Abstract
Injury to retinal ganglion cell (RGC) axons within the optic nerve causes apoptosis of the soma. We previously demonstrated that in vivo axotomy causes elevation of superoxide anion within the RGC soma, and that this occurs 1–2 days before annexin-V positivity, a marker of apoptosis. Pegylated superoxide dismutase delivery to the RGC prevents the superoxide elevation and rescues the soma. Together, these results imply that superoxide is an upstream signal for apoptosis after axonal injury in RGCs. We then studied metallocorroles, potent superoxide dismutase mimetics, which we had shown to be neuro-protective in vitro and superoxide scavengers in vivo for RGCs. RGCs were retrograde labeled with the fluorescent dye 4Di-10Asp, and then axotomized by intraorbital optic nerve transection. Iron(III) 2,17-bis-sulfonato-5,10,15-tris(pentafluorophenyl)corrole (Fe(tpfc)(SO3H)2) (Fe-corrole) was injected intravitreally. Longitudinal imaging of RGCs was performed and the number of surviving RGCs enumerated. There was significantly greater survival of labeled RGCs with Fe-corrole, but the degree of neuroprotection was relatively less than that predicted by their ability to scavenge superoxide-This implies an unexpected complexity in signaling of apoptosis by reactive oxygen species.
Keywords: retinal ganglion cells, neuroprotection, apoptosis, in vivo imaging, reactive oxygen species
1. Introduction
Retinal ganglion cell (RGC) death is the final result of injury to the optic nerve. It can occur as a result of transection from trauma, ischemia from ischemic optic neuropathy, inflammation from optic neuritis, or most commonly, glaucoma. In most cases the first critical event is thought to be RGC axonal damage, possibly mediated by glial dysfunction, following which there is signaling of cell body apoptosis, retrograde axonal degeneration, and Wallerian degeneration. Therapeutic strategies that could theoretically interfere with cascades of cell death are widely sought, as they might represent treatments for untreatable or difficult-to-treat optic neuropathies, including glaucoma. This has led to a search for “neuroprotective” therapies that could be translated from the laboratory into the clinical care of patients, similar to what has been attempted in diseases of the central nervous system (Danesh-Meyer and Levin, 2009).
In recent years we defined one particular signal transduction pathway by which RGC axonal injury could cause RGC apoptosis, namely a burst of intracellular superoxide anion. We were able to show that axotomized rat RGCs in primary culture greatly elevated their superoxide levels, and that this was potentiated by prior optic nerve transection (Lieven et al., 2006). Subsequently, we used in vivo confocal scanning laser ophthalmoscopy to visualize an increase in superoxide contained within retrograde-labeled RGCs after optic nerve transection, and could show that this preceded apoptosis, as defined by binding of fluorescent annexin-V (Kanamori et al., 2010a). The critical role of superoxide was demonstrated by partial amelioration of RGC death by the intra-vitreal injection of pegylated superoxide dismutase-1 (PEG-SOD) (Kanamori et al., 2010a).
Recently, we studied a group of novel superoxide scavengers based on a corrole (analogous to the corrin ring in Vitamin B12). The corresponding Fe and Mn complexes are potent reactive oxygen species scavengers (Eckshtain et al., 2009; Gershman et al., 2007; Mahammed and Gross, 2006, 2011, 2010), and have been shown to be cytoprotective in disease models outside of the optic nerve (Kupershmidt et al., 2010; Okun et al., 2009). We were able to use in vitro models of retinal neuronal cell death and in vivo imaging in the rat to show that they could abrogate superoxide signaling after serum deprivation or axonal injury (Kanamori et al., 2010b). Given our prior studies defining a role for superoxide in signaling apoptosis after axonal injury, we hypothesized that metallocorroles would be neuroprotective in vivo after optic nerve injury. We now report the testing of this hypothesis.
2. Materials and methods
2.1. Animals
All experiments on animals were carried out in accordance with the guidelines set out by the Maisonneuve-Rosemont Hospital Research Centre Animal Care Committee and Canadian Council for Animal Care. Long-Evans females rats weighing 225–250 g were from Charles River Canada.
