brown in the presence of active HRP. Top and bottom row image show macroscopic and microscopic appearance respectively of a control sample and a HRP- functionalized sample. (c) CLSM 3D projection images of hMSCs cultured on scaffolds containing collagen-I functionalized (`+COLL') and unfunctionalized (`−COLL') silk tubes. (d) DNA content of samples in functionalized (`+COLL) or unfunctionalized (`−COLL') silk tubes after 24 hours of culture (error bars=s.d.;*p<0.05; n=3). Scale bars are (a) 150 μm, (b) 100 μm, and (c) 300 μm.
To ensure that the bioactive agents introduced into silk tubes remain functionally active, HRP enzyme was introduced. Active HRP enzyme converts DAB substrate to its oxidized form, which appears as a brown precipitate. Upon application of DAB substrate to the scaffold, a brown stain was observed, indicating that HRP remained functional during the scaffold assembly process (Fig. 3b). The brown stain was considerably more prominent around the tubes, compared to the rest of the scaffold, but brown precipitate was also observed in the scaffold bulk. To demonstrate that this is the result of DAB precipitate diffusing out from the tubes rather than HRP present in the scaffold bulk, DAB was added directly onto cryosections of the scaffold and the reaction was observed under the microscope. In this case, DAB conversion to its brown oxidized form was clearly confined to silk tubes and was not present in the scaffold bulk (Fig. 3b).
To determine if the bioactive agents introduced into silk tubes could be recognized by cells, silk tubes were loaded with collagen-I and cultured with hMSCs. After 14 days post-seeding, CLSM revealed that collagen-I samples encouraged hMSCs proliferation deeper into the hollow tube compared to the unfunctionalized samples (Fig. 3c). Moreover, the DNA content assay revealed that the collagen-I samples supported significantly higher hMSC attachment at 24 hours post-seeding (Fig. 3d).
3.3 Morphological and mechanical properties of the silk scaffold bulk
Scaffold bulk pore size distribution, pore shape and pore orientation were a function of the freezing process. The ice crystals that formed in the silk solution during the freezing step acted as porogens and dictated the features of the pores once the scaffold was lyophilized. Pore shape and orientation were controlled by the freezing direction. When the silk solution was isotropically frozen at either −20°C or −80°C, the pores were relatively round and randomly oriented (Fig. 4a). According to MIP, samples frozen at −80°C had median pore diameter of 28 μm and an interquartile range (IQR) of 22 μm to 31 μm. Median pore diameter when frozen at −20°C was higher at 72 μm with an IQR of 48 μm to 93 μm (Fig. 4b). The average porosity was similar for the two scaffolds with 87.2% porosity when frozen at −20°C and 81.8% when frozen at −80°C (Fig. 4c).
Figure 4. Scaffold bulk pore morphology, size, porosity and mechanical properties.
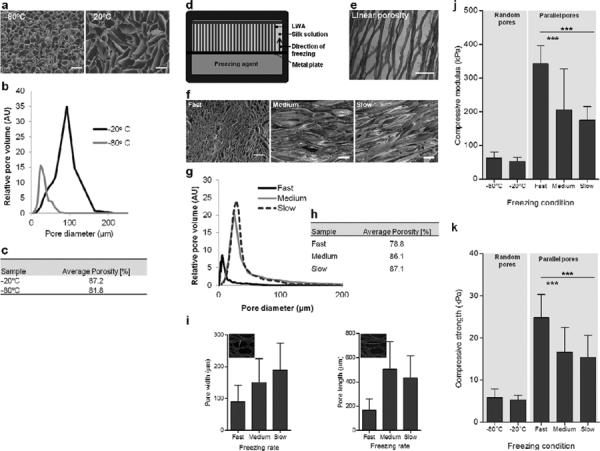
(a–c) Scaffolds formed by isotropic freezing at −20°C and −80°C produced pores that were randomly oriented. (a) SEM micrographs of scaffold cross-sections. (b) Pore size distribution determined by MIP. (c) Porosity determined by MIP. (d–i) Aligned pores were formed using an anisotropic freezing method. (d) Illustration of the anisotropic freezing setup. (e) Hematoxylin and eosin stained scaffold section showing aligned pores. (f) SEM micrographs of scaffold cross-sections created using three different freezing rates. `Fast' freezing rate was 2.9 mm/min, `Medium' freezing rate was 0.7 mm/min and `Slow' freezing rate was 0.4 mm/min. (g) Pore size distribution determined by MIP. (h) Porosity determined by MIP. (i) Pore width and length determined from CLSM images, as represented in inset images (error bars = s.d.; n=50; 10 measurements/image, 5 images/condition). (j–k) Compressive loading of scaffold bulk with random and aligned pores tested in hydrated state. (j) Compressive modulus and (k) compressive strength (error bars = s.d.; *** p<0.001 and n=8). Scale bars are 200 μm.
