Abstract
Recently we have shown that mouse and human meibomian glands undergo specific age-related changes, including decreased acinar cell proliferation, acinar atrophy, and altered peroxisome proliferator-activated receptor gamma (PPARγ) localization from cytoplasmic-vesicular/nuclear in young mice and humans to nuclear in old mice and humans. Since PPARγ is a lipid-sensitive, nuclear receptor implicated in regulating adipocyte and sebocyte differentiation and lipogenesis, our findings suggest that PPARγ may be involved in modulating meibomian gland differentiation during aging. Based on these findings, we propose that aging of the meibomian gland results in downregulation of PPARγ, leading to decreased meibocyte differentiation and lipid synthesis, gland atrophy, and a hyposecretory meibomian gland dysfunction.
Keywords: eyelid, meibomian gland, meibomian gland dysfunction, PPARγ
I. Introduction
PPARγ is a lipid-activated nuclear hormone receptor that regulates lipid synthesis and cell differentiation.1 It is a member of closely homologous genes including PPARγ and PPARβ and δ and is the major subtype expressed in adipocytes and sebocytes, where it regulates the expression of genes involved in lipogenesis.2, 3 Importantly, PPARγ has been shown to play a critical role in both fat and sebaceous gland development and differentiation with mice chimeric for PPARγ-null cells showing little or no contribution to the formation of these structures.2 Recently, we have evaluated the expression of PPARγ in the meibomian gland during mouse development and aging, as well as human meibomian gland dysfunction (MGD). In this paper, we review these findings and discuss the potential role of PPARγ in the development of MGD.
II. Role of PPARγ in the Development of the Mouse Meibomian Gland
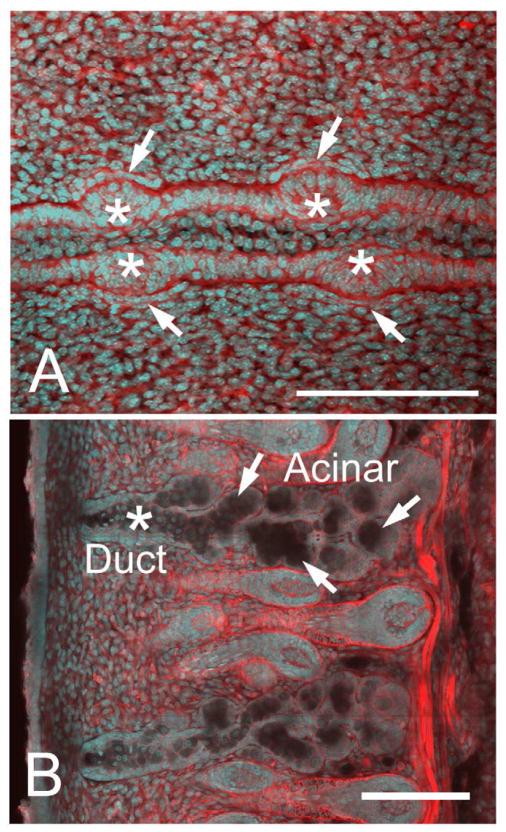
Previous studies of meibomian gland development have been limited,4, 5 and the most extensive study has focused on the human meibomian gland, which begins to form around embryonic month 3 following eyelid fusion.6, 7 Mouse meibomian gland development has recently been shown to begin around embryonic day (E)18.5 as an epithelial placode within the fused eyelid epithelium (Figure 1A).8 This structure is similar to that of the developing hair follicle and pilosebaceous unit,9 from which the meibomian gland is thought to share developmental similarities. Invagination of eyelid epithelium to form an epithelial cord within the lid mesenchyme is then apparent at birth (P0) with progressive lengthening of the epithelial cord from P1 to P3. Branching of the epithelial cord is first detected at P5, and by P8 there is distinct differentiation to form both acinar and ductal meibomian gland structures (Figure 1B).
Figure 1.
