Abstract
Purpose
An early age at menarche is associated with disordered eating in women. However, it is unclear whether they share genetic factors. The goal of the current study is to delineate the genetic correlation between age at menarche and disordered eating.
Methods
Participants included 427 monozygotic and 329 dizygotic 16-17 year-old female twins from the Swedish Twin Study of Child and Adolescent Development. Disordered eating was assessed with the Eating Disorder Inventory-2. Age at menarche was assessed via self-report. A bivariate correlated factors model was used to delineate the genetic correlation between age at menarche and disordered eating.
Results
The analysis revealed a negative genetic correlation of −.18 in the best-fit model indicating that the genetic factors that influence younger age at menarche are associated with increased liability for disordered eating.
Conclusion
Future research should examine possible causes for this correlation such as the estrogen system and gene-environment interactions.
Keywords: disordered eating, puberty, adolescence, twin study
Early pubertal timing increases risk for disordered eating [1]. For example, adolescents reporting a younger age at menarche are more likely to report bulimic symptomatology, and women with bulimia nervosa (BN) report an earlier age of menarche compared to women with anorexia nervosa or healthy controls [2-5]. A recent population based study of adolescents also indicated that the median ages of onset for AN, BN and subthreshold eating disorders ranged from 12.3-12.6 years-old [6], which are quite similar to the average age of menarche in the general population (12.4 years-old) [7]. Additionally, girls who are more developmentally advanced, but not significantly older, than peers in regard to secondary sex characteristics report significantly more disordered eating behaviors [8]. However, studies of the association between pubertal timing and disordered eating have not been consistent and are difficult to compare as the specific aspect of puberty under investigation often varies across studies [1, 5, 9-11].
The nature of the association between pubertal timing and disordered eating is unclear. By definition, adolescence and puberty are times of significant physical, emotional, and social change. For girls, pubertal onset involves increasing adiposity and breast development which contrasts with current ideals of beauty that emphasize a body shape typical of the prepubertal state. This could lead to increased body dissatisfaction and disordered eating behaviors [11, 12]. However, the association between pubertal timing and disordered eating could also be due to common biological phenomena. For example, the estrogen system has been shown to play an important role in body weight control and food intake [13], eating behaviors fluctuate across the menstrual cycle [14, 15], variants of the FTO (fat mass and obesity associated) gene are associated with age at menarche [16], and estrogen receptor genes are associated with eating disorder symptomatology [17]. In addition, twin studies show that eating disorder symptomatology is heritable [18, 19]. Taken together, these findings raise the possibility that the estrogen system and ovarian hormones play a role in the genetic influence on disordered eating.
Indeed, twin studies suggest that estradiol may enhance or moderate the genetic influence on disordered eating: the genetic influence on disordered eating is minimal in girls with low levels of estradiol whereas the genetic effects are substantial in girls with high levels of estradiol [20]. Genetic effects for disordered eating are also almost nonexistent (~0%) in prepubertal girls and increase markedly to approximately 50%-60% in advancing-pubertal girls and young adults [21, 22]. This has led some to hypothesize that estradiol may activate the expression of genes that influence eating disorders [21, 23]. In an investigation of whether the genetic influences on bulimic symptomatology differed between girls who had begun menstruation and girls who had not begun menstruation, no differences in the genetic influences between the two groups were reported [24]. Although this finding appears to contradict those suggesting ovarian hormones moderate the genetic effects on disordered eating, menarche occurs later in the pubertal process so subtle differences in genetic effects may be missed during the later stages of puberty.
As described above, research has almost exclusively focused on whether pubertal timing and ovarian hormones moderate the genetic risk for disordered eating. However, these hormones could mediate, as well as moderate, risk. The presence of mediation would suggest that the same genetic factors are responsible for both pubertal timing and disordered eating. We are aware of only one study that has examined the possibility of mediation and addressed whether pubertal timing and disordered eating share genetic factors [25]. In this report, authors examined whether age at menarche and dieting shared genetic factors and found that common genetic influences accounted for the association between early onset menarche and dieting in adolescence [25]. This could explain the finding noted above that the genetic effects on bulimic symptomatology are not different between girls who have begun menstruation and those who have not. It is possible that age at menarche does not moderate the genetic effects on bulimic symptomatology, but plays a mediational role. The goal of the current report is to address this important gap in the literature: evaluate whether aspects of pubertal timing and disordered eating share genetic factors. We investigate whether age at menarche and disordered eating share genetic effects using a bivariate twin design. Based on previous research, we hypothesize that age of menarche will have a negative genetic correlation with a measure of disordered eating such that a younger age at menarche will be associated with increased disordered eating.
