Abstract
Background
We examined survival associated with locally advanced esophageal squamous cell cancer (SCC) to evaluate if treatment without surgery could be considered adequate.
Study design
Patients in the SEER registry with stage II–III SCC of the mid or distal esophagus from 1998–2008 were grouped by treatment with definitive radiation versus esophagectomy with or without radiation. Information on chemotherapy is not recorded in SEER. Tumor stage was defined as first clinical tumor stage in case of neo-adjuvant therapy and pathological report if no neo-adjuvant therapy was performed. Cancer specific (CSS) and overall survival (OS) were analyzed using the Kaplan-Meier approach and propensity-score adjusted Cox proportional hazard models.
Results
Of the 2,431 patients analyzed, there were 844 stage IIA (34.7%), 428 stage IIB (17.6%), 1,159 stage III (47.7%) patients. Most were treated with definitive radiation (n=1,426, 58.7%). Of the 1,005 (41.3%) patients who underwent surgery, 369 (36.7%) had preoperative radiation, 160 (15.9%) had postoperative radiation, and 476 (47.4%) had no radiation. Five-year survival was 17.9% for all patients, and 22.1%, 18.5%, and 14.5% for stages IIA, IIB, and stage III, respectively. Compared to treatment that included surgery, definitive radiation alone predicted worse propensity-score adjusted survival for all patients (CSS Hazard Ratio (HR) 1.48, p<0.001; OS HR 1.46, p<0.001) and for stage IIA, IIB, and III patients individually (all p-values ≤0.01). Compared to surgery alone, surgery with radiation predicted improved survival for stage III patients (CSS HR 0.62, p=0.001, OS HR 0.62, p<0.001) but not stage IIA or IIB (all p-values>0.18).
Conclusions
Esophagectomy is associated with improved survival for patients with locally advanced SCC and should be considered as an integral component of the treatment algorithm if feasible.
Introduction
The estimated incidence of esophageal cancer in the United States (US) for 2012 is 17,460 new cases, with an increasing tendency.1–5 Overall five-year survival has improved significantly over the past few decades but remains poor at 19%.4, 5 The optimal treatment for locally advanced but potentially resectable esophageal cancer without distant metastases has not been definitively established by randomized controlled trials and varies in practice.6 Complete surgical resection likely provides the best chance for cure in patients who do not have distant disease.7–9 However, surgery is utilized in only 30–40% of resectable cases, perhaps because esophagectomy is historically associated with significant morbidity and mortality and disappointing long-term results.7, 10
The histology distribution has changed such that adenocarcinoma is now the most common type, but squamous cell carcinoma (SCC) still accounts for 37% of esophageal cancers.1, 3 Patients with adenocarcinoma and SCC have similar observed long-term survival across major treatment modalities, suggesting that both histologies respond similarly to treatment.11 Accordingly, esophageal cancer treatment guidelines are generally the same for both adenocarcinoma and SCC.12 However, the benefit of surgical resection in improving survival compared to definitive chemoradiotherapy for esophageal SCC has been questioned.13 In particular, several randomized trials have suggested that definitive chemoradiation could offer equivalent survival to treatment that involves surgery for locally advanced, non-metastatic esophageal SCC.14–16
Because these studies have suggested that surgery does not improve survival for esophageal SCC compared to definitive chemoradiation, we sought to examine outcomes after different treatment regimens of potentially resectable mid and lower esophageal SCC using a population-based registry. The Surveillance, Epidemiology, and End Results (SEER) program is the largest population-based US cancer registry, with the latest 2008 release containing 17 registries that cover 28% of the US population.1 SEER routinely collects radiation and surgery data but lacks any information on chemotherapy use. This study tested the hypothesis that esophagectomy with or without radiation is associated with improved survival compared to definitive radiation in patients with resectable SCC of the mid and distal esophagus.
Methods
The Institutional Review Board at Duke University approved the performance of this secondary analysis of the SEER database, which contains patient demographic, tumor characteristic, treatment (surgery, radiotherapy), survival, and cause of death data. SEER*Stat 7.0.5 was used to extract data of patients 18 years or older with locally advanced SCC of the esophagus from year 1998 to 2008. Patients with SCC of the esophagus were identified using the histology SEER codes ranging from 8050 to 8089. Patients who were diagnosed by their autopsy report or death certificate only were excluded.