Confocal images using actin (red) and nuclei (DAPI, cyan) staining of eyelids at different time points of meibomian gland morphogenesis. At E18.5 (A), we observed the formation of an epithelial condensation (asterisk) within the fused lid margin. Epithelial placodes in the superior and inferior lids also appeared opposite to each other, and were associated with condensation of mesenchyme as detected by cell alignment and increased actin staining of mesenchyme directly adjacent to the placode (arrows). Extension of epithelium into the lid mesenchyme was observed from postnatal day 0 (P0) to P8 (B) showing extensive branching to form individual acinar tissue (arrows) and the central duct (asterisk). Bar = 250 micron. (Adapted from Nien et al.8)
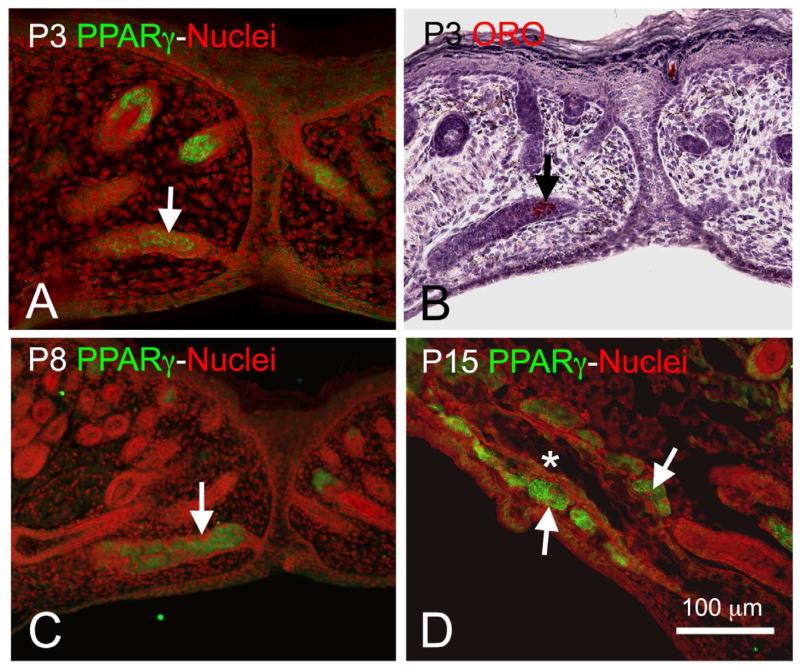
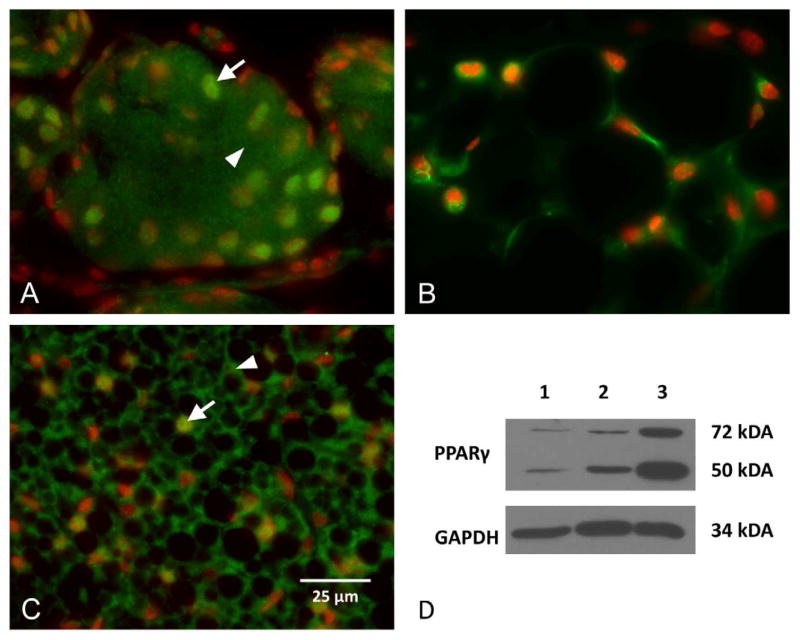
During development, PPARγ expression is first detected at P3, localized to the inner portion of the invaginating epithelial cord and prior to the formation of acinar structures (Figure 2A, arrow). Using oil-red-O staining, lipid is also detected at this stage of development and localized to the inner portion of the epithelial cord where cells are expressing PPARγ (Figure 2B, arrow). As morphogenesis progresses, PPARγ expression continues to be localized to the invaginating epithelial cord with expression extending to developing acinar tissue by P5 and P8 (Figure 2C, arrow). Following eyelid opening (Figure 2D, P15), PPARγ expression is lost in the ductal epithelium following formation of the central duct (asterisk) and becomes limited specifically to the acinar compartment. Higher magnification of adult acinar tissue shows that PPARγ is localized to cytoplasmic vesicles and nuclei of acinar cells (Figure 3A, Green = PPARγ, Red = nuclei). This pattern is similar to that obtained for immunostaining of white fat and brown fat (Figure 3B and 1C, respectively), and western blots of cellular extracts from the meibomian gland, white fat and brown fat probed with antibodies specific for PPARγ show the presence of a 50 kDa/72 kDa band (Figure 1D, lane 1, 2 and 3, respectively). While the molecular weight of PPARγ is known to be 50 