Methods
Participants
The current sample included female-female twins from the Swedish Twin Study of Child and Adolescent Development (TCHAD) [26]. TCHAD includes 1,480 twin pairs born between May 1985 and December 1986. Twins were recruited through the Swedish Medical Birth Registry and have been followed since 1994. TCHAD has had four assessment waves and only those twins who participated in Wave 3 were included in the present study as this is when the variables of interest were assessed. Wave 3 occurred in 2001-2002 when the twins were 16-17 years-old. Eighty-two percent (N = 2,369) of all twins contacted to participate in Wave 3 completed questionnaires [26]. Assignment of Twin 1 and Twin 2 in each pair was made randomly. Study questionnaires were approved by The Ethics Committee of Karolinska Institutet, Stockholm, Sweden. In Sweden, participants indicate consent by responding to and returning assessments. The University of North Carolina at Chapel Hill Institutional Review Board approved this study.
Zygosity of twins was determined based on computer algorithms that were applied to responses from questions about the twins’ physical similarity and the frequency with which people confuse them with one another. These algorithms were obtained from a discriminant analysis of 106 same-sex pairs where zygosity had been determined by typing 16 polymorphic DNA markers [26].
Measures
Disordered eating was assessed using the drive for thinness (i.e., excessive concern with dieting, preoccupation with weight and an extreme pursuit of thinness), bulimia (i.e., tendency toward episodes of binge eating that may be followed with the impulse to induce vomiting), and body dissatisfaction (i.e., belief that specific parts of the body are too large) subscales of the Eating Disorder Inventory-2 (EDI) [27]. The EDI has been translated and validated on a Swedish female population [28]. The measure was scored as indicated by the EDI manual [27] and the items comprising the drive for thinness, bulimia, and body dissatisfaction subscales were summed to create the Eating Disorder Risk Composite (EDRC). This composite score was used due to the low, subscale-specific mean scores often found in nonclinical samples [27]. If participants responded to fewer than 75% of the total items, scores were considered missing. For those participants who had missing items but responded to more than 75% of the items, the mean score for the missing question was imputed. Internal reliability of the EDRC for this sample was good (α = 0.90). Scores were log transformed prior to analysis due to a positive skew.
Age at menarche was assessed through self-report. Girls were asked if they had begun their menstrual period. If they responded ‘Yes’ they were then asked how many years and months old they were when they first got their period. Self-reported age at menarche is reliable, and accuracy is increased as the time interval between age at menarche and recall is reduced [29]. However, instability in accuracy has been suggested for the exact month menstruation began [29]. In addition, menarche is thought to be a pubertal event that occurs as part of a developmental process that includes progressive endocrine changes occurring over a prolonged period of time [30]. Therefore, the current report defined age at menarche by age in years only. Girls who had not begun menstruation were excluded from analysis.
Statistical Analysis
Bivariate correlated factor models fitted using Mx [31] were used to decompose the correlation between age at menarche and disordered eating into genetic and environmental components. Analyses were conducted using a raw data approach which allows information from both complete and incomplete pairs to be utilized. The bivariate correlated factors model estimates the proportion of variance for both age at menarche and disordered eating accounted for by additive genetic (a2), shared environmental (c2), and unique environmental (e2) factors. Additive genetic effects represent the cumulative impact of many genes (A). Shared environment refers to those environmental factors which make twins similar (C) whereas unique environment refers to those environmental factors which serve to make twins dissimilar and includes measurement error (E).
The model also yields the estimates of the genetic and environmental correlations between disordered eating and age at menarche. These correlations indicate the extent to which the same genetic (or environmental) factors influence each trait (Figure 1). For example, if the genetic correlation, ra, is estimated at unity, this would indicate that age at menarche and disordered eating share all of their genetic factors. This method does not provide information on the number or identify which genes (or environmental factors) contribute to the variance of the traits.