2.2. Materials
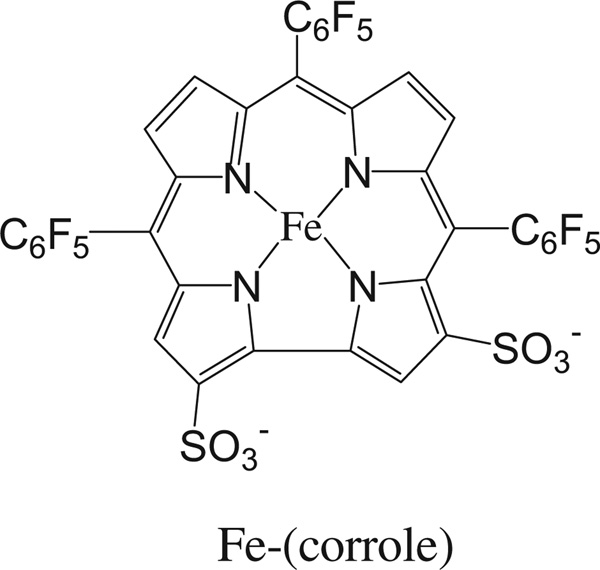
4Di-10Asp, [4-(4-(didecylamino)styryl)-N-methylpyridinium iodide] was from Anaspec (Fremont.CA, USA), Rhodamine-conjugated Griffonia simplicifolia lectin I (GSL-I) was from Vector Laboratories (Burlingame, CA). Iron(III) 2,17-bis-sulfonato-5,10,15-tris(pentafluorophenyl)corrole (Fe(tpfc)(SO3H)2) (Fe-corrole; Fig. 1) was synthesized as described previously (Mahammed and Gross, 2005; Saltsman et al., 2002).
Fig. 1.
Iron(III) 2,17-bis-sulfonato-5,10,15-tris(pentafluorophenyl)corrole (Fe(tpfc)(SO3H)2), the corrole used in this study.
2.3. Retrograde labeling of RGCs
RGCs were retrogradely labeled by stereotactic injection of 4Di-10Asp into both superior colliculi. Briefly, anesthetized rats were placed in a stereotactic apparatus and the cortex surface was exposed by drilling the parietal bone to facilitate dye injection. A total volume of 2.8 µL of 100 mM 4Di-10Asp was injected bilaterally at 6 mm caudal to bregma and 1.2 mm lateral to the midline to a depth of 4.5 mm from the skull surface.
2.4. Optic nerve transection
Animals were maintained for 4–7 days after 4Di-10Asp injection into the superior colliculi in order for the dye to be transported to RGC somas. After labeling, animals were re-anesthetized as described previously (Kanamori et al., 2010a) under ketamine (50 mg/kg) and xylazine (100 mg/kg) and intrameningeal optic nerve transection on the right eye was performed. All animals were examined by indirect ophthalmoscopy after optic nerve transaction in order to ensure the integrity of the retinal blood supply. Rats with abnormal retinal vessels or retinal edema were not used further.