The tumor-node-metastasis (TNM) stage was either directly extracted from SEER or manually recoded from available SEER variables, using the 6th edition of the AJCC Cancer Staging Manual.17 For patients where tumor stage T1 and T2 were not distinguishable, an additional category T1/2 was introduced. For every patient, a single tumor stage in SEER is enclosed; it is defined as clinical tumor stage if any neo-adjuvant therapy including radiotherapy, chemotherapy, hormone therapy, or immunotherapy was performed while for patients without neo-adjuvant therapy, the pathological tumor stage is reported. Additional tumor characteristics collected included tumor grade and tumor location based on the ICD-O-3 codes. Only patients with the following tumor sites were considered and grouped for the analyses; mid esophagus [C15.1 (thoracic esophagus) and C15.4 (middle third of esophagus)]; and lower esophagus [C15.2 (abdominal esophagus) and C15.5 (lower third of esophagus)]. In addition, only patients with tumor stages IIA, IIB, and III were kept for analysis, because treatment guidelines include surgical resection as a potential therapy.12 The following patient characteristics were also extracted: gender, race, ethnicity, age (age>90 was recoded to 90 to meet patient’s health identifier regulations), marital status, and time to last available reported survival time-point. Patients living longer than 5-years were right-censored.
While SEER does contain information about surgery and radiation therapy, no information is available in relation to chemotherapy administration. Therefore, four distinct groups were defined based on the following treatments: definitive radiotherapy, preoperative radiotherapy with esophagectomy, esophagectomy only, and esophagectomy with postoperative radiotherapy. Only patients who underwent beam radiation and/or esophagectomy were analyzed; patients who had missing treatment information or had any other type of radiation or endoscopic tumor resection/local destruction were excluded. For the main analysis, we grouped patients undergoing esophagectomy +/− radiation therapy together (others) while definitive radiation was the comparator group. We then performed a priori defined subgroup analysis comparing (1) patients undergoing esophagectomy only with patients undergoing esophagectomy and radiation therapy and (2) patients undergoing preoperative with patients undergoing postoperative radiotherapy.
Comparisons of patient characteristics among the treatment groups of interest were performed using chi-square test for categorical and t-test or one-way ANOVA for continuous variables. Cancer specific survival (CSS) was assessed focusing on patients with a cause of death originating from esophageal etiology while all other deaths were right censored. In contrast, investigating overall survival (OS), all patients dying during the follow-up period were considered an event. Unadjusted survival analyses were performed both with the Kaplan Meier method comparing survival curves with the log-rank test and with unadjusted Cox proportional hazard models. Propensity score adjusted multivariable Cox proportional hazards models for CSS and OS were calculated for all treatment comparisons of interest while the propensity score was recalculated using a logistic regression model according to the underlying hypothesis. For the main analysis, surgery+/−radiation therapy served as the control group and definitive radiotherapy as the exposure group. For the comparison of surgery with radiotherapy versus surgery without radiotherapy, the latter served as the exposure group while for the comparison of postoperative versus preoperative radiotherapy, preoperative radiotherapy was considered the exposure group. The following covariates were included into the propensity score calculation: gender, age (continuous), marital status, race, ethnicity, tumor grade, location of the primary tumor, T-stage, N-stage, and year of diagnosis (grouped in 5 time-periods).18 Because all the variables used to calculate the propensity score were also potentially associated with survival, we a priori included the same set of variables in addition to the propensity score to adjust the Cox proportional hazard regression models.
To account for the potential bias that the decision to perform adjuvant radiotherapy was made intraoperatively rather than preoperatively, we performed one set of sensitivity analyses. We therefore excluded patients with postoperative radiotherapy from the main analysis comparing radiotherapy with surgery±preoperative radiotherapy.
The significance level alpha was set at 0.05. Two-sided p-values were calculated for all analyses. Data are presented as counts (percentages), means (standard deviations), or hazard ratios (95% confidence intervals) where appropriate. Statistical analyses were performed using STATA/SE version 11.2 (Stata Corporation, College Station, TX, USA).
Results
Overall, 2,431 patients with squamous cell carcinoma of the mid or distal esophagus who met the study inclusion criteria from 1998 to 2008 were identified in the SEER registry. Table 1 shows patient, treatment, and tumor characteristics for all patients and stratified by treatment strategy. Most tumors were located in the middle third of the esophagus and were treated with radiation alone. Compared to patients undergoing surgery with or without radiation, patients who received definitive radiation more often had mid-esophageal tumors, were older, and more likely male, black, and non-married. Of the patients treated with surgery, slightly more than half also had radiation. Most of the patients who had both surgery and radiation received radiation in the preoperative setting.