Silk scaffolds frozen in a custom designed PDMS mold (Fig. 4d), in which the freezing process proceeded in a single direction, contained pores that had an elongated shape and were aligned parallel to one another (Fig. 4e). Three different freezing agents were employed to achieve three different freezing rates referred to as `fast', `medium' and `slow'. The pores within the resultant scaffolds reflected the differences in the freezing rate, with pore size increasing with decreased freezing rate as observed by SEM micrographs and measured by MIP (Fig. 4f–g). Similarly, porosity increased with decreasing freezing rate where the `fast' freezing rate produced 78.8% porosity, the `medium' freezing rate produced 86.1%, and the 'slow' freezing rate produced 87.1% (Fig. 4h). Pore dimensions were further analyzed by measuring pore width and length in CLSM images of scaffold cross-sections (Fig. 4i insets). Both parameters increased with decreased freezing rate. Pore cross-section width in the middle of the scaffold was 89.1 ± 22.5 μm (range: 22.0 μm– 305.0 μm) at `fast' freezing rate, 150.2 ± 28.8 μm (range: 41.2 μm– 328.9 μm) at `medium' freezing rate and 189.3 ± 25.1 μm (range: 51.8 μm– 497.0 μm) at `slow' freezing rate. Pore cross-section length was 168.3 ± 42.9 μm (range: 46.6 μm– 406.8 μm) at `fast' freezing rate, 421.6 ± 81.1 μm (range: 127.4 μm– 1240.0 μm) at `medium' freezing rate and 601.9 ± 27.0 μm (range: 248.6 μm– 1121.5 μm) at `slow' freezing rate (Fig. 4i). For all freezing rates, the pore size increased with increasing distance from the freezing source.
Compressive properties of the silk scaffold bulk differed significantly depending on the freezing system employed. Scaffolds frozen anisotropically displayed significantly higher (p<0.001) compressive modulus (Fig. 4j) and compressive strength (Fig. 4k) compared to scaffolds created using the isotropic freezing system when compressed in the direction of pore alignment.
3.4 Channel endothelialization
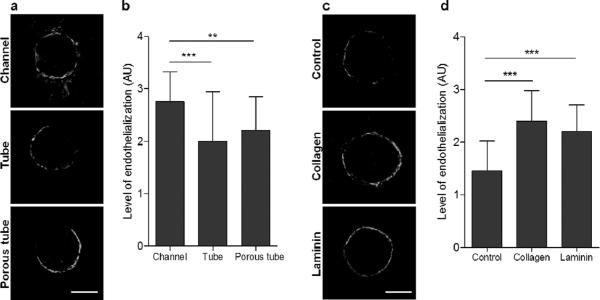
To pre-vascularize silk scaffolds in vitro, human arterial endothelial cells (hAECs) were introduced into the hollow channels with different morphologies and in the presence of bioactive agents. The channel morphologies investigated were hollow channels with open wall porosity, silk tubes and porous silk tubes. In the hollow channels with open porosity, hAECs formed a nearly contiguous layer around the channel wall by day seven post-seeding. This was observed for hollow channels with diameters ranging from 152 μm to 787 μm (Fig. S2). Cells spanned the entire channel length, as the imaging was done in the middle of the channel. Porous and non-porous silk tubes supported hAEC attachment to a lesser extent. The cells attached to the silk tube walls, but did not form fully contiguous tubes by day seven post-seeding (Fig. 5a–b). Longitudinal representation of channel endothelialization by hAECs is presented in Fig. S3.
Figure 5. The effect of hollow channel morphology and functionalization on endothelialization by hAECs.