2.5. Intravitreal injections
Intravitreal injections of 100 nM Fe-corrole were performed as described previously (Kanamori et al., 2010b).
2.6. Retinal ganglion cell enumeration by confocal scanning laser ophthalmoscopy
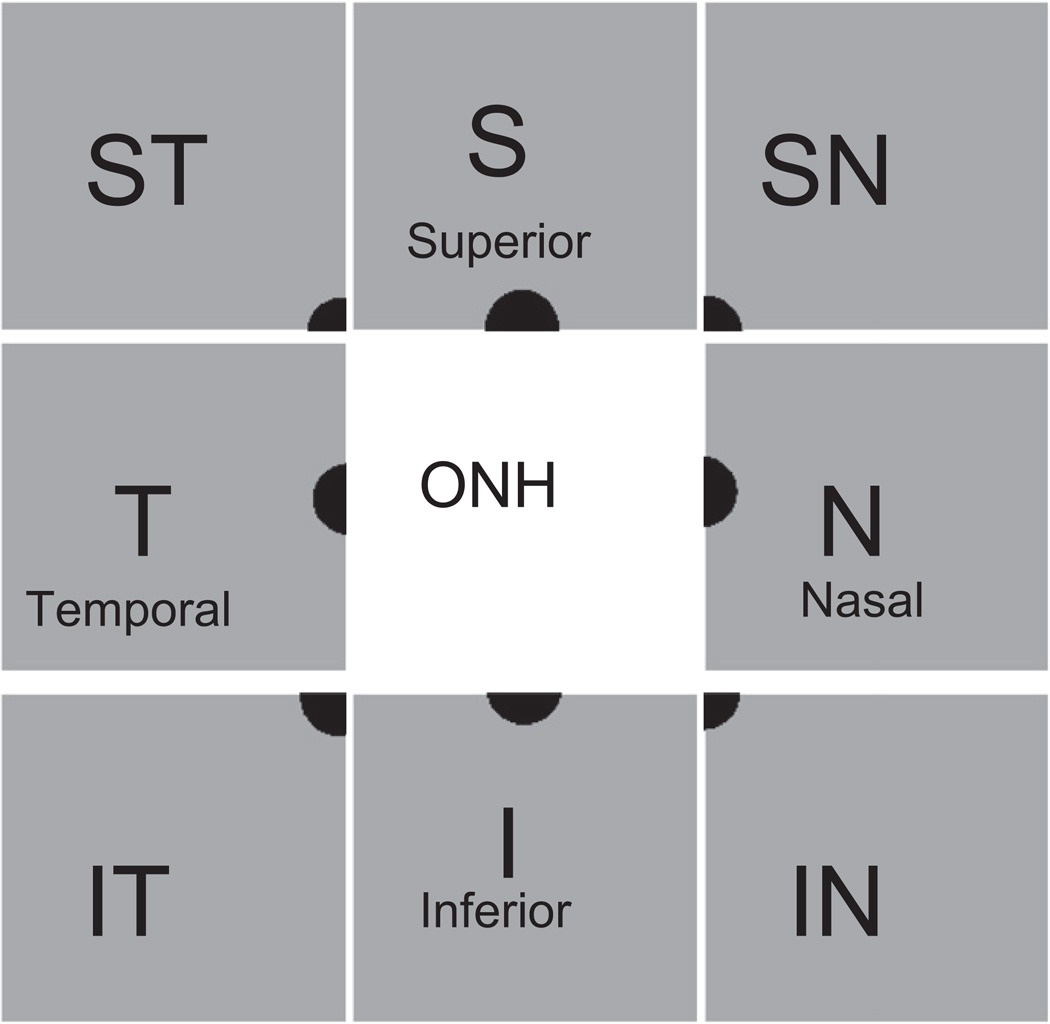
Retrograde-labeled RGCs were counted in 8 retinal areas immediately adjacent to the optic nerve head (Fig. 2). Retinal images were obtained using a 30° field of view and real-time averaging of at least 70 images at 97% sensitivity on a Heidelberg Retina Angiograph II (Heidelberg Engineering, Germany). The plane of focus was adjusted to the inner retina by imaging the nerve fiber layer at 488 nm.
Fig. 2.
Schematic drawing of the topography of retinal imaging by CSLO. To quantify the number of cells positive for the retrograde label 4Di-10Asp, 8 images were taken at each retinal octant adjacent to the optic nerve head.
Counting was performed before optic nerve transection, and at 5 and 7 days after transection. Areas for counting were the same for each session in order to maintain consistency, and were achieved using intersections of retinal vessels as demarcation points. All counting was done by an investigator masked to treatment status.
2.7. Statistics
Results are presented as mean ± standard deviation (SD). Comparisons are by unpaired t-test.
3. Results