Table 1.
Patient characteristics including demographics, tumor -, and treatment features
| Overall | Definitive RT | Surgery +/− RT | p-value* | Preoperative RT | Surgery only | Postoperative RT | p-value† | |
|---|---|---|---|---|---|---|---|---|
| No of pts. | 2,431 | 1,426 (58.7) | 1,005 (41.3) | 369 (36.7) | 476 (47.4) | 160 (15.9) | ||
| Age (mean, SD), years | 66.5 (11.1) | 68.1 (11.1) | 64.2 (10.6) | <0.001 | 61.3 (9.4) | 67.0 (10.9) | 62.1 (10.3) | 0.008‡ |
| Female (n, %) | 871 (35.8) | 486 (34.1) | 385 (38.3) | 0.03 | 128 (34.7) | 199 (41.8) | 58 (36.3) | 0.09 |
| Race | ||||||||
| White | 1,577 (64.9) | 877 (61.5) | 700 (69.7) | <0.001 | 266 (72.1) | 333 (70.0) | 101 (63.1) | 0.33 |
| Black | 557 (22.9) | 369 (25.9) | 188 (18.7) | 61 (16.5) | 90 (18.9) | 37 (23.1) | ||
| Other/Unknown | 297 (12.2) | 180 (12.6) | 117 (11.6) | 42 (11.4) | 53 (11.1) | 22 (13.8) | ||
| Ethnicity | ||||||||
| Hispanic | 2,283 (93.9) | 1,330 (93.3) | 953 (94.8) | 0.11 | 14 (3.8) | 30 (6.3) | 8 (5.0) | 0.26 |
| Non-Hispanic | 148 (6.1) | 96 (6.7) | 52 (5.2) | 355 (96.2) | 446 (93.7) | 152 (95.0) | ||
| Marital Status | ||||||||
| Married | 1,279 (52.6) | 693 (48.6) | 586 (58.3) | <0.001 | 251 (68.0) | 245 (51.5) | 90 (56.3) | <0.001 |
| Other/Unknown | 1,152 (47.4) | 733 (51.4) | 419 (41.7) | 118 (32.0) | 231 (48.5) | 70 (43.8) | ||
| Tumor location | ||||||||
| Mid esophagus | 1,469 (60.4) | 948 (66.5) | 521 (51.8) | <0.001 | 208 (56.4) | 227 (47.7) | 86 (53.8) | 0.04 |
| Lower esophagus | 962 (39.6) | 478 (33.5) | 484 (48.2) | 161 (43.6) | 249 (52.3) | 74 (46.3) | ||
| Primary tumor (T) | ||||||||
| T1 | 164 (6.7) | 121 (8.5) | 43 (4.3) | <0.001 | 13 (3.5) | 25 (5.3) | 5 (3.1) | 0.02 |
| T2 | 508 (20.9) | 259 (18.2) | 249 (24.8) | 97 (26.3) | 127 (26.7) | 25 (15.6) | ||
| T3 | 1,167 (48.0) | 585 (41.0) | 582 (57.9) | 205 (55.6) | 274 (57.6) | 103 (64.4) | ||
| T4 | 509 (20.9) | 395 (27.7) | 114 (11.3) | 45 (12.2) | 43 (9.0) | 26 (16.3) | ||