(a–b) Effect of channel morphology on hAEC interactions. (a) Representative CLSM images of hAECs stained with calcein AM interacting with 508 μm diameter silk channels, silk tubes or porous silk tubes at day seven post-seeding. (b) Quantification of tube endothelialization by hAECs. (c–d) Effect of channel wall functionalization on hAEC interactions. (c) Representative CLSM images of hAECs stained with calcein AM interacting with 508 μm diameter silk tubes (control) or silk tubes functionalized with collagen type I or laminin at day seven post-seeding. (d) Quantification of tube endothelialization by hAECs. The level of tube endothelialization in images (b) and (d) was quantified by assigning each tube an arbitrary value between 1 and 4 corresponding to the level of endothelialization. No cells or only an occasional cell in the tube was given a score of 1. A score of 2 indicated sporadic cell coverage, with no areas of contiguous endothelialization. A score of 3 indicated >75% endothelialization with only small areas where the cell layer was not contiguous and 4 indicated complete endothelialization with a completely contiguous layer of cells around the silk tube. Error bars = s.d.;***p<0.001 and **p<0.01; n=48 (16 tubes/scaffold, 3 scaffolds/treatment). Scale bars are 300 μm.
hAEC growth in silk tubes was significantly improved by loading the tubes with collagen-I or laminin. hAECs formed nearly contiguous cell layers around the silk tubes by day seven post-seeding when these matrix proteins were present. Interestingly, there was no significant difference in the level of endothelialization between the two bioactive agents, even though laminin concentration in the silk tubes was 10-fold lower than collagen-I (Fig. 5c–d).
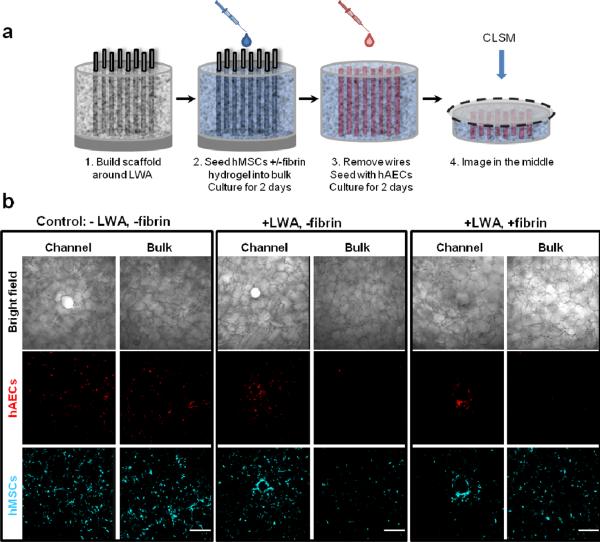
3.5 Cell compartmentalization using LWAs
LWAs were used to localize two different cell types within the scaffold, namely hMSCs into the scaffold bulk and hAECs into the hollow channels. hMSCs were introduced into the scaffold bulk in the presence or absence of a fibrin hydrogel, which was expected to reinforce compartmentalization to the bulk. Two days post-hMSC seeding, LWAs were removed and hAECs were seeded and cultured for two days (Fig. 6a). A cross-section in the middle of each sample was imaged, both where a hollow channel was present and also in the scaffold bulk, between channels to assess the extent of cell compartmentalization (Fig. 6b). Control samples were seeded in the absence of LWAs and fibrin confinement. In the control samples (−wires, −fibrin), there were no differences in cell localization. Both cell types were evenly dispersed around the hollow channels and in the bulk space. In contrast, the presence of LWAs localized hAECs to silk channels, while hMSCs remained evenly dispersed in the scaffold bulk, with some localization to the channel periphery. When fibrin confinement was not implemented (+ wires, −fibrin), hAECs were predominantly localized to the hollow channels, with cells also found in the scaffold bulk near the channels. However, when both LWAs and fibrin confinement were implemented (+wires, +fibrin), hAECs were strictly localized to the hollow channels. Regardless of fibrin confinement, when the LWA was used no hAECs were found in the scaffold bulk away from the hollow channels.
Figure 6. Cell compartmentalization using LWAs and fibrin confinement.
(a) Schematic of the experimental setup. hMSCs were initially seeded onto the scaffold, followed by LWA removal and hAEC seeding two days later. (b) Representative CLSM images of hAEC (red) and hMSC (cyan) localization around the channels and the bulk space between channels. To localize hMSCs to the scaffold bulk and hAECs to the hollow channels, hMSC were seeded in the presence or absence of a fibrin hydrogel (i.e. +/− fibrin) and with or without the LWA in place (i.e. +/− wires). Scale bars are 300 μm.
