Abstract
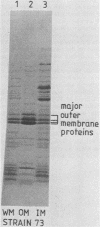
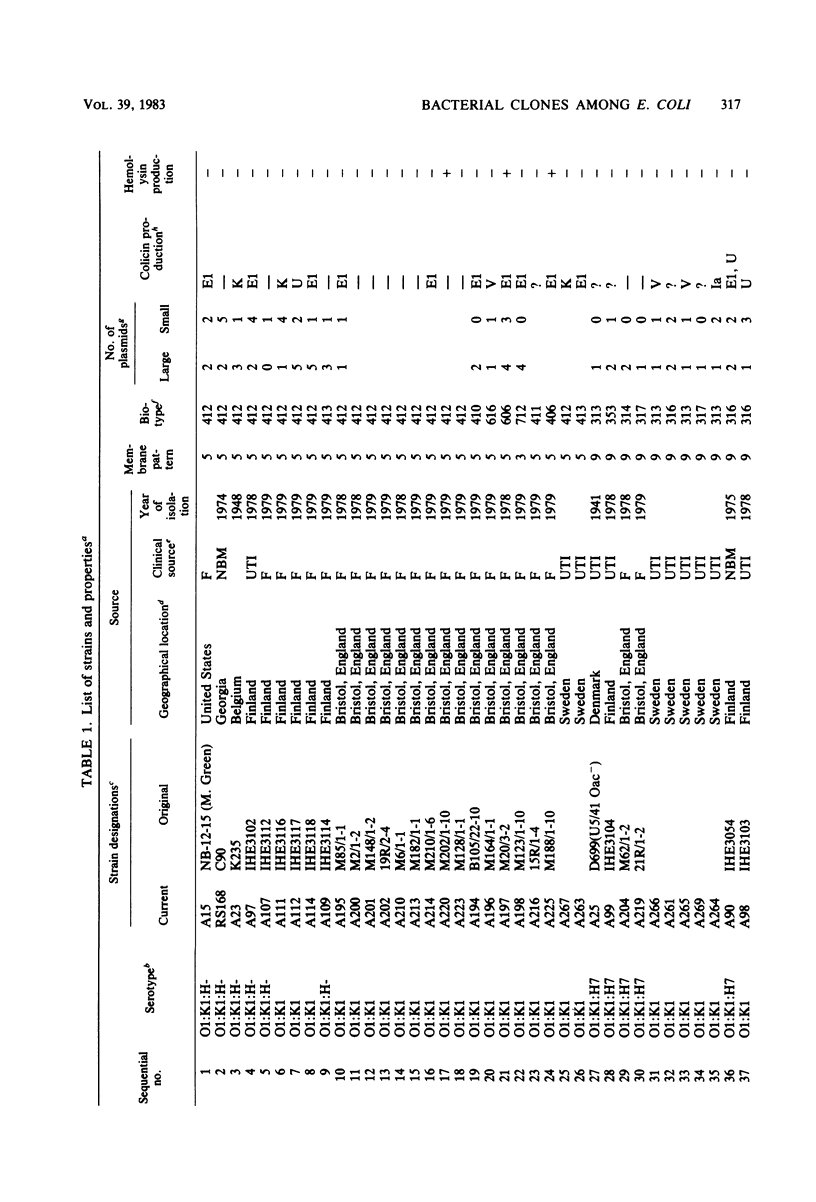
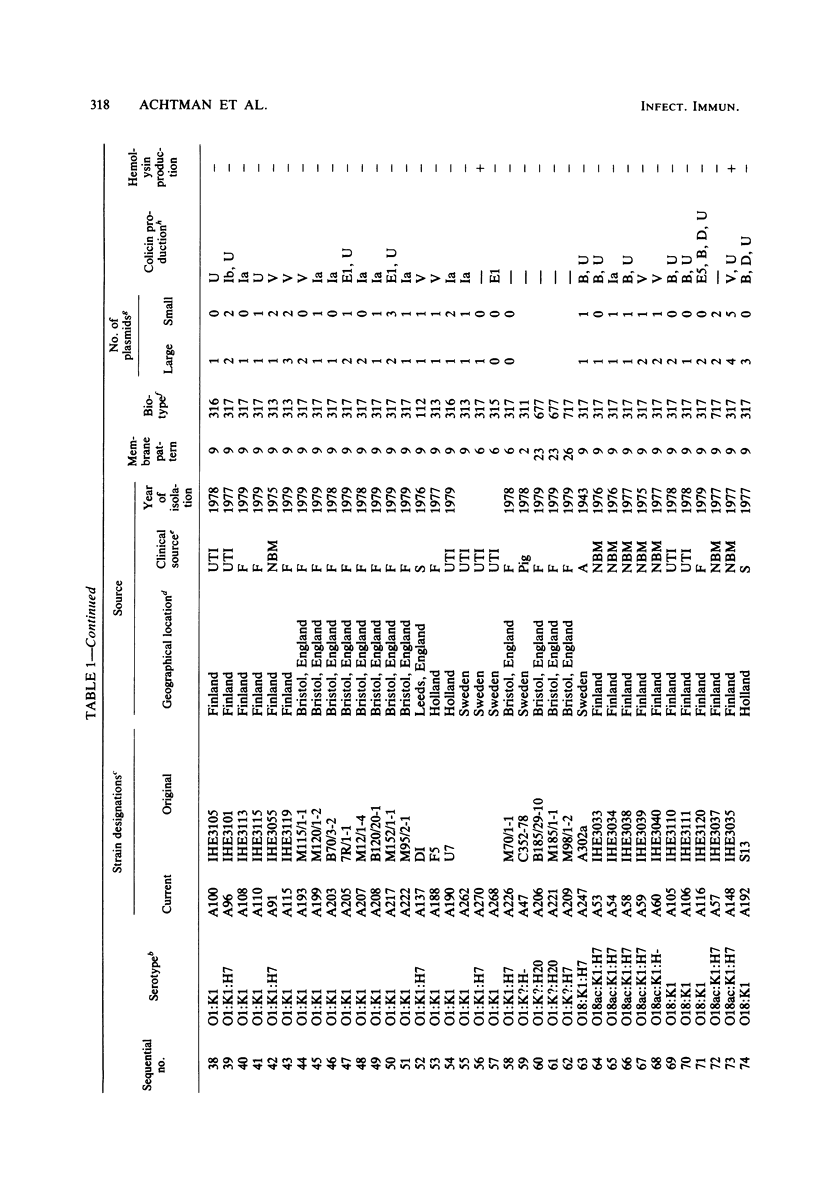
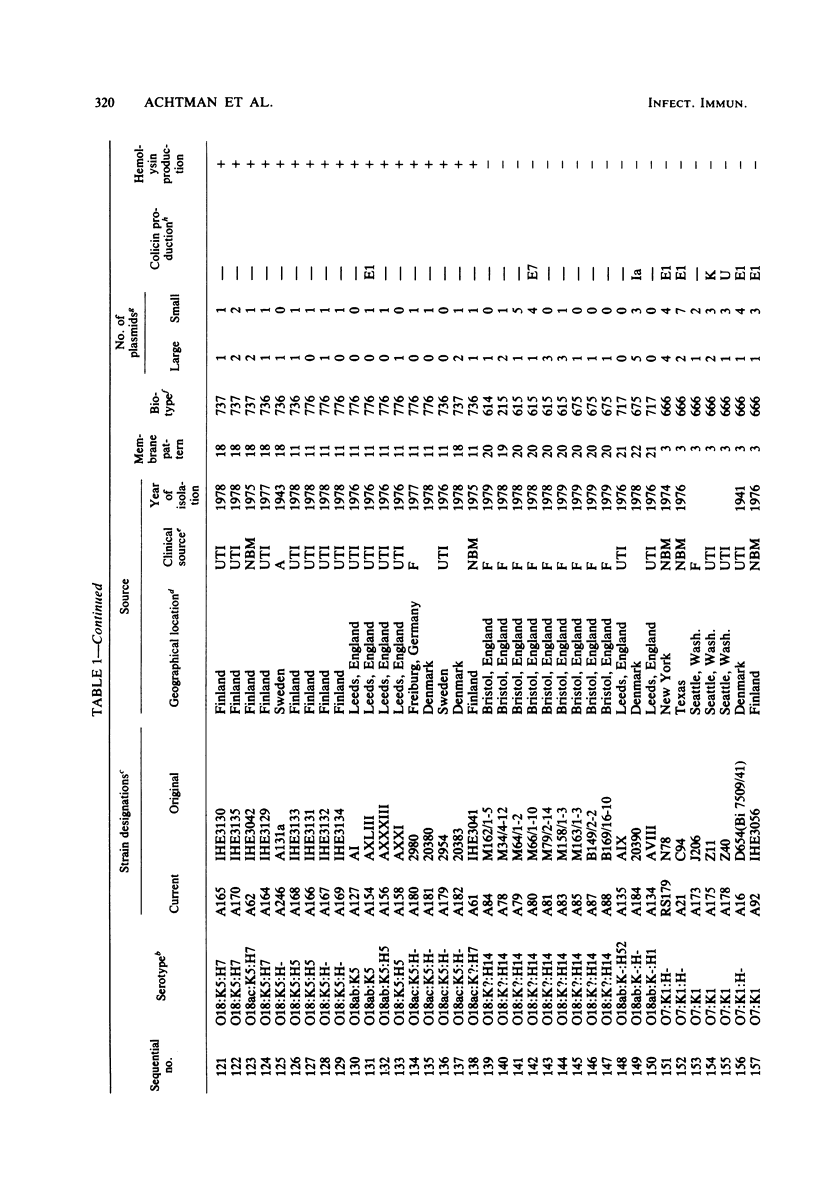
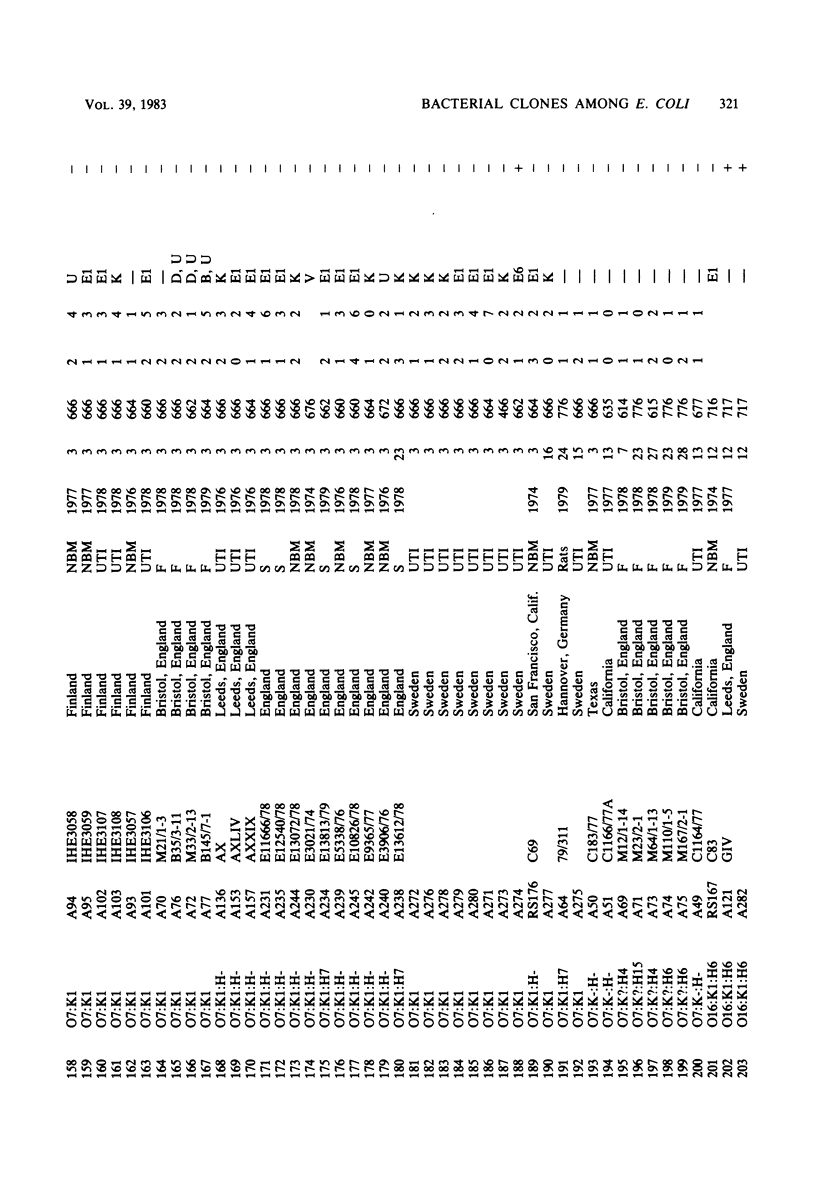
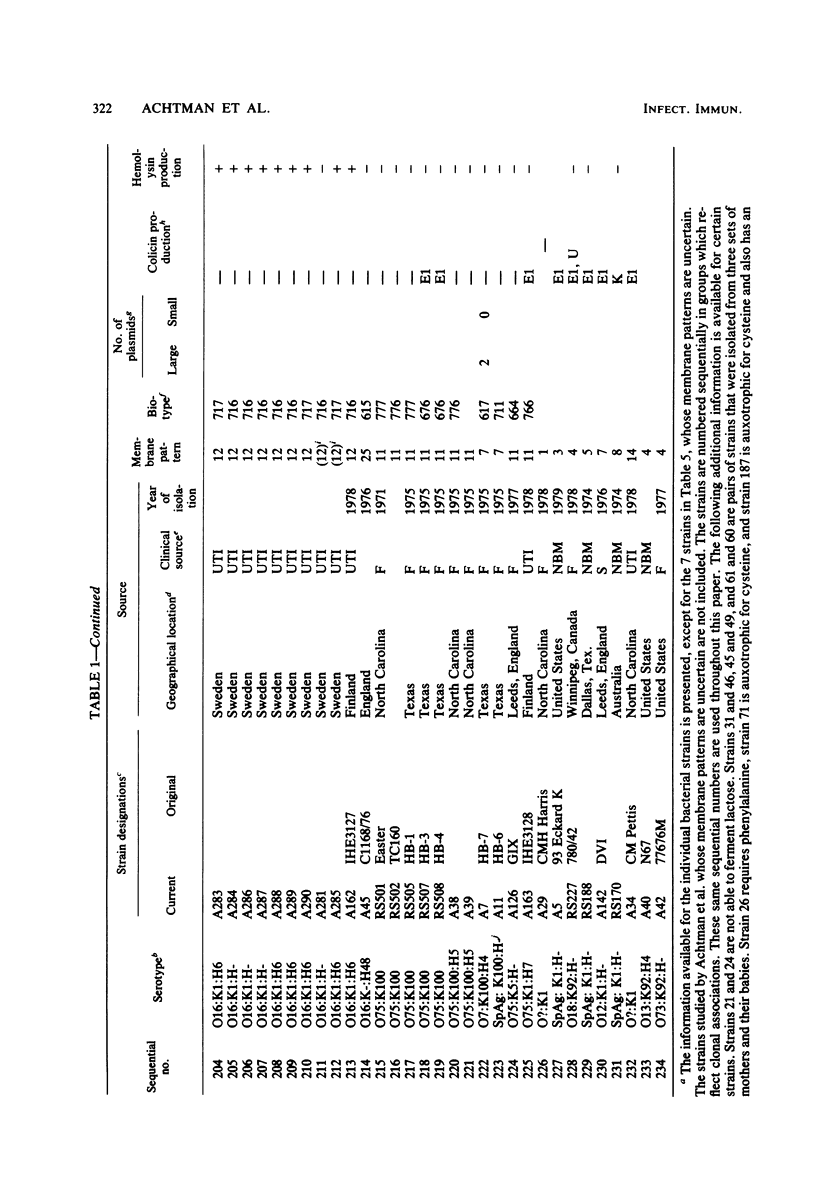
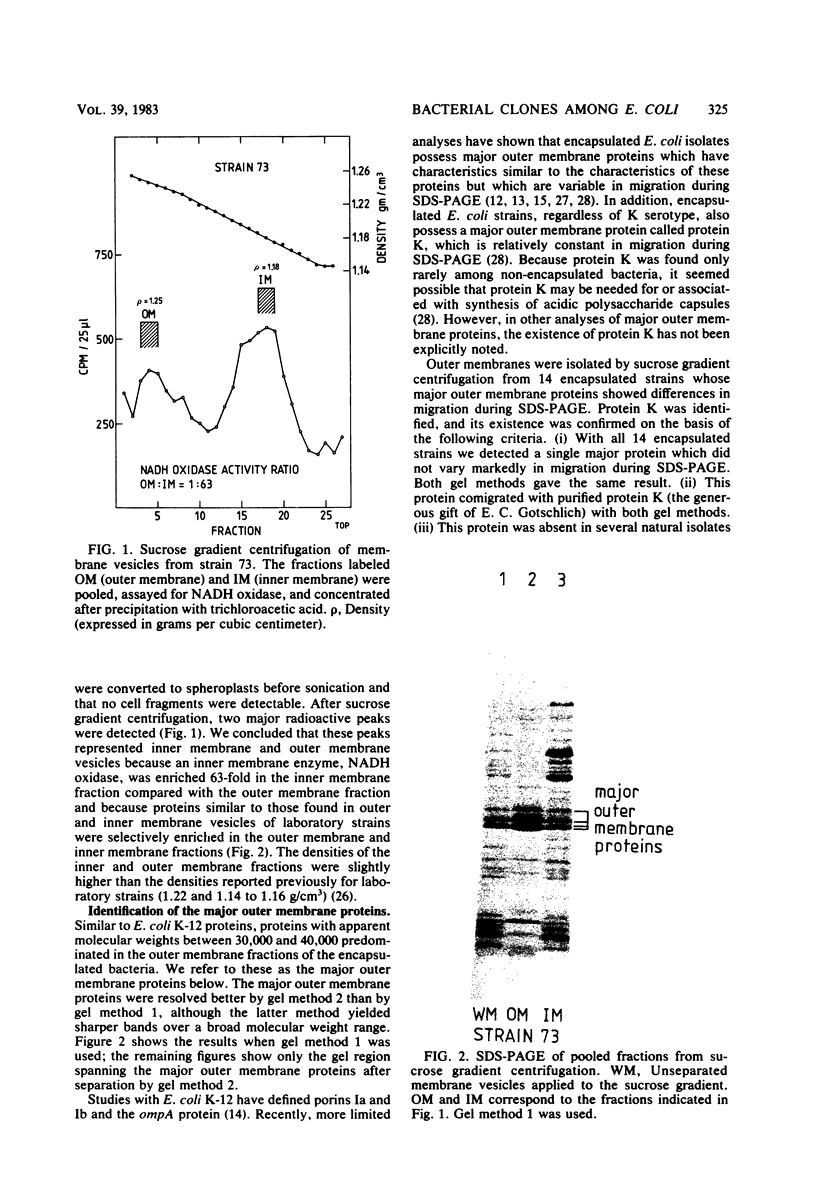
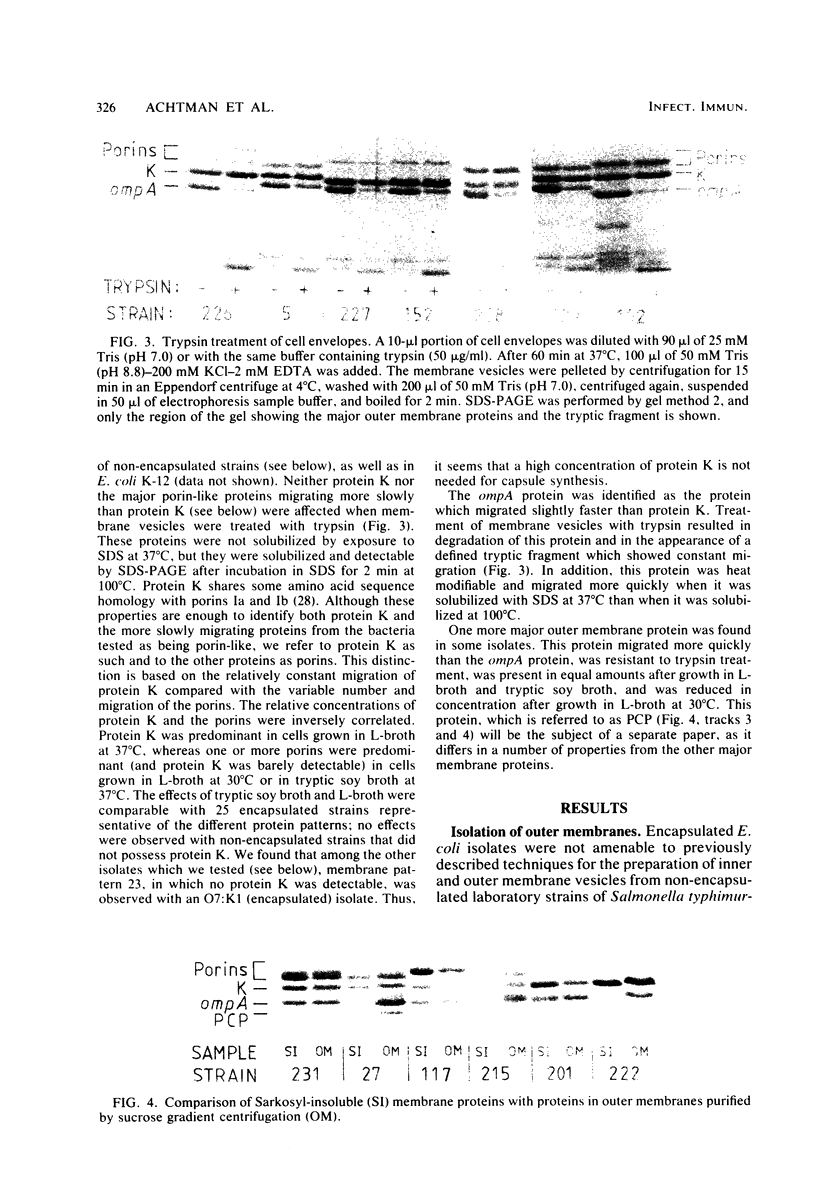
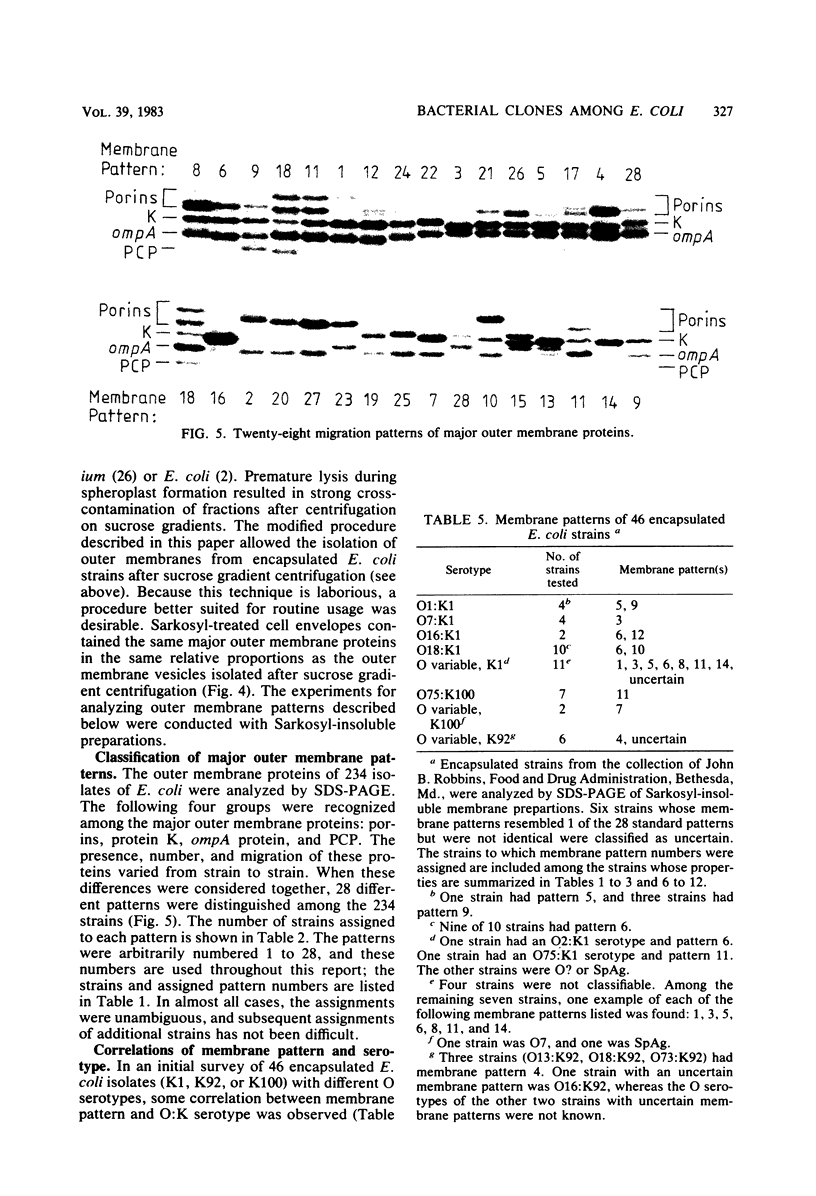
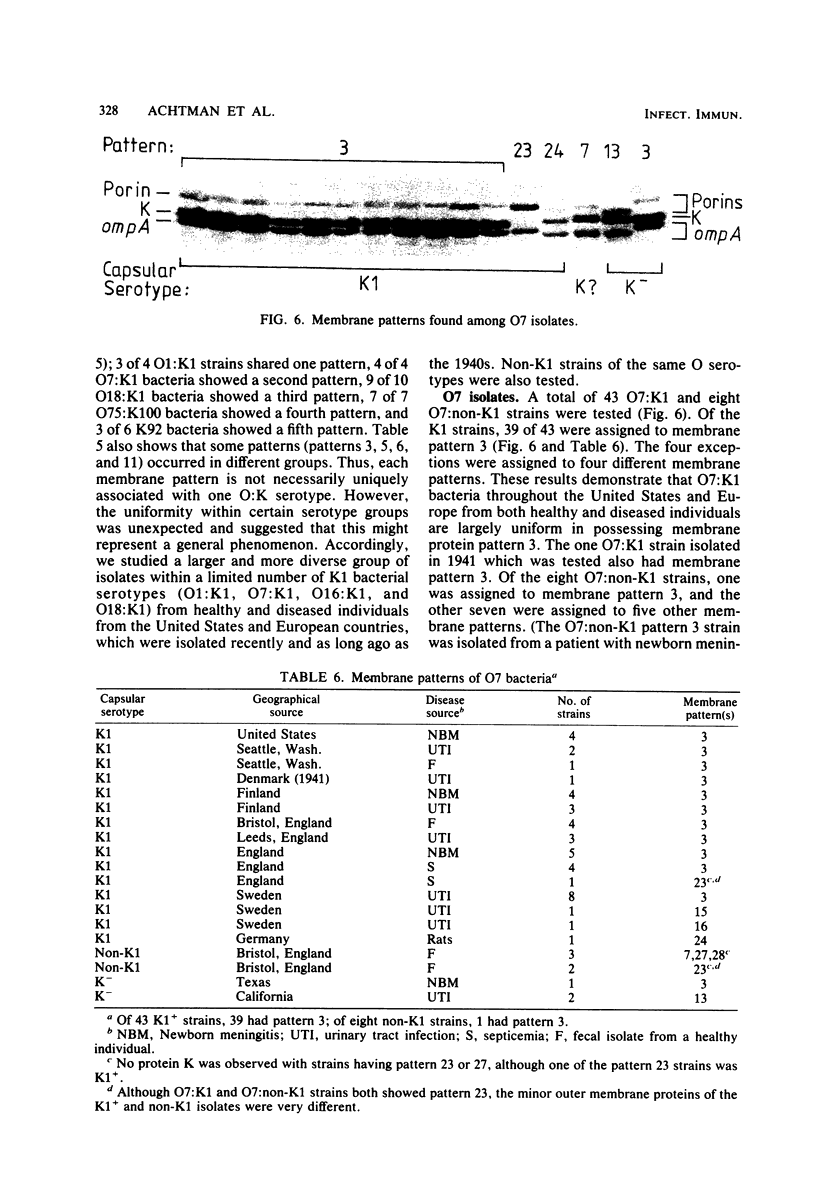
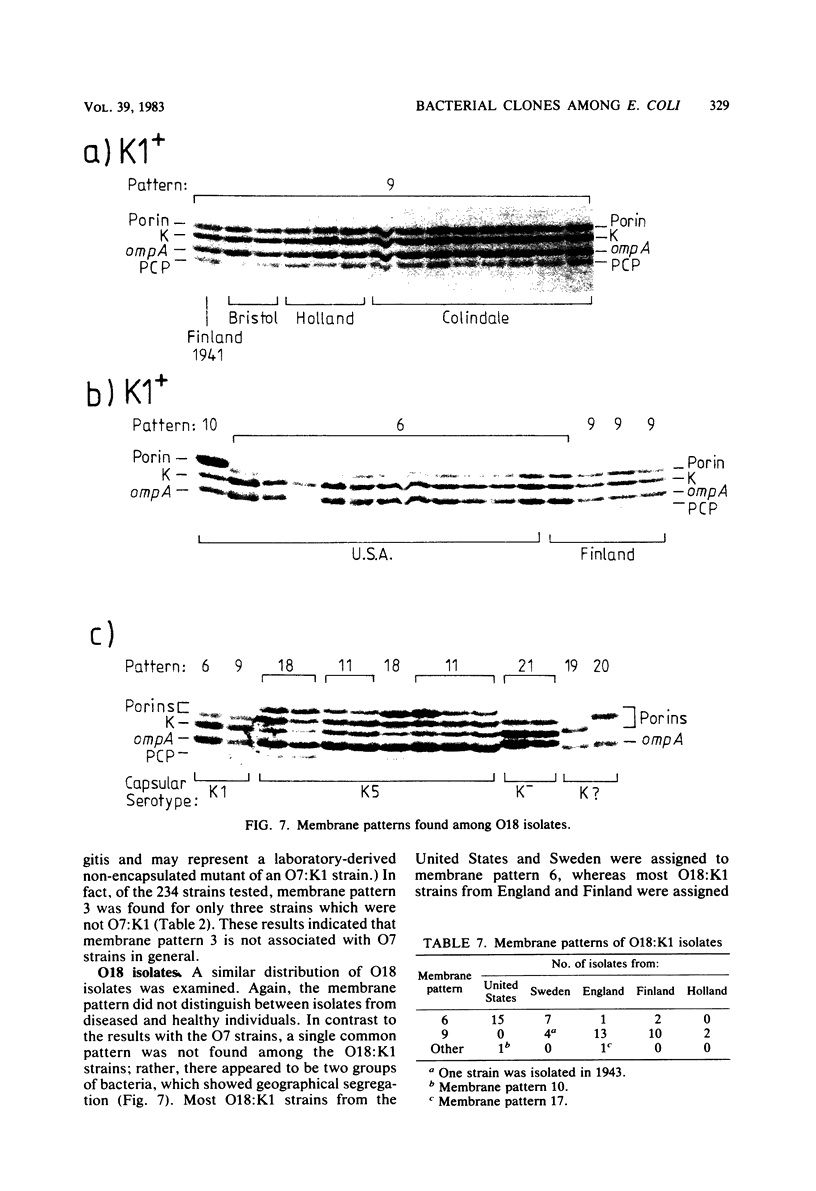
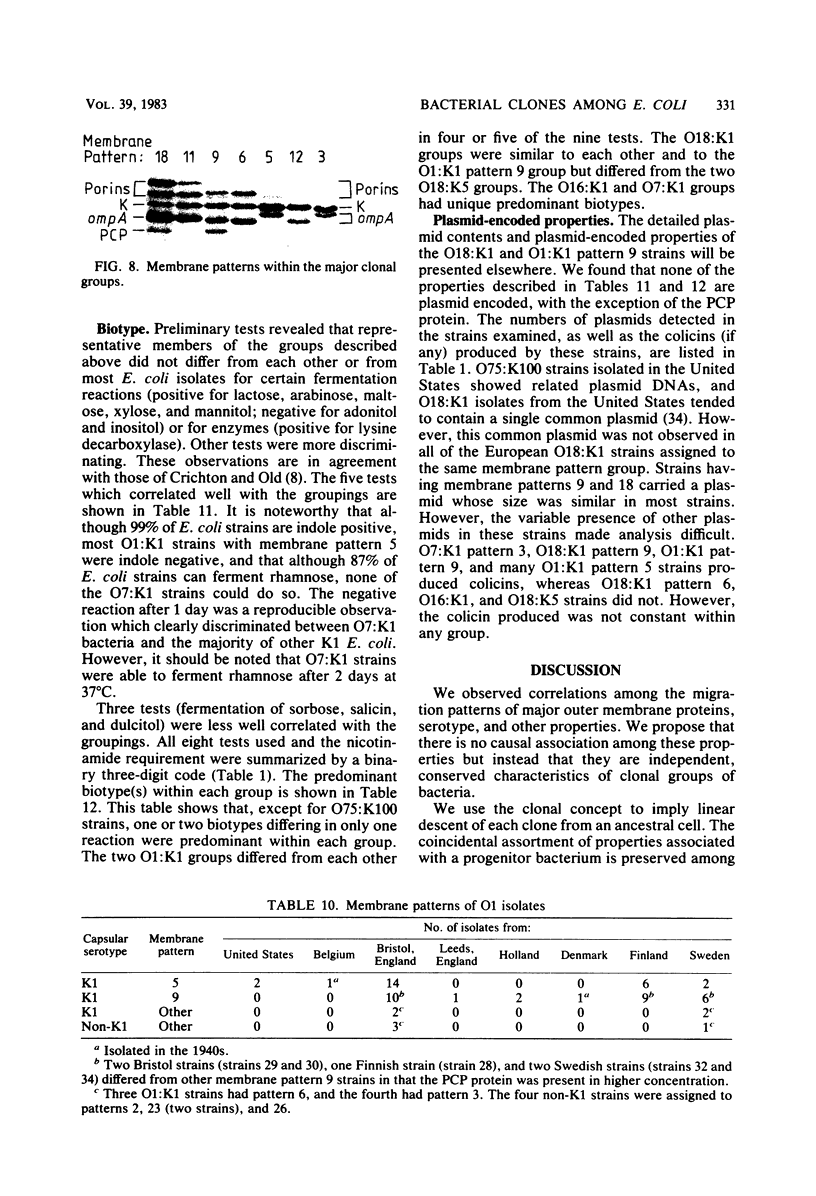
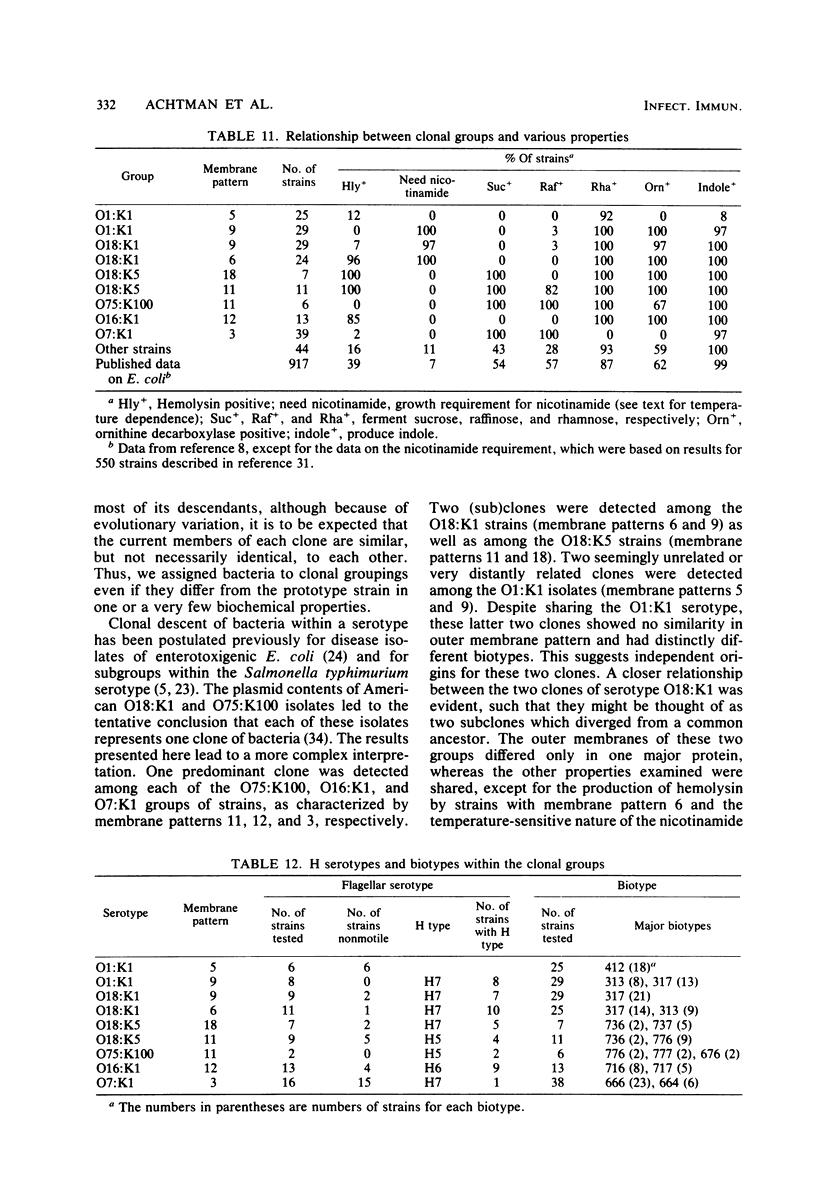