| T1/T2 | 83 (3.4) | 66 (4.6) | 17 (1.7) | 9 (2.4) | 7 (1.5) | 1 (0.6) | ||
| Lymph node status (N) | ||||||||
| N0 | 1,046 (43.0) | 594 (41.7) | 452 (45.0) | <0.001 | 161 (43.6) | 235 (49.4) | 56 (35.0) | 0.01 |
| N1 | 1,311 (53.9) | 761 (53.4) | 550 (54.7) | 206 (55.8) | 241 (50.6) | 103 (64.4) | ||
| NX | 74 (3.0) | 71 (5.0) | 3 (0.3) | 2 (0.5) | 0 (0) | 1 (0.6) | ||
| Stage | ||||||||
| IIA | 844 (34.7) | 440 (30.9) | 404 (40.2) | <0.001 | 144 (39.0) | 216 (45.4) | 44 (27.5) | <0.001 |
| IIB | 428 (17.6) | 269 (18.9) | 159 (15.8) | 62 (16.8) | 75 (15.8) | 22 (13.8) | ||
| III | 1,159 (47.7) | 717 (50.3) | 442 (44.0) | 163 (44.2) | 185 (38.9) | 94 (58.8) | ||
| Tumor grade | ||||||||
| G1/2 (well/moderate) | 1,137 (46.8) | 652 (45.7) | 485 (48.3) | <0.001 | 170 (46.1) | 233 (49.0) | 82 (51.3) | 0.05 |
| G3/4 (poor/undifferentiated) | 944 (38.8) | 493 (34.6) | 451 (44.9) | 163 (44.2) | 221 (46.4) | 67 (41.9) | ||
| Unknown | 350 (14.4) | 281 (19.7) | 69 (6.9) | 36 (9.8) | 22 (4.6) | 11 (6.9) | ||
| Cause of death | ||||||||
| Alive | 648 (26.7) | 307 (21.5) | 341 (33.9) | <0.001 | 160 (43.4) | 131 (27.5) | 50 (31.3) | <0.001 |
| Esophagus | 1,410 (58.0) | 893 (62.6) | 517 (51.4) | 165 (44.7) | 264 (55.5) | 88 (55.0) | ||
| Other cause of death | 373 (15.3) | 226 (15.8) | 147 (14.6) | 44 (11.9) | 81 (17.0) | 22 (13.8) |
Values are counts and % if not otherwise indicated.
Comparison of radiotherapy only versus others;
Comparison of surgery only, preoperative, and postoperative radiotherapy;
One-way ANOVA. RT: radiation therapy.
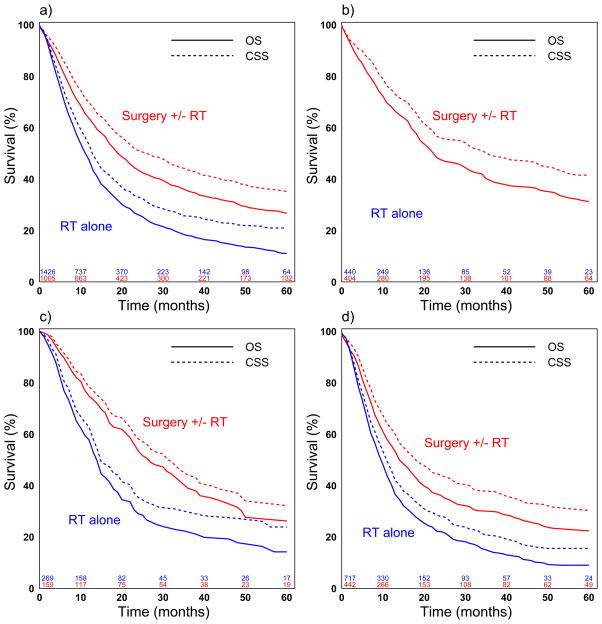
Median and five-year survival is shown in table 2 and figure 1a–d. Overall 5-year survival was 17.9% (95% CI: 16.1–19.7) while 5-year survival stratified by stage was 22.1% (CI: 18.8–25.5) for stage IIA, 18.5% (CI: 14.2–23.3) for stage IIB, and 14.5% (CI: 12.2–17.0) for stage III patients.
Table 2.