3.1. Effect of Fe-corrole on RGC loss following optic nerve transection
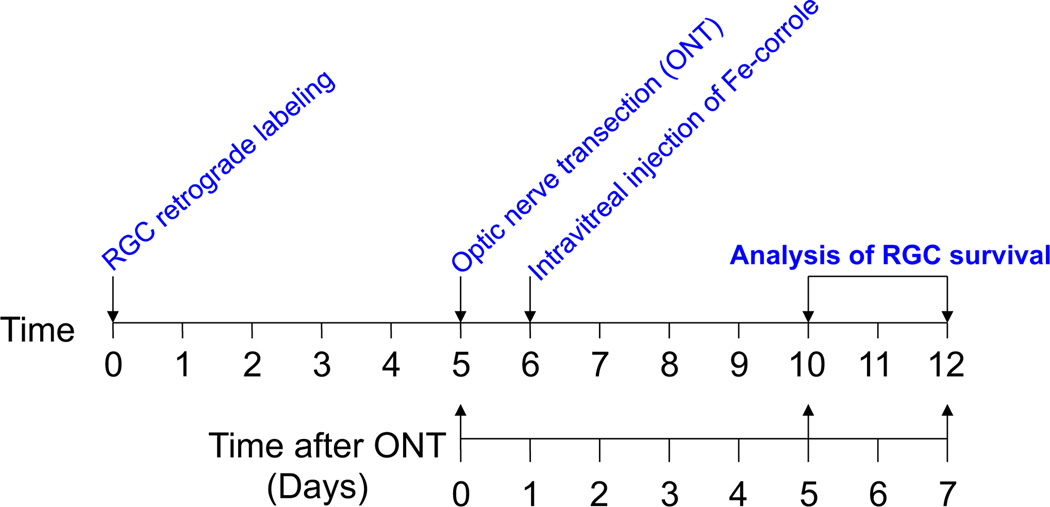
We investigated whether Fe-corrole was neuroprotective in vivo in the rat optic nerve transection model. RGCs were visualized by retrograde labeling with the fluorescent dye 4Di-10Asp using in vivo confocal scanning laser ophthalmoscopy. This technique allows serial counting of RGC numbers over time. Serial imaging of fluorescent RGCs in defined areas of retina was performed before axotomy as a baseline. One day after optic nerve transection, intravitreal injection of Fe-corrole was performed. Serial imaging of fluorescent RGCs in the same defined areas of retina was then repeated at 5 and 7 days after axotomy in order to quantify RGC survival. The 5-day time point was chosen to approximate the peak of apoptosis in rat RGCs after optic nerve transection in our hands (Kanamori et al., 2010a), and the 7-day time point because it is one of the commonly used end-points for RGC survival after transaction or crush experiments in the optic nerve (Almasieh et al., 2011; Mansour-Robaey et al., 1994). The timing of the experimental events is depicted in Fig. 3.
Fig. 3.
Flowchart of RGC retrograde labeling, drug treatment, and RGC counting by confocal scanning laser ophthalmoscopy.
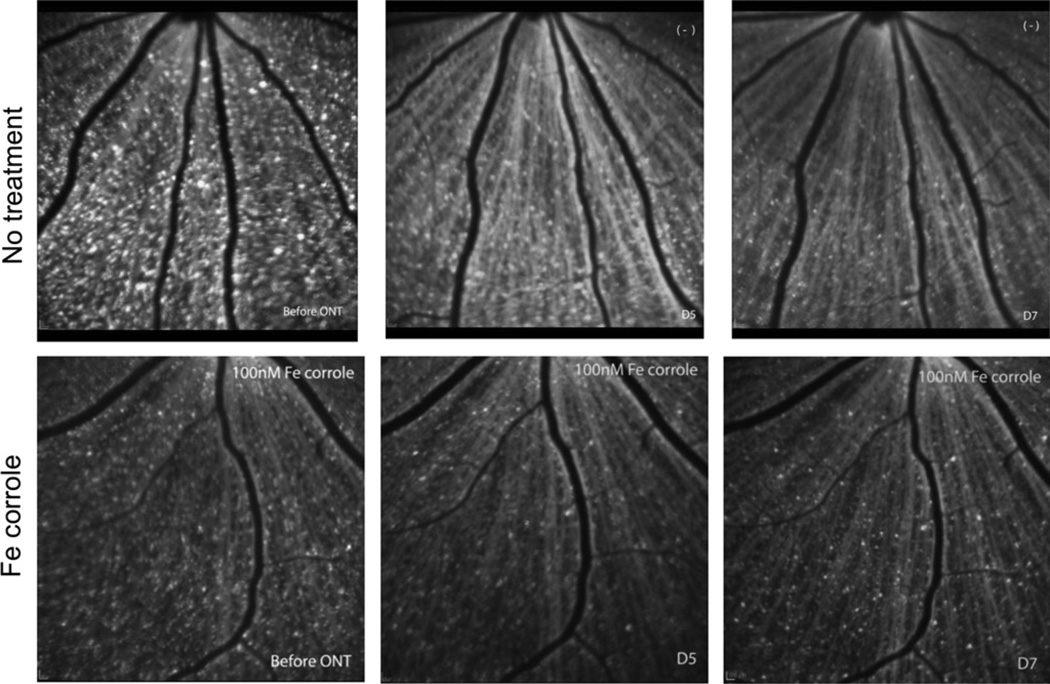
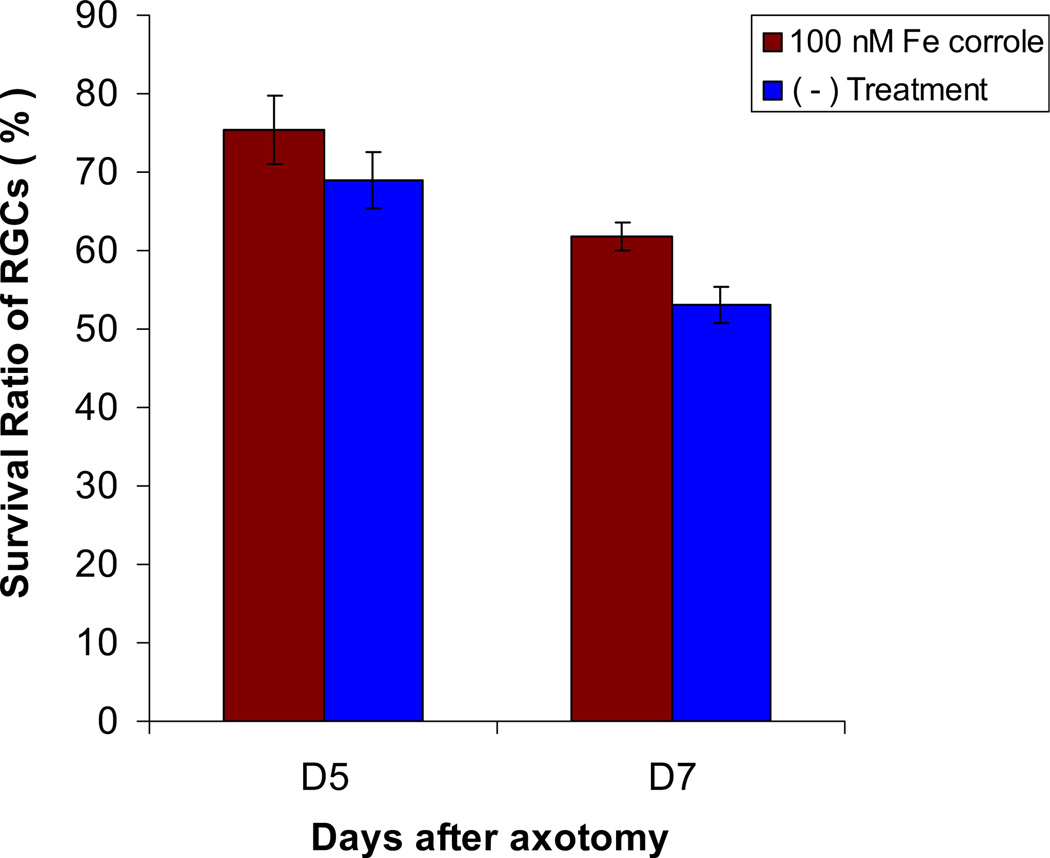