kDa, PPARγ is also modified through serine phosphorylation and sumoylation,10 suggesting that the 72 kDa band may represent a post-translational modification. Overall, these findings indicated that meibomian glands express PPARγ in lipid synthesizing cells and that PPARγ is a biomolecular marker for meibocyte differentiation.
Figure 2.
PPARγ expression (Green = PPARγ, Red = nuclei) at P3 (A) was localized to the central portion of the invaginating epithelial cord (arrow) and correlated with oil-red-O staining (B, Red) indicating the presence of neutral lipid. PPARγ expression at P8 (C) was also localized to the epithelial cord (arrow) and developing acini, while at P15 (D) after eyelid opening expression was absent in the central duct (asterisk) but present in the acini (arrows). (Adapted from Nien et al.8)
Figure 3.
PPARγ (Green) and nuclei (DAPI, Red) staining of meibomian gland acini (A), white fat (B), and brown fat (C). Note that PPARγ stains both the nuclei (arrow) and cytoplasm (arrowhead). Western blotting (D) identifies both a 50 kDa and 72 kDa proteins in all three tissues (lane 1 = meibomian gland, lane 2 = white fat, lane 3 – brown fat). (Adapted from Nien et al.8)
III. Age-Related Changes in the Mouse Meibomian Gland
Recently, we have used nonlinear optical (NLO) imaging and array tomography to volumetrically reconstruct the mouse meibomian gland by simultaneously collecting second harmonic generated (SHG) signals from extracellular collagen and two photon excited fluorescence signals (TPEF) from cellular structures.11 Briefly, NLO array tomography involves plastic embedding and serial sectioning of the mouse eyelids and the collection of stitched images of the SHG and TPEF signals. The SHG signal provides an outline of the interstitium that surrounds the meibomian gland that can be used to automatically threshold the gland area to create a total gland mask, which can then be used to cut the meibomian gland out from the TPEF cellular signal. These images are then reconstructed using digital image processing software to provide volumetric rendering of the entire meibomian gland divided into the cellular and lipid compartments.