Median and 5-year CSS and OS for overall, stage IIA, IIB, and III patients (in months)
| Cancer specific survival | Overall survival | |||
|---|---|---|---|---|
| Median survival (95% CI) | 5-year survival (95% CI) | Median survival (95% CI) | 5-year survival (95% CI) | |
| Overall | 17 (16–19) | 27.1 (24.8–29.4) | 14 (13–15) | 17.9 (16.1–19.7) |
| Definitive RT | 13 (13–15) | 21.0 (18.2–23.9) | 12 (11–13) | 11.2 (9.2–13.4) |
| Surgery ± RT | 27 (23–32) | 35.3 (31.6–39.0) | 20 (18–22) | 26.9 (23.7–30.2) |
| Preoperative RT | 35 (25–50) | 43.7 (37.4–49.8) | 25 (20–33) | 35.8 (30.1–41.6) |
| Postoperative RT | 26 (20–36) | 30.1 (21.5–39.1) | 21 (17–26) | 23.9 (16.7–31.9) |
| Surgery only | 22 (19–31) | 31.0 (25.8–36.3) | 17 (15–19) | 21.8 (17.6–26.2) |
|
| ||||
| Stage IIA | 22 (20–27) | 34.6 (30.6–38.7) | 17 (16–19) | 22.1 (18.8–25.5) |
| Definitive RT | 16 (14–19) | 28.2 (22.9–33.7) | 13 (12–15) | 12.9 (9.2–17.3) |
| Surgery ± RT | 36 (27–52) | 41.7 (35.7–47.6) | 23 (20–30) | 31.4 (26.3–36.7) |
| Preoperative RT | 33 (23–84) | 42.5 (32.4–52.2) | 24 (19–35) | 35.6 (26.7–44.6) |
| Postoperative RT | 51 (21-NA) | 42.5 (25.5–58.4) | 35 (19–70) | 35.0 (20.2–50.1) |
| Surgery only | 36 (22–57) | 41.2 (32.9–49.3) | 21 (17–31) | 28.5 (21.7–35.6) |
|
| ||||
| Stage IIB | 21 (18–24) | 26.6 (21.2–32.3) | 17 (15–20) | 18.5 (14.2–23.3) |
| Definitive RT | 15 (14–20) | 23.9 (17.4–30.9) | 14 (13–16) | 14.2 (9.5–19.7) |
| Surgery ± RT | 32 (24–39) | 32.3 (23.0–41.9) | 27 (22–36) | 26.2 (18.1–35.1) |
| Preoperative RT | 36 (23-NA) | 37.4 (21.2–53.6) | 31 (17–50) | 33.5 (19.0–48.8) |
| Postoperative RT | 26 (14–42) | 13.8 (1.0–42.3) | 24 (14–34) | 12.1 (0.9–38.3) |
| Surgery only | 30 (21–49) | 32.2 (19.7–45.3) | 27 (18–38) | 24.3 (13.8–36.3) |
|
| ||||
| Stage III | 13 (12–15) | 21.6 (18.6–24.8) | 11 (11–12) | 14.5 (12.2–17.0) |
| Definitive RT | 11 (10–13) | 15.7 (12.2–19.5) | 10 (9–11) | 9.1 (6.7–11.9) |
| Surgery ± RT | 19 (16–24) | 30.6 (25.3–36.1) | 15 (13–18) | 22.8 (18.4–27.5) |
| Preoperative RT | 31 (19-NA) | 46.5 (37.4–55.1) | 25 (15–45) | 36.5 (27.9–45.1) |
| Postoperative RT | 21 (15–32) | 26.0 (15.5–37.7) | 16 (13–23) | 20.1 (11.8–30.1) |
| Surgery only | 13 (10–18) | 18.7 (12.0–26.7) | 10 (8–13) | 13.1 (8.2–19.2) |
RT: radiation therapy.
Figure 1a–d.
Cancer specific (CSS) and overall survival (OS) for radiation therapy alone versus others; overall (a), stage IIA (b), IIB (c), and III (d)
Logrank test: p<0.001 for CSS and OS for all comparisons (a–d)
Definitive radiotherapy versus surgery±radiotherapy
Overall, patients treated with surgery±radiotherapy had significantly improved median CSS (27 versus 13 months, p<0.001) and median OS (20 versus 12 months, p<0.001) compared to definitive radiation (Table 2). In both unadjusted and propensity-score adjusted Cox proportional hazard regression analysis of all patients, definitive radiation therapy was associated with worse CSS and OS compared to surgery±radiotherapy (Table 3, Figure 1a). In propensity-score adjusted subgroup analyses stratified by tumor stage, patients undergoing definitive radiation therapy had worse CSS and OS for all individual stages IIA, IIB, and III (Table 4, Figure 1b–d).
Table 3.
Cancer specific and overall survival (overall)
| Cancer specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p-value | PS-adjusted HR″ (95% CI) | p-value | Unadjusted HR (95% CI) | p-value | PS-adjusted HR″ (95% CI) | p-value | |
| Definitive RT versus others (reference) | 1.65 (1.48–1.84) | <0.001 | 1.48 (1.31–1.67) | <0.001 | 1.65 (1.49–1.82) | <0.001 | 1.46 (1.31–1.63) | <0.001 |
| Surgery+RT versus surgery only (reference) | 0.75 (0.63–0.89) | 0.001 | 0.76 (0.63–0.92) | 0.005 | 0.71 (0.61–0.83) | <0.001 | 0.75 (0.63–0.89) | 0.001 |
| Preoperative RT vs. postoperative RT (reference) | 0.81 (0.63–1.06) | 0.12 | 0.89 (0.67–1.17) | 0.39 | 0.83 (0.65–1.05) | 0.11 | 0.91 (0.71–1.16) | 0.45 |
Included into the propensity score calculation: gender, age, race, ethnicity, marital status, tumor grade, tumor location, T-stage, N-stage, and year of diagnosis (5 groups).