There was a decrease in survival of RGCs at 5 days and 7 days after optic nerve transection, respectively (Fig. 4). The RGC survival rate (as a percentage of baseline) in untreated rats was 69.0% ± 11.0% (p = 0.011; n = 9) at 5 days and 53.1% ± 6.9% (p = 0.011; n = 9) at 7 days (Fig. 5). These results are consistent with our previously published data on labeling of RGCs quantified by retrograde labeling with Alexa Fluor 488-dextran (Kanamori et al., 2010a). Analysis of labeled RGCs in retinal whole mounts at 7 days after optic nerve transection in no treatment group of animals showed a similar survival rate of 53.7% (n = 9) compared with counts on CSLO images, confirming the validity of the in vivo imaging technique for assessing RGC survival.
Fig. 4.
Examples of longitudinally collected images of RGCs retrograde labeled with 4Di-10Asp and detected by confocal scanning laser ophthalmoscopy. Left panels, before optic nerve transection. Middle panels, 5 days after optic nerve transection. Right panels, 7 days after optic nerve transection. The same fields are imaged in the top row or bottom row.
Fig. 5.
Survival ratio of RGCs after transection compared to that before transection in no treatment eyes (n = 9, blue) and eyes injected with Fe-corrole (n = 5, red).
Intravitreal injection of Fe-corrolewas associated with increased RGC viability at 5 days and 7 days after transection. In Fe-corrole treated rats, the survival rate at 5 days was 75.5% ± 8.7% (n = 5) of baseline, and at 7 days was 61.7% ± 3.5% (n = 5) of baseline (p = 0.287 and 0.012 compared with control, respectively; Fig. 5).