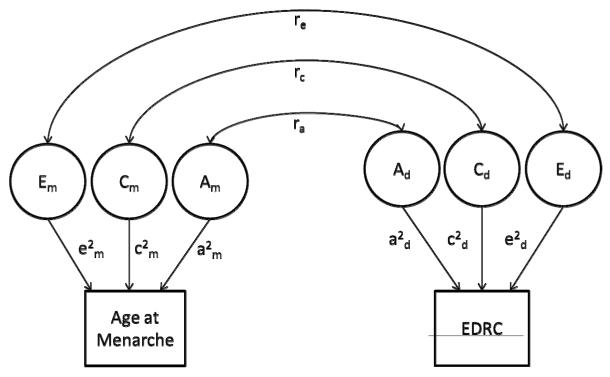
Figure 1.
Graphical depiction of full correlated factors bivariate model for age at menarche and disordered eating. The variance in liability to age at menarche is parsed into additive genetic (Am), shared environmental (Cm), and unique environmental (Em) factors. The variance in liability to the Eating Disorder Risk Composite (EDRC) is also parsed into additive genetic (Ad), shared environmental (Cd), and unique environmental (Ed) factors. Paths which are squared, to estimate the proportion of variance accounted for by genetic and environmental factors, are represented by lowercase letters (a2, c2, e2). ra indicates the correlation between the genetic effects responsible for age at menarche and the genetic effects responsible for disordered eating. rc indicates the correlation between the shared environmental effects responsible for age at menarche and the shared environmental effects responsible for disordered eating. re indicates the correlation between the unique environmental effects responsible for age at menarche and the unique environmental effects responsible for disordered eating.
The fit of the full ACE model, which estimates the genetic, shared environmental, and unique environmental influences for each trait as well as the genetic, shared environmental, and unique environmental correlations (model I), was compared to the following nested submodels: (II) setting the genetic correlation to zero, or AmCmEm, AdCdEd, rc re (hypothesis: age at menarche and EDRC share no genetic factors); (III) setting the shared environmental correlation to zero, or AmCmEm, AdCdEd, ra re (hypothesis: age at menarche and EDRC do not have shared environmental factors in common); (IV) estimating the full ACE model for age at menarche and the AE model for the EDRC, or AmCmEm, AdEd, ra re (hypothesis: twin resemblance for EDRC is due solely to genetic factors); (V) setting all shared environmental components to zero, or AmEm,AdEd, ra re (hypothesis: shared environmental factors are unimportant in twin resemblance for age at menarche, EDRC, and their overlap); (VI) setting all shared environmental components to zero and with ra = 0, or AmEm, AdEd, re (hypothesis: twin resemblance for age at menarche and EDRC is due to genetic factors only, the phenotypic correlation is due to unique environmental factors); (VII) setting all shared environmental components to zero and with re = 0, or AmEm, AdEd, ra (hypothesis: twin resemblance for age at menarche and EDRC is due to genetic factors only, and the phenotypic correlation is due to shared genetic factors); (VIII) setting all genetic components to zero, or CmEm, CdEd, rc re ( hypothesis: twin resemblance for age at menarche, EDRC, and their overlap is due to environmental factors); and (IX) setting all genetic and shared environmental components to zero or Em, Ed, re, (hypothesis: there is no twin resemblance for either trait).
Models were compared using the difference in twice the negative log-likelihood of the models, which, given certain regularity conditions, is distributed as a chi-square. A significant (p < .05) change in chi-square indicates that setting the respective parameters to zero significantly worsens the fit of the model and the parameters should be retained. Models with fewer parameters are preferable, if they do not result in a significantly worse fit, because they are more parsimonious. The Akaike Information Criterion (AIC; 2*log-likelihood+2*k where k is the number of estimated parameters) [32] allows the fit of two models to be compared and was used to compare the full model (model I) to the nested submodels (models II-IX). The model with lowest AIC is retained as the best-fitting model [33] because lower values indicate a better balance between parsimony and goodness-of-fit.