Covariates: propensity score, gender, age, race, ethnicity, marital status, tumor grade, tumor location, T-stage, N-stage, and year of diagnosis (5 groups).
Table 4.
Cancer specific and overall survival for stage IIA, IIB, and III
| Cancer specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p-value | PS-adjusted HR* (95% CI) | p-value | Unadjusted HR (95% CI) | p-value | PS-adjusted HR* (95% CI) | p-value | |
| Stage IIA | ||||||||
| Definitive RT versus others (reference) | 1.65 (1.36–2.00) | <0.001 | 1.54 (1.25–1.91) | <0.001 | 1.68 (1.42–1.99) | <0.001 | 1.56 (1.30–1.88) | <0.001 |
| Surgery+RT versus surgery only (reference) | 0.87 (0.65–1,17) | 0.37 | 0.99 (0.72–1.37) | 0.97 | 0.80 (0.62–1.03) | 0.08 | 0.92 (0.69–1.21) | 0.54 |
| Preoperative RT vs. postoperative RT (reference) | 1.14 (0.69–1.87) | 0.61 | 1.00 (0.59–1.69) | 0.99 | 1.14 (0.73–1.78) | 0.56 | 1.06 (0.66–1.71) | 0.81 |
|
| ||||||||
| Stage IIB | ||||||||
| Definitive RT versus others (reference) | 1.61 (1.23–2.12) | 0.001 | 1.46 (1.08–1.97) | 0.01 | 1.72 (1.34–2.21) | <0.001 | 1.51 (1.15–1.99) | 0.003 |
| Surgery+RT versus surgery only (reference) | 0.90 (0.58–1.41) | 0.65 | 0.68 (0.39–1.21) | 0.19 | 0.89 (0.59–1.34) | 0.58 | 0.79 (0.47–1.33) | 0.38 |
| Preoperative RT vs. postoperative RT (reference) | 0.66 (0.33–1.32) | 0.24 | 0.45 (0.19–1.07) | 0.07 | 0.75 (0.39–1.44) | 0.38 | 0.72 (0.32–1.62) | 0.43 |
|
| ||||||||
| Stage III | ||||||||
| Definitive RT versus others (reference) | 1.60 (1.37–1.87) | <0.001 | 1.45 (1.22–1.72) | <0.001 | 1.55 (1.35–1.79) | <0.001 | 1.37 (1.17–1.60) | <0.001 |
| Surgery+RT versus surgery only (reference) | 0.56 (0.44–0.73) | <0.001 | 0.62 (0.47–0.81) | 0.001 | 0.55 (0.44–0.69) | <0.001 | 0.62 (0.48–0.79) | <0.001 |
| Preoperative RT vs. postoperative RT (reference) | 0.73 (0.51–1.04) | 0.08 | 0.93 (0.63–1.36) | 0.70 | 0.72 (0.53–1.00) | 0.05 | 0.91 (0.64–1.28) | 0.59 |
RT: radiation therapy. Included into the propensity score calculation: gender, age, race, ethnicity, marital status, tumor grade, tumor location, T-stage, N-stage, and year of diagnosis (5 groups).
Covariates: propensity score, gender, age, race, ethnicity, marital status, tumor grade, tumor location, T-stage, N-stage, and year of diagnosis (5 groups).
Surgery only versus surgery+radiotherapy
Among all patients, CSS and OS were worse in patients undergoing esophagectomy only compared to patients undergoing esophagectomy and radiation therapy for both, unadjusted and propensity-score adjusted analysis (Table 3). While this CSS and OS benefit of the combination therapy was also detected in propensity-score adjusted results for stage III patients, survival of patients with stage IIA and IIB tumors did not significantly improve when radiation was used in addition to surgery (Table 4).
Preoperative versus postoperative radiation therapy
In subgroup analyses focusing on the comparison of preoperative and postoperative radiotherapy, no overall difference was detected for CSS and OS in both, unadjusted and propensity-score adjusted analyses of all patients (Table 3). In unadjusted analysis stratified by stage, only stage III patients showed better OS when preoperative radiotherapy was used. However, this survival benefit was not present after propensity-score adjustment as well (Table 4).