4. Discussion
Efficient and reproducible introduction of oxygen and nutrient delivery conduits into critically-sized tissue engineered constructs will significantly advance the options in the field of regenerative medicine by 1) allowing long term construct culture in vitro and 2) promoting rapid vascularization and construct integration in vivo. We present a versatile method of introducing an array of hollow channels into silk scaffolds toward this goal. A major advantage of the present system is the high level of control over the properties of the hollow channels and the surrounding bulk silk matrix, which enables the formation of `tailored' scaffolds for a variety of tissue engineering purposes, where degradation rate, mechanical properties, bioactive components and localized features are all controllable (Fig. 7).
Figure 7. The silk scaffold platform contains a hollow channel space and a porous bulk space that can be exploited for engineering complex tissues.
The hollow channel space serves as nutrient and oxygen delivery conduits and offers localized cell compartmentalization for a variety of applications including scaffold prevascularization. The hollow channel dimensions and functionalization can be tailored to suit the design criteria of a range of tissue types. The scaffold bulk space is composed of an interconnected porous network that can be fabricated with a range of materials properties including pore size and mechanical properties.
In this scaffold system, the hollow channel dimensions and channel wall functionalization are highly tunable. Hollow channel diameter and arrangement is determined by the wire diameter and placement within the LWA, so conceptually any channel diameter or arrangement is feasible. Wire array parameters are designed using a CAD program, which can be easily modified and precisely controlled, and are transferred to a placeholder via a laser cutter. Importantly, the hollow channels can be built into large, porous silk scaffolds. This is in contrast to similar hollow channel systems in which the scaffold thickness was limited to less than one millimeter[22, 33]. Hollow channel scaffold systems that have thickness similar to the system presented here lacked control over other morphological features, including channel spacing[24, 25]. One compelling feature of the current system that distinguishes it from previously reported similar systems is the ability to control channel morphology by introducing thin silk tubes around the hollow channel periphery and to functionalize the channels with bioactive agents. We demonstrate that functionalized hollow channel walls can localize and pattern bioactive agents within a porous scaffold bulk. These tube structures are a means of modulating cellular activity and can act as vehicles for localized presentation and delivery of drugs and other bioactive molecules. Sustained and controlled drug delivery from silk-based constructs and stabilizing effects of silk on enzymes and therapeutic products are well established (for review, please refer to Pritchard et al., 2011).
In addition to the range of tunable morphological and functionalization properties of the hollow channels, physical and mechanical properties of the scaffold bulk can be tuned, further distinguishing this platform from similar channeled systems. In particular we demonstrate control over the pore size, shape and orientation and mechanical properties of the silk scaffolds, which are known to affect cellular function and resulting tissue formation. Different tissue engineering applications have different requirements with regard to these properties[2]. For example, pore size requirements for engineering bone tissue are distinct from those required for cartilage or skin [34]. Similarly, many tissues, such as muscle and tendon, possess inherent anisotropy that needs to be engineered into the scaffold material to meet physiologic design requirements. Our ability to engineer isotropically or anisotropically arranged pores into silk scaffolds makes this system a more comprehensive platform. Furthermore, combinations of the two pore orientations may assist in engineering of important tissue interfaces such as bone-ligament and muscle tendon junctions. Scaffold freezing method had a significant effect on the stiffness and ultimate tensile strength of the scaffold bulk, but the mechanical properties of this scaffold system can be further tailored to a specific tissue engineering purpose by altering the silk molecular weight[30], concentration[11, 35] or by blending silk with other materials. For example, loading silk scaffolds with micro-scale silk fibers increases the compressive modulus of the construct by up to 100-fold [36]. In contrast, combining silk with elastic materials, such as tropoelastin, decreases the construct stiffness and modulates cell proliferation and differentiation [37]. Additionally, controlling the β-sheet content of silk scaffolds is a powerful means of fine-tuning the mechanical properties and degradation rates of silk constructs to an extent that is yet to be demonstrated with other biopolymers [8, 28]. Further, this feature is accomplished in the absence of requirements for chemical cross-linking. The high level of control over the mechanical properties of the scaffold bulk can be utilized to engineer constructs with mechanical properties that match tissues of interest. Mechanical properties are an important consideration when engineering in vivo tissue replacements and in vitro tissue models, one example being engineered healthy and diseased myocardium, which have different matrix stiffness [38].