4. Discussion
These data demonstrate that the metallocorrole Fe-corrole partly rescued RGCs from apoptosis induced by optic nerve transection, likely based on its ability to decrease intracellular superoxide levels in vitro (Kanamori et al., 2010b), in vivo (Kanamori et al., 2010b), and prevent cell death in a variety of disease models (Kupershmidt et al., 2010; Okun et al., 2009). This result is consistent with previous findings that intracellular superoxide increases in axotomized RGCs (Kanamori et al., 2010a; Lieven et al., 2006), mice deficient in SOD-1 lose RGCs over time (Yuki et al., 2011), and intravitreal delivery of pegylated-SOD rescues RGCs from death after axotomy (Kanamori et al., 2010a). However, given that Fe-corrole is a superoxide scavenger (Eckshtain et al., 2009), including in axotomized RGCs (Kanamori et al., 2010b), the relative dissociation between its activity in the RGC axotomy model is unexpected, and likely indicates a greater complexity to the pathophysiological process by which superoxide signals RGC death after axonal injury.
There are several potential reasons for these findings. An obvious explanation would be that the bioavailability of Fe-corrole at the level of the RGC was insufficient, e.g. because of failure to diffuse in the vitreous or penetrate the cell membrane. Against this is that the levels of intracellular superoxide were substantially decreased when studied by in vivo confocal imaging, similar in magnitude to that achieved with pegylated-SOD (Kanamori et al., 2010b).
A second possibility is that our previous measurements (Kanamori et al., 2010b) of superoxide levels were confounded by other reactive oxygen species. We used HEt, which reacts with superoxide to form 2-hydroxy-ethidium, a molecule with a distinct excitation spectrum when compared to the oxidation product ethidium (Robinson et al., 2006; Zhao et al., 2003). Although in previous work we showed that axotomy induces the superoxide product 2-hydroxy-ethidium and not ethidium in RGCs using ex vivo techniques (Kanamori et al., 2010a), and that Fe-corrole and Mn-corrole but not the redox-inactive Ga-corrole decreased 2-hydroxy-ethidium formed in neurons after serum deprivation in vitro (Kanamori et al., 2010b), the in vivo imaging that we used cannot distinguish 2-hydroxy-ethidium from ethidium. Potentially our measurements showing decreased oxidized HEt (i.e. 2-hydroxy-ethidium or ethidium) in vivo with Fe-corrole could actually reflect a contribution from decreasing another reactive oxygen species. For example, Fe-corrole also has peroxynitrite decomposition activity (Eckshtain et al., 2009), and possibly it is the generation of peroxynitrite that is the signal associated with RGC death after axotomy. This would necessarily involve superoxide reacting with nitric oxide, and perhaps the RGC neuroprotection from PEG-SOD observed in our previous study (Kanamori et al., 2010a) was a result of decreased peroxynitrite generation. However, if this were the case, then Fe-corrole, which both dismutates superoxide and decomposes peroxynitrite (Eckshtain et al., 2009), should be more neuroprotective than was found in the present study.
A third possibility is that the Fe-corrole superoxide dismutase activity is neuroprotective, but that there is another catalytic activity that is somewhat deleterious to RGC survival in vivo. If this were the case, then the other activity would have to be relatively specific to RGCs after axotomy, because metallocorroles are cytoprotective in a variety of other paradigms (Kanamori et al., 2010b; Kupershmidt et al., 2010; Okun et al., 2009).
A fourth possibility is that the RGC counts were confounded by microglia phagocytosing dying RGCs, and that there actually was more RGC neuroprotection by Fe-corrole than what we measured. However, this could not be the case for two reasons: First, this would result in greater counts in the Fe-corrole group, not less. Second, the staining for microglia did not reveal any effect of Fe-corrole on microglia numbers.