Sensitivity analysis for definitive radiotherapy versus surgery±preoperative radiotherapy
In sensitivity analysis comparing radiation therapy with surgery±preoperative radiotherapy, overall, patients undergoing radiation only had worse CSS (adjusted HR: 1.45, 95% CI: 1.28–1.65, p<0.001) and OS (adjusted HR: 1.43, 95% CI: 1.28–1.60, p<0.001). This survival benefit hold true also for patients with stage IIA, IIB, and III tumors.
Discussion
Using the population-based SEER dataset, we show that patients with locally advanced but potentially resectable squamous cell cancer tumors of the esophagus have better CSS and OS for esophagectomy+/−radiation therapy over definitive radiotherapy. This finding also holds true for the tumor subgroups stage IIA, IIB, and III, even after performing propensity score adjusted multivariable Cox proportional hazard regression analyses. We also showed that using radiation in addition to surgery improved CSS and OS for stage III tumors, no such difference was found for stage IIA and IIB tumors.
Esophagectomy has historically been considered the mainstay of treatment but has disappointing long-term outcomes.20 Many studies involving various combinations of surgery, chemotherapy, and radiation have been conducted in efforts to improve the relatively poor long-term outcomes associated with esophageal cancer, often with conflicting results.8, 21–29 The NCCN guidelines reflect the variable evidence, and allow a variety of treatments.12 Esophagectomy is recommended for non-cervical tumors that invade the submucosa. For more locally advanced tumors that at least invade the muscularis propria, the guidelines allow for the entire spectrum of possible treatment, including esophagectomy, definitive chemoradiation, preoperative chemoradiation, and preoperative chemotherapy.
Studies have suggested that definitive chemoradiation could offer equivalent survival to treatment that involves surgery for locally advanced, non-metastatic esophageal SCC.13–16 However, this study using a population-based registry with analysis limited to locally advanced but potentially resectable SCC of the mid or distal esophagus shows that surgical resection is associated with significantly improved survival. Patients in the SEER registry from 1998 to 2008 undergoing definitive radiation therapy had worse CSS and OS compared to patients undergoing esophagectomy with or without radiation in analyses of all patients as well as analyses stratified by stage. We also found that combining radiation with surgery improved survival of stage III but not stage IIA and IIB patients. The timing of radiation did not impact survival in any of the analyses, though use in the induction setting has potential advantages of tumor downstaging that may improve resectability, improved tolerance as compared to postoperative treatment, and avoiding potential radiation damage to the esophageal replacement conduit.
The long-term survival benefit of surgical resection can be offset by increased treatment mortality when surgery is used, which may explain why randomized studies involving relatively small numbers of patients do not show a statistically significant survival benefit for surgery.14, 15, 24 Considering the increased short-term risks of surgery, a treatment regimen that selectively uses surgery only for non-complete responses or recurrences after definitive chemoradiation may avoid unnecessary morbidity in some patients. However, the use of surgery in this manner does not necessarily mean that outcomes will be equivalent as when surgery is used immediately following the administration of chemoradiation, and should be considered only in the context of a specific protocol or trial. Regardless of how surgery is utilized, minimizing morbidity and mortality is clearly critical to optimizing outcomes.
Analyses using the population-based SEER-cancer registry have the advantage of achieving wide generalizability and enough power to examine differences even among specific subgroups of non-frequent cancer sites. While the application of propensity scoring techniques to adjust for differences in treatment allocation does allow equalizing the chance of getting either one of the treatments under consideration and improves the precision of calculated estimates, these investigations also have inherent limitations. First, data on chemotherapy administration is missing, which could account for differences in survival in the groups studied if the use of chemotherapy was not balanced between the groups. The impact of this limitation on our finding that surgical resection with or without radiation is associated with better outcomes than radiation alone is not clear. Many if not most of the patients in our study population who received radiation were likely to also have had chemotherapy, given that relatively early randomized trials failed to show a survival advantage for preoperative or postoperative radiation alone and definitive chemoradiation has been shown to be superior to radiation alone.30–33 In contrast, patients undergoing surgery without radiation may have been less likely to have received chemotherapy. Given an expected benefit of chemotherapy use, one might speculate that balancing for chemotherapy use between the two groups would even have enlarged the survival difference. Second, retrospective cohort studies are impacted by selection bias. Propensity score adjustment is a tool to mitigate this potential bias by improving precision of the results. However, propensity-score adjustment can only be based on measured covariates and lacks the inclusion of unmeasured potential confounders like comorbidities, smoking status, or provider volume. In particular, one cannot rule out that patients who underwent definitive radiotherapy had more severe comorbidities and therefore were less likely to undergo esophagectomy, an assumption that could negatively bias the definitive radiotherapy approach. Third, information regarding beam-radiation doses and fields are not contained in the database, which might have biased outcomes comparing preoperative and postoperative radiotherapy. Fourth, tumor staging is defined by clinical assessment in patients who underwent neo-adjuvant therapy while it is based on pathological examination in patients where esophagectomy was performed without neo-adjuvant therapy. This might have led to under- or over-staging in some of the patients who underwent neo-adjuvant therapy.