Variable properties among Escherichia coli isolates include serotype, electrophoretic migration of major outer membrane proteins, metabolic properties, production of hemolysin or colicin or both, and plasmid content. These characteristics were compared in E. coli strains of capsular types K1, K5, K92, and K100 and in non-encapsulated isolates. The 234 bacterial strains from the United States and Europe which we studied had been isolated from healthy or diseased individuals recently or as long ago as 1941. Regardless of source, most O7:K1, O16:K1, and O75:K100 isolates could be assigned to three unique, serotype-specific groups, which were interpreted as representing three bacterial clones. Two bacterial (sub)clones each were discerned among the O18:K1 and O18:K5 isolates, and two further, distinct clones were discerned among the O1:K1 isolates. The implications of these results for epidemiological analyses and for virulence are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATWOOD K. C., SCHNEIDER L. K., RYAN F. J. Periodic selection in Escherichia coli. Proc Natl Acad Sci U S A. 1951 Mar;37(3):146–155. doi: 10.1073/pnas.37.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S., Ward L. R., De Saxe M. J., Old D. C., Barker R., Duguid J. P. Correlation of phaga type, biotype and source in strains of Salmonella typhimurium. J Hyg (Lond) 1978 Oct;81(2):203–217. doi: 10.1017/s0022172400025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Crichton P. B., Old D. C. Biotyping of Escherichia coli. J Med Microbiol. 1979 Nov;12(4):473–486. doi: 10.1099/00222615-12-4-473. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., McNelis R. M., Gotschlich E. C. Strain-specific variation in the protein and lipopolysaccharide composition of the group B meningococcal outer membrane. J Bacteriol. 