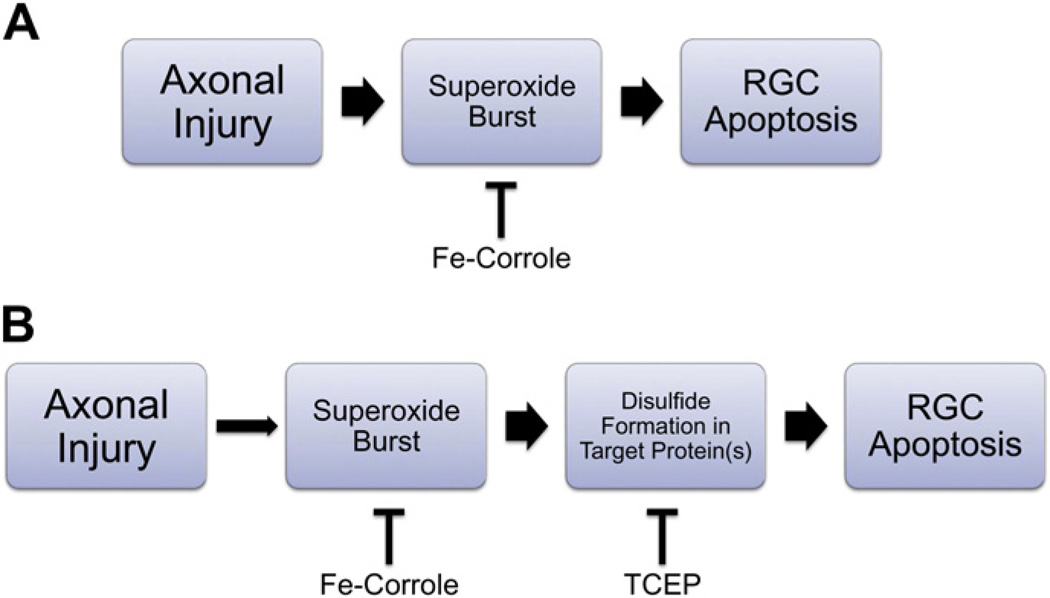
The fifth, and most likely, possibility is that the timing of reduction of superoxide scavenging by Fe-corrole within the relevant intracellular compartment was not the same as that achieved with pegylated-SOD. Although our previous study showed that there was a 3–5 day delay between optic nerve transection and the burst of intracellular superoxide in RGCs (Kanamori et al., 2010a), and that the effect of Fe-corroles on superoxide levels can be seen within 24 h (Kanamori et al., 2010b), it may be that an interaction between the timing and degree of scavenging with the threshold for signaling RGC apoptosis after axotomy resulted in a failure to observe neuroprotection. It is also possible that localization of drug to the relevant subcellular compartment, e.g. mitochondria, could be different between Fe-corrole and pegylated-SOD. If there were even a slight difference in the timing of superoxide scavenging, then downstream effectors such as redox-dependent oxidative steps (Almasieh et al., 2011; Geiger et al., 2002; Nguyen et al., 2003; Seidler et al., 2010) depicted in Fig. 6 could become activated more rapidly with Fe-corroles compared to pegylated-SOD in axotomized RGCs.
Fig. 6.
Schematic for the signaling of retinal ganglion cell death after axonal injury. A. Axonal injury induces a burst of superoxide within the retinal ganglion cell (RGC) soma (Kanamori et al., 2010a; Lieven et al., 2006). However, the failure of Fe-corrole to inhibit most RGC death despite effective scavenging of superoxide (Kanamori et al., 2010b) implies that this pathway incompletely reflects the pathophysiology of axonal injury. B. Another possibility is that the time course for superoxide induction is slow and stochastic, but the induction of downstream oxidative events (e.g. disulfide formation) and subsequent apoptosis is rapid. If this were the case, even a small amount of superoxide may induce downstream signaling. Inhibiting downstream signaling events, e.g. with redox modulating agents such as TCEP (Almasieh et al., 2011; Swanson et al., 2005) may be necessary for effective neuroprotection.
In summary, we found that Fe-corroles are neuroprotective of RGCs after axotomy in vivo, but that the degree of protection was less than would be expected based on its ability to scavenge superoxide. The most likely reason is probably the timing of the superoxide burst with respect to RGC axotomy and its downstream effectors. Elucidation of this critical post-superoxide pathway would be important for developing more effective neuroprotective agents for RGC axonal disease.
Acknowledgments
This manuscript is dedicated to the memory of Carl Camras. His dedication to complete objectivity and critical examination of scientific results was well known to all who counted him as a colleague. He is sorely missed.
Grant Support: Supported by Canadian Institutes for Health Research, Canadian Foundation for Innovation, Canadian Research Chairs program, Fonds de recherche en ophtalmologie de l’Université de Montréal, and National Institutes of Health (R21EY017970). Work at the Technion was supported by the Johnson & Johnson COSAT-Technion fund. Dr. Levin has assigned to the Wisconsin Alumni Research Foundation patents issued and pending on phosphine–borane complexes, which are also neuroprotective, but do not share the mechanism of metallocorroles.