A major advantage of introducing hollow channels into 3D scaffolds is the ability to endothelialize the channels and therefore pre-vascularize the scaffold. Scaffold pre-vascularization has been shown to aid construct integration with the host tissue[39–41]. Unlike previously reported linear channeled systems, this silk-based platform allows the channel walls to take on a variety of surface morphologies. This can be utilized for tuning the channel morphology for optimal cell attachment and proliferation. Human aortic endothelial cells interacted preferentially with highly porous channels, likely due to greater contact with the surrounding fibrin hydrogel, and with silk tubes loaded with either collagen-I or laminin. It is important to note that the utility of hollow channels is not limited to nutrient delivery and scaffold pre-vascularization, but can be employed in designing tissue replacements or models of other tubular structures such as renal proximal tubules, lymphatic tubules and peripheral nerves.
Finally, unlike previously reported linear channeled systems, this silk-based platform allows for tunable cell compartmentalization into hollow channels versus the bulk scaffold. The coordinated use of the LWA and a confining hydrogel enables cells to be cultured separately while allowing for cell-cell contact. In this study the presence of both the LWA and confinement of the fibrin were required for defined separation of mesenchymal and endothelial cells. This seeding regime is useful for preparing scaffolds with endothelialized channels. Other users may favor a less defined separation for increased interaction between the cell types or may prefer to induce cell compartmentalization through chemical cues loaded into different scaffold compartments.
The ability to engineer functional, critically-sized tissue constructs would provide significant benefits to the field of tissue engineering and regenerative medicine. In particular, healing large critically-sized wounds, such as large bone defects and soft tissue injuries remains a major challenge. Current standards of care, such as skin substitutes, commonly fail to regenerate the wound site due to insufficient vascular infiltration and nutrient delivery[42]. The methodology presented here offers a unique, efficient and reproducible means of introducing hollow channels that span scaffolds with thickness on the order of several centimeters. The high level of control over the morphological and functionalization features of the hollow channels and the surrounding bulk silk matrix make these scaffolds unique in the field, providing a highly tunable platform for a wide range of needs in the tissue engineering and regenerative medicine fields.
5. Conclusion
We report the development of a tissue engineering platform, which contains arrays of hollow channels that span the bulk of a porous scaffold and act as conduits for oxygen and nutrient delivery throughout the scaffold. The hollow channels are incorporated into the scaffold using an efficient and reproducible method and their dimensions and material properties are highly tunable. For example, the nutrient delivery conduits can be designed with a variety of channel diameters, wall-to-wall spacing, and patterns. The channel walls can be designed with variable thickness and porosity and can be loaded with bioactive agents. By altering these channel wall properties, in vitro endothelialization of the channels can be modulated. The bulk of the scaffold can be systematically designed with porosity and mechanical properties that will be optimal for building desired tissue equivalents. Depending on how the scaffolds are seeded, different cell types can be localized to the scaffold bulk and the hollow channels with varying degrees of compartmentalization. Future work will further demonstrate the utility of these scaffold platforms in building functional tissue equivalents.