Results
Descriptive Statistics
A majority of the sample (98%, N = 756) reported experiencing menarche, which resulted in a final sample of 427 monozygotic (MZ) and 329 dizygotic (DZ) female twins (pairs with complete data: MZ = 154; DZ= 120). The average age at menarche was 12.7 (SD = 1.1) years, which is similar to the United States population average of 12.4 years-old for girls born between 1980-1984 [7], and the 1996 reported Swedish population average of 13.2 years-old [34]. The mean EDRC for the final sample was 9.2 (SD = 10.0). In general, as age at menarche increased, EDRC scores decreased (Table 1).
Table 1.
Mean (SD) of Eating Disorder Risk Composite (EDRC) Score by Age at Menarche
| Age at Menarche | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| N | 3 | 9 | 61 | 196 | 239 | 110 | 29 | 2 | 1 |
| EDRC | 17.67 | 14.71 | 11.43 | 9.66 | 9.12 | 7.27 | 8.00 | 3.5 | 15.00 |
| Mean (SD) | (6.35) | (13.67) | (10.26) | (9.90) | (10.51) | (8.10) | (9.00) | (4.95) | (0.00) |
Twin Analysis
Twin correlations are presented in Table 2. The within-twin, cross-trait correlations are negative indicating an inverse relation between age at menarche and disordered eating such that an earlier age at menarche is associated with a higher EDRC score; the Pearson correlation between age at menarche and the EDRC across zygosity groups was r(650) = −.12, p < .01. The cross-twin, within-trait correlations (i.e., intraclass correlations) for both age at menarche and the EDRC were stronger in MZ twins (age at menarche = .76; EDRC = .68) than DZ twins (age at menarche = .32; EDRC = .42). This suggests that genetic influences are important for both variables. The cross-twin, cross-trait correlations were small yet stronger for MZ twins (−.16; − .07) than DZ twins (−.02; −.04). This pattern is consistent with common genetic factors contributing to the association between age at menarche and disordered eating.
Table 2.
Twin Correlations Between Age at Menarche and Eating Disorder Risk Composite (EDRC). Monozygotic (MZ) Twins Above Diagonal, Dizygotic (DZ) Twins Below Diagonal
| Age at Menarche | Age at Menarche | EDRC | EDRC | |
|---|---|---|---|---|
| Twin 1 | Twin 2 | Twin 1 | Twin 2 | |
| Age at Menarche -Twin 1 | 1.00 | −.06 | −.16* | |
| Age at Menarche - Twin 2 | .32** | 1.00 | −.07 | −.14 |
| EDRC – Twin 1 | −.16* | −.02 | 1.00 | .68** |
| EDRC Index – Twin 2 | −.04 | −.10 | .42** | 1.00 |
p < .05
p < .01
As indicated by the change in chi-square, only models VI, VIII, and IX could be rejected as fitting significantly worse than model I (Table 3). The AIC values indicated that model VII was the best-fitting model, which included genetic and unique environment estimates for age at menarche and the EDRC score, and an estimate for the genetic correlation between age at menarche and the EDRC score. The genetic and environmental estimates for age at menarche and the EDRC score from the full and best-fitting models are presented in Table 4. In the full model for age at menarche, genetic factors accounted for 75% (95% CI: 53, 80) of variance, shared environmental factors for 1% (95% CI: 0, 21) of variance, and unique environmental factors for 25% (95% CI: 20, 31) of variance. For disordered eating, the full model estimated genetic factors at 43% (95% CI: 16, 70), shared environment at 24% (95% CI: 0, 47), and unique environment at 34% (95% CI: 27, 43). Best-fitting model results were nearly identical to the full model for age at menarche. In the best-fit model for disordered eating, genetic factors accounted for approximately 67% (95% CI: 59, 80) of variance and unique environmental factors for 33% (95% CI: 27, 41) of the variance.
Table 3.