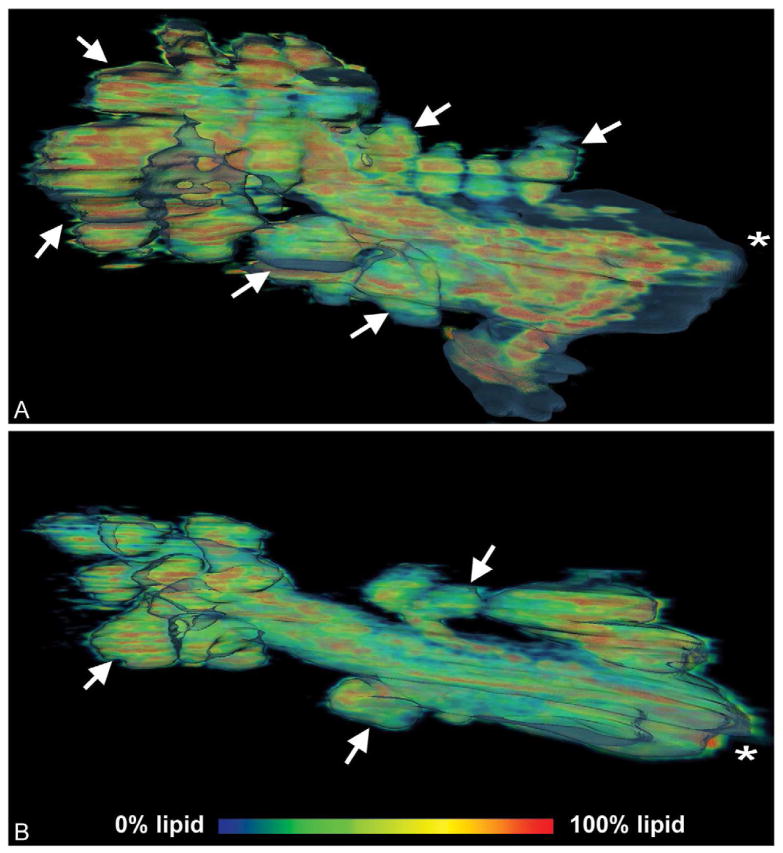
Volume renderings of the mouse meibomian gland are shown in Figure 4, which represents the lipid distribution as a heat map (Red = lipid, Blue = cells) inside the total gland volume rendering from a 2-month-old (A) and 2-year-old (B) gland (asterisk = orifice). Interestingly, the meibomian gland from the older mouse showed remarkably fewer acini compared to the gland from the younger mouse (arrows = acini). This loss of acini is similar to what is detected in older human patients as “dropout” using transillumination meibography. Since these are volume renderings, the total volume of the different gland compartments were measured with femtoliter sensitivity, and a 27.1% reduction in total volume was noted between the 2-month-old and 2-year-old glands, which was almost entirely associated with a 54.4% decrease in lipid volume.
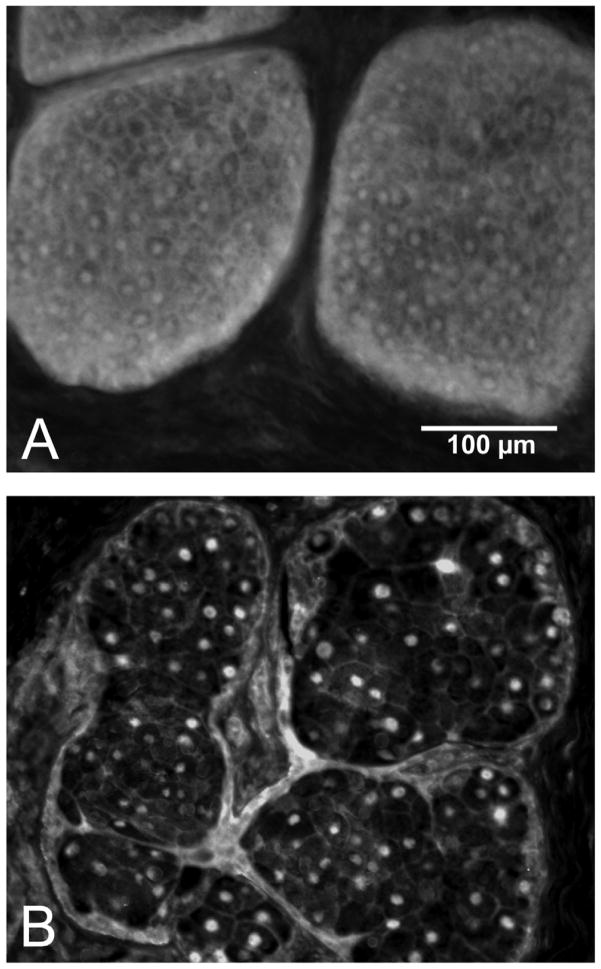
Figure 4.