References
- Almasieh M, Lieven CJ, Levin LA, Di Polo A. A cell-permeable phosphineborane complex delays retinal ganglion cell death after axonal injury through activation of the pro-survival extracellular signal-regulated kinases 1/2 pathway. J. Neurochem. 2011;118:1075–1086. doi: 10.1111/j.1471-4159.2011.07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Levin LA. Neuroprotection: extrapolating from neurologic diseases to the eye. Am. J. Ophthalmol. 2009;148:186–191. doi: 10.1016/j.ajo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Eckshtain M, Zilbermann I, Mahammed A, Saltsman I, Okun Z, Maimon E, Cohen H, Meyerstein D, Gross Z. Superoxide dismutase activity of corrole metal complexes. Dalton Trans. 2009:7879–7882. doi: 10.1039/b911278b. [DOI] [PubMed] [Google Scholar]
- Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- Gershman Z, Goldberg I, Gross Z. DNA binding and catalytic properties of positively charged corroles. Angew. Chem. Int. Ed. Engl. 2007;46:4320–4324. doi: 10.1002/anie.200700757. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010a;133:2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori A, Catrinescu MM, Mahammed A, Gross Z, Levin LA. Neuroprotection against superoxide anion radical by metallocorroles in cellular and murine models of optic neuropathy. J. Neurochem. 2010b;114:488–498. doi: 10.1111/j.1471-4159.2010.06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt L, Okun Z, Amit T, Mandel S, Saltsman I, Mahammed A, Bar-Am O, Gross Z, Youdim MB. Metallocorroles as cytoprotective agents against oxidative and nitrative stress in cellular models of neurodegeneration. J. Neurochem. 2010;113:363–372. doi: 10.1111/j.1471-4159.2010.06619.x. [DOI] [PubMed] [Google Scholar]
- Lieven CJ, Schlieve CR, Hoegger MJ, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest. Ophthalmol. Vis. Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- Mahammed A, Gross Z. Albumin-conjugated corrole metal complexes: extremely simple yet very efficient biomimetic oxidation systems. J. Am. Chem. Soc. 2005;127:2883–2887. doi: 10.1021/ja045372c. [DOI] [PubMed] [Google Scholar]
- Mahammed A, Gross Z. Iron and manganese corroles are potent catalysts for the decomposition of peroxynitrite. Angew. Chem. Int. Ed. Engl. 2006;45:6544–6547. doi: 10.1002/anie.200601399. [DOI] [PubMed] [Google Scholar]
- Mahammed A, Gross Z. Highly efficient catalase activity of metallocorroles. Chem. Commun. (Camb.) 2010;46:7040–7042. doi: 10.1039/c0cc01989e. [DOI] [PubMed] [Google Scholar]
- Mahammed A, Gross Z. The importance of developing metal complexes with pronounced catalase-like activity. Catal. Sci. Technol. 2011;1:535–540. [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SM, Alexejun CN, Levin LA. Amplification of a reactive oxygen species signal in axotomized retinal ganglion cells. Antioxid. Redox Signal. 2003;5:629–634. doi: 10.1089/152308603770310293. [DOI] [PubMed] [Google Scholar]
- Okun Z, Kupershmidt L, Amit T, Mandel S, Bar-Am O, Youdim MB, Gross Z. Manganese corroles prevent intracellular nitration and subsequent death of insulin-producing cells. ACS Chem. Biol. 2009;4:910–914. doi: 10.1021/cb900159n. [DOI] [PubMed] [Google Scholar]
- Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltsman I, Mahammed A, Goldberg I, Tkachenko E, Botoshansky M, Gross Z. Selective substitution of corroles: nitration, hydroformylation, and chlorosulfonation. J. Am. Chem. Soc. 2002;124:7411–7420. doi: 10.1021/ja025851g. [DOI] [PubMed] [Google Scholar]
- Seidler EA, Lieven CJ, Thompson AF, Levin LA. Effectiveness of novel borane-phosphine complexes in inhibiting cell death depends on the source of superoxide production induced by blockade of mitochondrial electron transport. ACS Chem. Neurosci. 2010;1:95–103. doi: 10.1021/cn900024r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest. Ophthalmol. Vis. Sci. 2005;46:3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- Yuki K, Ozawa Y, Yoshida T, Kurihara T, Hirasawa M, Ozeki N, Shiba D, Noda K, Ishida S, Tsubota K. Retinal ganglion cell loss in superoxide dismutase 1 deficiency. Invest. Ophthalmol. Vis. Sci. 2011;52:4143–4150. doi: 10.1167/iovs.10-6294. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]