Correlated Factor Model Fit Statistics for Age at Menarche and Eating Disorder Risk Composite.
| Model No. |
Parameters Estimated | −2lnL | Df | χ2 diff (df) | AIC |
|---|---|---|---|---|---|
| I | AmCmEm, AdCdEd, ra rc re: All genetic and environmental estimates for age at menarche and disordered eating; all genetic and environmental correlations |
3874.11 | 1387 | -- | 1100.11 |
| II | AmCmEm, AdCdEd, rc re: All genetic and environmental estimates for age at menarche and disordered eating; shared and unique environmental correlations |
3877.46 | 3.35 (1) | 1101.46 | |
| III | AmCmEm, AdCdEd, ra re: All genetic and environmental estimates for age at menarche and disordered eating; genetic and unique environmental correlations |
3874.30 | 1388 | 0.19 (1) | 1098.30 |
| IV | AmCmEm, AdEd, ra re: All genetic and environmental estimates for age at menarche; genetic and unique environmental estimates for disordered eating; genetic and unique environmental correlations |
3876.82 | 1389 | 2.72 (2) | 1098.82 |
| V | AmEm, AdEd, ra re: Genetic and unique environmental estimates for age at menarche and disordered eating; genetic and unique environmental correlations |
3876.82 | 1390 | 2.72 (3) | 1096.82 |
| VI | AmEm, AdEd, re: Genetic and unique environmental estimates for age at menarche and disordered eating; unique environmental correlation |
3885.50 | 1391 | 11.39 (4)* | 1103.50 |
| VII |
AmEm, AdEd, ra: Genetic and unique environmental estimates for age at menarche and disordered eating; genetic correlation |
3876.87 | 1391 | 2.76 (4) | 1094.87 |
| VIII | CmEm, CdEd, rc rc: Shared and unique environmental estimates for age at menarche and disordered eating; shared and unique environmental correlations |
3925.14 | 1390 | 51.02 (3)* | 1145.14 |
| IX | Em, Ed, re: Unique environmental estimates for age at menarche and disordered eating; unique environmental correlation |
4182.16 | 1393 | 308.10 (6)* | 1396.16 |
Best-fit model shown in bold. −2lnL = twice the negative log-likelihood. Df = degrees of freedom. χ2 diff = Chi-square difference from full model. AIC = Akaike information criterion. Those estimates with the subscript m refer to age at menarche. Those estimates with the subscript d refer to disordered eating. A = additive genetic. C = shared environment. E = unique environment. ra = genetic correlation. rc = shared environmental correlation. re = unique environmental correlation.
p < .05
Table 4.
Bivariate Model Parameter Estimates, Correlations, and Confidence Intervals for Full and Best-fit Models for Age at Menarche and Eating Disorder Risk Composite (EDRC)
| Age at Menarche | EDRC Score | Correlations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model No. | a2 (95% CI) |
c2 (95% CI) |
e2 (95% CI) |
a2 (95% CI) |
c2 (95% CI) |
e2 (95% CI) |
ra (95% CI) |
rc (95% CI) |
re (95% CI) |
| I AmCmEm, AdCdEd, ra rc re |
75% (53, 80) |
1% (0, 21) |
25% (20, 31) |
43% (16, 70) |
24% (0, 47) |
34% (27, 43) |
−.30 (−.77, −.02) |
+1.00 (−1, 1) |
+.02 (−.13, .17) |
|
VII AmEm, AdEd, ra |
75% (69, 80) |
-- |
25% (20, 31) |
67% (59, 80) |
-- |
33% (27, 41) |
−.18 (−.30, −.07) |
-- |
-- |
Best-fit model shown in bold. AmCmEm, AdCdEd, ra rc re = All genetic and environmental estimates for age at menarche and disordered eating; all genetic and environmental correlations. AmEm, AdEd, ra = Genetic and unique environmental estimates for age at menarche and disordered eating; genetic correlation. A = additive genetic. C = shared environment. E = unique environment. a2 = additive genetic variance. c2 = shared environmental variance. e2 = unique environmental variance. ra = genetic correlation. rc = shared environmental correlation. re = unique environmental correlation. 95% CI = 95% confidence interval.
Estimates from the best-fitting model indicate that genetic factors entirely account for the phenotypic association between age at menarche and disordered eating as we were able to constrain the shared and unique environmental correlations to zero without significantly worsening the fit of the model. In this model, the genetic correlation was estimated to be −.18 (95% CI: −.30, −.07) suggesting a small overlap in genetic factors. Approximately 5% of the total genetic variance in disordered eating is accounted for by genetic factors shared with age at menarche. However, the significantly worse fit of those models setting the genetic correlation to zero indicates that although small, shared genetic factors are significant contributors to the phenotypic association between age at menarche and disordered eating.