In conclusion, esophageal cancer is a relatively uncommon disease that is most commonly found when locally advanced or with distant metastases. Treatment strategies vary widely and, independent of treatment, survival is generally poor. Recommended treatment of mid and distal esophageal cancers generally does not depend on histological subtype, though some recent studies have suggested that definitive chemoradiation offers similar outcome benefits for treatments that involve surgical resection for patients with SCC. In this study using a large population-based database, we show that patients with locally advanced but potentially resectable mid and distal esophageal SCC have better outcomes when surgery is added to the treatment regimen. Further investigations in patients with stage IIA/B tumors are warranted to decide if radiation therapy is beneficial. Therefore, esophagectomy should be considered as an integral component of the treatment algorithm whenever feasible.
Acknowledgments
Supported by grant PBBEP3-131567 from the Swiss National Science Foundation (M.W.) and the NIH funded Cardiothoracic Surgery Trials Network (M.F.B).
Footnotes
Disclosure information: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Luketich JD. Resection for esophageal cancer: strategies for optimal management. Ann Thorac Surg. 2008;85(2):S751–6. doi: 10.1016/j.athoracsur.2007.11.078. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 5.Dubecz A, Gall I, Solymosi N, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol. 2012;7(2):443–7. doi: 10.1097/JTO.0b013e3182397751. [DOI] [PubMed] [Google Scholar]
- 6.Smith GL, Smith BD, Buchholz TA, et al. Patterns of care and locoregional treatment outcomes in older esophageal cancer patients: The SEER-Medicare Cohort. Int J Radiat Oncol Biol Phys. 2009;74(2):482–9. doi: 10.1016/j.ijrobp.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143(12):1198–203. doi: 10.1001/archsurg.143.12.1198. discussion 1203. [DOI] [PubMed] [Google Scholar]
- 8.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 9.Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009;115(21):4924–33. doi: 10.1002/cncr.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubecz A, Sepesi B, Salvador R, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg. 2010;211(6):754–61. doi: 10.1016/j.jamcollsurg.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Chang DT, Chapman C, Shen J, et al. Treatment of esophageal cancer based on histology: a Surveillance Epidemiology and End Results analysis. Am J Clin Oncol. 2009;32(4):405–10. doi: 10.1097/COC.0b013e3181917158. [DOI] [PubMed] [Google Scholar]
- 12.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9(8):830–87. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Nakagawa K, Yamada K, et al. A single institutional non-randomized retrospective comparison between definitive chemoradiotherapy and radical surgery in 82 Japanese patients with resectable esophageal squamous cell carcinoma. Dis Esophagus. 2008;21(5):430–6. doi: 10.1111/j.1442-2050.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 14.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–8. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 15.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–7. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 16.Chiu PW, Chan AC, Leung SF, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE) J Gastrointest Surg. 2005;9(6):794–802. doi: 10.1016/j.gassur.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL American Joint Committee on Cancer and American Cancer Society. AJCC cancer staging manual. 6. New York: Springer-Verlag; 2002. p. xiv.p. 421. [Google Scholar]
- 18.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 20.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116(16):3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 22.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–7. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 23.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 24.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337(3):161–7. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 26.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–68. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 27.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 28.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 30.Teniere P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet. 1991;173(2):123–30. [PubMed] [Google Scholar]
- 31.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 32.Arnott SJ, Duncan W, Kerr GR, et al. Low dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol. 1992;24(2):108–13. doi: 10.1016/0167-8140(92)90287-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys. 1989;16(2):325–7. [PubMed] [Google Scholar]