1976 Aug;127(2):973–981. doi: 10.1128/jb.127.2.973-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bulling E., van Leeuwen W. J., van Embden J. D., Guinée P. A., Portnoy D., Falkow S. R-factor cointegrate formation in Salmonella typhimurium bacteriophage type 201 strains. J Bacteriol. 1981 May;146(2):444–452. doi: 10.1128/jb.146.2.444-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Antigenic cross-reactivity of major outer membrane proteins in enterobacteriaceae species. J Gen Microbiol. 1979 Apr;111(2):293–302. doi: 10.1099/00221287-111-2-293. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Major outer membrane proteins: common antigens in enterobacteriaceae species. J Gen Microbiol. 1980 Jul;119(1):123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- Kaijser B. Immunology of Escherichia coli: K antigen and its relation to urinary-tract infection. J Infect Dis. 1973 Jun;127(6):670–677. doi: 10.1093/infdis/127.6.670. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Transduction of fimbriation demonstrating common ancestry in FIRN strains of Salmonella typhimurium. J Gen Microbiol. 1979 Jun;112(2):251–259. doi: 10.1099/00221287-112-2-251. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Paakkanen J., Gotschlich E. C., Mäkelä P. H. Protein K: a new major outer membrane protein found in encapsulated Escherichia coli. J Bacteriol. 1979 Sep;139(3):835–841. doi: 10.1128/jb.139.3.835-841.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson J. P., Goldschmidt R. M., Curtiss R., 3rd Potential of Escherichia coli isolated from nature to propagate cloning vectors. Nature. 1980 Jan 3;283(5742):104–106. doi: 10.1038/283104a0. [DOI] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Sutton A., Schneerson R. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect Immun. 1980 Jul;29(1):200–206. doi: 10.1128/iai.29.1.200-206.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunert A. Escherichia coli in laboratory animals. Z Versuchstierkd. 1978;20(1):19–27. [PubMed] [Google Scholar]
- Welch W. D., Spurgeon L., Kitts D., Moyed H. S., Thrupp L. D. Unique temperature-sensitive nutritional requirements of bacteremic Escherichia coli isolates. J Clin Microbiol. 1981 Mar;13(3):606–608. doi: 10.1128/jcm.13.3.606-608.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]