Discussion
The mechanism by which pubertal timing increases risk for disordered eating is unclear. We applied bivariate twin modeling to determine the extent to which shared genetic factors account for the association between age at menarche and disordered eating in girls. Results showed that a modest proportion of the genetic factors that predispose girls to early menarche also increase disordered eating. Although the overlap in genetic liability between early age at menarche and increased disordered eating was small, it was significant.
Our results are consistent with the growing body of literature suggesting that biological factors contribute to the phenotypic association between puberty and disordered eating [21-23, 25]. Several studies have suggested that pubertal timing and ovarian hormones moderate the genetic risk for disordered eating [20, 23]. Our results suggest that in addition to moderating genetic risk, pubertal timing may play a mediational role. Taken together with previous research showing that proper functioning of the estrogen system is critical for normal food intake and weight regulation and that estrogen receptors are involved in the regulation of food intake [13], and that the genes involved in weight regulation are associated with age at menarche [16], we hypothesize that the genes involved in the proper functioning of the estrogen system, specifically those involved in ovarian hormones and weight regulation account for, at least part of, the underlying shared genetic factors between age at menarche and disordered eating.
Importantly, along with biological changes in the brain, puberty also involves many physical, psychological, and emotional changes all of which also likely play an important and complex role in the association between pubertal timing and disordered eating. In contrast, or in combination with the hypothesized genetic mediation of the estrogen system, the secondary effects of puberty may influence the genetic effects on disordered eating at puberty through gene-environment interactions. Gene-environment interactions occur when genetic predispositions are expressed differently in different environments [35]. For example, exposure to the cultural thin ideal and the departure from it that occurs during puberty may only increase risk for disordered eating in those girls with a genetic predisposition towards disordered eating. In other words, when puberty-related increases in adiposity occur, internalization of the cultural thin ideal may have a more significant impact on those girls with a genetic predisposition towards disordered eating. Moreover, girls who begin menstruating earlier than their friends may experience psychological distress [36] and negative feelings such as embarrassment. Negative feelings associated with an early onset menarche may again have a more significant impact on those girls with a genetic predisposition towards disordered eating. Gene-environment interactions can increase the observed additive genetic influences of a trait if the environmental factor is shared between the co-twins. Gene-environment interactions could account for the observed increase in genetic effects on disordered eating at puberty, but would not contribute to the genetic correlation. The genes involved in the estrogen system and in weight regulation likely interact with aspects of the environment to further increase vulnerability to eating disorders at puberty.
The exact mechanism by which the estrogen system and ovarian hormones may influence the genetic vulnerability to disordered eating has not been elucidated. However, ovarian hormones may both mediate and moderate the genetic risk for disordered eating. As noted, we posit that the genetic factors involved in ovarian hormones partially underlie the shared genetic factors observed in this study. Ovarian hormones, specifically estradiol, may also directly moderate genetic vulnerability by activating the genetic risk for disordered eating and/or indirectly influence risk through the physical, psychological, and emotional changes that occur with puberty. We also speculate that ovarian hormones influence the genetic vulnerability of disordered eating, at least in part, through epigenetic moderating mechanisms. Reproductive hormones play a role in epigenetic changes in the brain by altering DNA methylation, which alters gene expression [37]. Preliminary studies suggest profound DNA methylation changes at pubertal onset in humans [38] and rats [39]. Although purely speculative, epigenetic changes at puberty could, in part, be responsible for the association between reproductive hormones and disordered eating. Moreover, these epigenetic changes could also explain the differences in genetic effects observed between prepubertal and postpubertal girls.
There are limitations to this study that warrant discussion. First, the age range of our sample (16-17 years-old) is limited. Menarche onset occurs in the later stages of puberty so the majority of the sample is in advanced or postpubertal stages. Second, this sample comes from a single birth cohort. Third, the prevalence of eating disorders in this Swedish cohort may differ from other countries. However, the United States, Sweden and other Scandinavian countries have reported similar prevalence of eating disorders [40]. Fourth, this study was cross-sectional in nature therefore we are unable to determine causality. Finally, we used a measure of disordered eating attitudes and behaviors and not eating disorder diagnoses. It is possible these results would not translate to a clinical population.