Supplementary Material
Acknowledgements
We thank the NIH Tissue Engineering Resource Center (TERC) (NIH P41 EB002520) and the National Science Foundation Graduate Research Fellowship Program (NSF DGE 0806676) for support. We are grateful to G. Leisk and J. Trombetta for assistance in LWA assembly, and C. Preda for assistance in anisotropic scaffold construction. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under NSF award no. ECS-0335765. CNS is part of the Faculty of Arts and Sciences at Harvard University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Carletti E, Motta A, Migliaresi C, Haycock J. Scaffolds for tissue engineering and 3 D cell culture. Methods Mol Biol. 2011;695:17–39. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- [2].Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–39. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL. Macrophage responses to silk. Biomaterials. 2003;24:3079–85. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- [4].Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- [5].Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, et al. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–93. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- [6].Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–28. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghaznavi AM, Kokai LE, Lovett ML, Kaplan DL, Marra KG. Silk fibroin conduits: A cellular and functional assessment of peripheral nerve repair. Ann Plast Surg. 2011;66:273–9. doi: 10.1097/SAP.0b013e3181e6cff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Numata K, Cebe P, Kaplan DL. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. 2010;31:2926–33. doi: 10.1016/j.biomaterials.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–92. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- [10].Jin HJ, Chen J, Karageorgiou V, Altman GH, Kaplan DL. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 2004;25:1039–47. doi: 10.1016/s0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- [11].Kim UJ, Park J, Joo Kim H, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–8. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- [12].Lu Q, Wang X, Lu S, Li M, Kaplan DL, Zhu H. Nanofibrous architecture of silk fibroin scaffolds prepared with a mild self-assembly process. Biomaterials. 2011;32:1059–67. doi: 10.1016/j.biomaterials.2010.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pritchard EM, Kaplan DL. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv. 2011;8:797–811. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- [14].Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, et al. Stabilization of enzymes in silk films. Biomacromolecules. 2009;10:1032–4. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu Q, Wang X, Hu X, Cebe P, Omenetto F, Kaplan DL. Stabilization and release of enzymes from silk films. Macromol Biosci. 2010;10:359–68. doi: 10.1002/mabi.200900388. [DOI] [PubMed] [Google Scholar]
- [16].Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, Omenetto FG. Fabrication of silk microneedles for Controlled - Release drug delivery. Adv Funct Mater. 2011;22:1–6. [Google Scholar]
- [17].Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Pt B-Rev. 2008;14:87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- [18].Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26:434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [19].Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Pt B-Rev. 2009;15:353–70. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, et al. Endothelialized networks with a vascular geometry in microfabricated poly (dimethyl siloxane) Biomed Microdevices. 2004;6:269–78. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- [21].Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–5. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- [22].Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, et al. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- [23].Flynn L, Dalton PD, Shoichet MS. Fiber templating of poly (2-hydroxyethyl methacrylate) for neural tissue engineering. Biomaterials. 2003;24:4265–72. doi: 10.1016/s0142-9612(03)00334-x. [DOI] [PubMed] [Google Scholar]
- [24].Nazhat SN, Neel EAA, Kidane A, Ahmed I, Hope C, Kershaw M, et al. Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers. Biomacromolecules. 2007;8:543–51. doi: 10.1021/bm060715f. [DOI] [PubMed] [Google Scholar]
- [25].Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–2. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- [26].Bagnaninchi P, Yang Y, Zghoul N, Maffulli N, Wang R, Haj AJE. Chitosan microchannel scaffolds for tendon tissue engineering characterized using optical coherence tomography. Tissue Eng. 2007;13:323–31. doi: 10.1089/ten.2006.0168. [DOI] [PubMed] [Google Scholar]
- [27].Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu X, Shmelev K, Sun L, Gil ES, Park SH, Cebe P, et al. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules. 2011;12:1686–9. doi: 10.1021/bm200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lovett M, Cannizzaro C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL. Silk fibroin microtubes for blood vessel engineering. Biomaterials. 2007;28:5271–9. doi: 10.1016/j.biomaterials.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wray LS, Hu X, Gallego J, Georgakoudi I, Omenetto FG, Schmidt D, et al. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J Biomed Mater Res B Appl Biomater. 2011;99:89–101. doi: 10.1002/jbm.b.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gil ES, Kluge JA, Rockwood DN, Rajkhowa R, Wang L, Wang X, et al. Mechanical improvements to reinforced porous silk scaffolds. J Biomed Mater Res A. 2011;99:16–28. doi: 10.1002/jbm.a.33158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–2. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- [33].Chin CD, Khanna K, Sia SK. A microfabricated porous collagen-based scaffold as prototype for skin substitutes. Biomed Microdevices. 2008;10:459–67. doi: 10.1007/s10544-007-9155-2. [DOI] [PubMed] [Google Scholar]
- [34].Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010;16:371–83. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lv Q, Feng QL. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J Mater Sci Mater Med. 2006;17:1349–56. doi: 10.1007/s10856-006-0610-z. [DOI] [PubMed] [Google Scholar]
- [36].Mandal BB, Grinberg A, Seok Gil E, Panilaitis B, Kaplan DL. High-strength silk protein scaffolds for bone repair. Proceedings of the National Academy of Sciences. 2012;109:7699–704. doi: 10.1073/pnas.1119474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials. 2010;31:8121–3. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- [40].Rouwkema J, Boer JD, Blitterswijk CAV. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- [41].Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Pt A. 2009;16:115–2. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- [42].Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–75. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.