Our results confirm the association between puberty and disordered eating and suggest this association is due to genetic factors. Adolescence and puberty are well-documented vulnerability markers for eating disorders in girls, and our results suggest this is in part due to shared genetic factors between pubertal timing and disordered eating. These shared genetic factors may be due to reproductive hormones. Ideally, future twin studies could extend these findings through prospective, longitudinal examinations, including direct measurements of reproductive hormones, across early childhood and adolescent development in order to fully characterize the developmental trajectory of disordered eating during puberty.
Acknowledgements
Dr. Baker was supported by National Institute of Health grant T32MH076694 (PI:Bulik). This project was also supported by funds from the Swedish Council for Working Life and Social Research and the Swedish Research Council provided to Dr. Paul Lichtenstein.
Abbreviations
- AN
Anorexia Nervosa
- BN
Bulimia Nervosa
- TCHAD
Swedish Twin Study of Child and Adolescent Development
- EDI
Eating Disorder Inventory-2
- EDRC
Eating Disorder Risk Composite
- AIC
Akaike Information Criterion
- MZ
Monozygotic twins
- DZ
Dizygotic twins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript were presented at the International Conference on Eating Disorders, April 2011, Miami, Florida
Implications and Contributions
This report indicates that a proportion of the genetic factors responsible for disordered eating are shared with the genetic factors responsible for early age of menarche. This is one of the first reports to examine whether features of puberty and eating disorders share genetic factors.
References
- [1].Jacobi C, Hayward C, de Zwaan M, Kraemer H, Agras W. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- [2].Kaltiala-Heino R, Rimpela M, Rissanen A, Rantanen P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J Adolesc Health. 2001;28:346–352. doi: 10.1016/s1054-139x(01)00195-1. [DOI] [PubMed] [Google Scholar]
- [3].Kaltiala-Heino R, Marttunen M, Rantanen P, Rimpela M. Early puberty is associated with mental health problems in middle adolescence. Soc Sci Meds. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- [4].Fairburn CG, Cooper Z, Doll H, Welch SL. Risk factors for anorexia nervosa. Arch Gen Psychiatry. 1999;56:468–476. doi: 10.1001/archpsyc.56.5.468. [DOI] [PubMed] [Google Scholar]
- [5].Fairburn CG, Welch SL, Doll HA, Davies BA, O’Connor ME. Risk factors for bulimia nervosa. A community-based case-control study. Arch Gen Psychiatry. 1997;54:509–517. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- [6].Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 68:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999-2004. J Adolesc Health. 2007;40:227–231. doi: 10.1016/j.jadohealth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- [8].Killen J, Hayward C, Hammer L, et al. Is puberty a risk factor for eating disorders? AJDC. 1992;146:323–325. doi: 10.1001/archpedi.1992.02160150063023. [DOI] [PubMed] [Google Scholar]
- [9].Ackard DM, Peterson CB. Association between puberty and disordered eating, body image, and other psychological variables. Int J Eat Disord. 2001;29:187–194. doi: 10.1002/1098-108x(200103)29:2<187::aid-eat1008>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [10].Leon G, Fulkerson J, Perry C, Cudeck R. Personality and behavioral vulnerabilities associated with risk status for eating disorders in adolescent girls. J Abnorm Psychol. 1993;102:438–444. doi: 10.1037//0021-843x.102.3.438. [DOI] [PubMed] [Google Scholar]
- [11].Stice E, Presnell K, Bearman S. Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Dev Psychol. 2001;37:608–619. doi: 10.1037//0012-1649.37.5.608. [DOI] [PubMed] [Google Scholar]
- [12].Attie I, Brooks-Gunn J. Development of eating problems in adolescent girls: a longitudinal study. Dev Psychol. 1989;25:70–79. [Google Scholar]
- [13].Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- [15].Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elks CE, Perry JR, Sulem P, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eastwood H, Brown K, Markovic D, Pieri L. Variation in the ESR1 and ESR2 genes and genetic susceptibility to anorexia nervosa. Mol Psychiatry. 2002;7:86–89. doi: 10.1038/sj.mp.4000929. [DOI] [PubMed] [Google Scholar]
- [18].Bulik C, Sullivan P, Wade T, Kendler K. Twin studies of eating disorders: a review. Int J Eat Disord. 2000;27:1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [19].Klump KL, Kaye WH, Strober M. The evolving genetic foundations of eating disorders. Psychiatr Clin North Am. 2001;24:215–225. doi: 10.1016/s0193-953x(05)70218-5. [DOI] [PubMed] [Google Scholar]
- [20].Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med. 2010;40:1745–1753. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. J Abnorm Psychol. 2009;118:788–796. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Klump KL, McGue M, Iacono WG. Differential heritability of eating attitudes and behaviors in prepubertal versus pubertal twins. Int J Eat Disord. 2003;33:287–292. doi: 10.1002/eat.10151. [DOI] [PubMed] [Google Scholar]
- [23].Klump KL, Gobrogge KL, Perkins PS, et al. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- [24].Rowe R, Pickles A, Simonoff E, Bulik CM, Silberg JL. Bulimic symptoms in the Virginia Twin Study of Adolescent Behavioral Development: correlates, comorbidity, and genetics. Biol Psychiatry. 2002;51:172–182. doi: 10.1016/s0006-3223(01)01257-4. [DOI] [PubMed] [Google Scholar]
- [25].Harden KP, Mendle J, Kretsch N. Environmental and genetic pathways between early pubertal timing and dieting in adolescence: distinguishing between objective and subjective timing. Psychol Med. 2012;42:183–193. doi: 10.1017/S0033291711000961. [DOI] [PubMed] [Google Scholar]
- [26].Lichtenstein P, Tuvblad C, Larsson J, Carlström E. The Swedish Twin Study of CHild and Adolescent Development: The TCHAD-Study. Twin Res Hum Genet. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- [27].Garner D. Eating Disorders Inventory-2: Professional Manual. Psychological Assessment Resources, Inc; Odessa, FL: 1991. [Google Scholar]
- [28].Nevonen L, Clinton D, Norring C. Validating the EDI-2 in three Swedish female samples: eating disorder patients psychiatric outpatients and normal controls. Nord J Psychiatry. 2006;60:44–50. doi: 10.1080/08039480500504537. [DOI] [PubMed] [Google Scholar]
- [29].Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- [30].Dorn LD, Nottelmann ED, Susman EJ, et al. Variability in hormone concentrations and self-reported menstrual histories in young adolescents: menarche as an integral part of a developmental process. J Youth Adolesc. 1999;28:283–304. [Google Scholar]
- [31].Neale MC, Boker S, Xie G, Maes H. Mx: Statistical Modeling. 5th edition Medical College of Virginia, Department of Psychiatry; Richmond, VA: 1999. [Google Scholar]
- [32].Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- [33].Schermelleh-Engel K, Moosbrugger H, Muller H. Evaluating the fit of structural equation models: test of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online. 2003;8:23–74. [Google Scholar]
- [34].Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- [35].Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- [36].Ge X, Conger RD, Elder GH., Jr. Coming of age too early: pubertal influences on girls’ vulnerability to psychological distress. Child Dev. 1996;67:3386–3400. [PubMed] [Google Scholar]
- [37].Kaminsky Z, Wang SC, Petronis A. Complex disease, gender and epigenetics. Ann Med. 2006;38:530–544. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- [38].Lintas C, Persico AM. Neocortical RELN promoter methylation increases significantly after puberty. Neuroreport. 2010;21:114–118. doi: 10.1097/WNR.0b013e328334b343. [DOI] [PubMed] [Google Scholar]
- [39].Ojeda SR, Lomniczi A, Sandau U, Matagne V. New concepts on the control of the onset of puberty. Endocr Dev. 2010;17:44–51. doi: 10.1159/000262527. [DOI] [PubMed] [Google Scholar]
- [40].Ghaderi A, Scott B. Prevalence, incidence and prospective risk factors for eating disorders. Acta Psychiatr Scand. 2001;104:122–130. doi: 10.1034/j.1600-0447.2001.00298.x. [DOI] [PubMed] [Google Scholar]