Meibomian gland lipid volume heat map of 2-month-old gland (A) and 2-year-old gland (B). Lipid components are seen in red to blue and represent a voxel volume of 100% to 0% lipid, respectively. Note the loss of acini (arrows) in the older gland (B) compared to the younger gland (A). (Adapted from Jester et al.11)
These volumetric changes noted by NLO array tomography were also consistent with the findings in a separate study that measured the cross-sectional areas of the meibomian gland in young and old mice and detected a significant 70% reduction in average gland area in eyelids from 2-year-old compared to 2-month-old mice.12 Additionally, decreased gland area was associated with a significant loss in the proliferative potential of acinar basal cells as measured by Ki67 labeling, a nuclear antigen expressed in actively cycling cells. When expression of PPARγ was evaluated, younger mice (2–6 months of age) showed both a cytoplasmic/nuclear localization, while older mice (1–2 years of age) showed only a nuclear localization of PPARγ (Figure 5). Taken together, these changes suggest that meibomian glands in older mice undergo an age-related atrophy that is associated with decreased acinar cell proliferation, differentiation, and lipid synthesis. Furthermore, the changes in PPARγ localization suggest that gene expression controlling lipid synthesis is altered in older mice, which may be the direct cause of the loss of lipid volume identified by array tomography.
Figure 5.
PPARγ localization (Green) in adult mice. We observed cytoplasmic and nuclear anti-PPARγ staining in 6 month old glands (A) that changed to nuclear staining in 2 year old mice (B). (Red – DAPI). (Adapted from Nien et al.12
IV. Age-Related Changes in Human Meibomian Gland Dysfunction
In a recent study, we evaluated excess eyelid tissue obtained from 36 patients ranging in age from 18 to 95 years following canthoplasty procedures.13 Prior to surgery, patients were evaluated for the presence of MGD dropout and changes in the quality of lipid expression using standard clinical scoring criteria. Tissue samples were then evaluated using immunocytochemistry to evaluate meibocyte differentiation using antibodies to PPARγ, cell proliferation using antibodies to Ki67, and meibomian gland inflammation using antibodies against the common leukocyte antigen, CD45.
In this surgical patient population, meibomian gland dropout was highly correlated with altered meibomian gland expression (r = 0.882, P < 0001), and both measures of MGD were highly correlated with patient age (r = 0.618 and r = 0.749, P < 0.001, respectively). Similar to our findings in the mouse, older patients showed significantly less Ki67 labeling (r = 0.591, P < 0.001), indicating reduced basal acinar cell proliferation. Additionally, as shown in Figure 6, there was a significant increase in nuclear PPARγ staining in older compared to younger individuals (r = 0.664, P < 0.001). The degree of MGD dropout and expression was also significantly correlated with nuclear PPARγ staining (r = 0.401 and r = 0.441, P < 0.02, respectively). Interestingly, infiltration of the meibomian gland ductal epithelium and acini with CD45+ cells was also detected, and the degree of infiltration significantly correlated with severity of MGD for both dropout and expression (r = 0.361 and r = 0.414, P < 0.05, respectively). Taken together, these findings indicate that the mouse and human meibomian gland show similar age-related changes that involve altered acinar cell proliferation (Ki67 labeling) and localization of PPARγ that appear to be associated with age-related MGD.
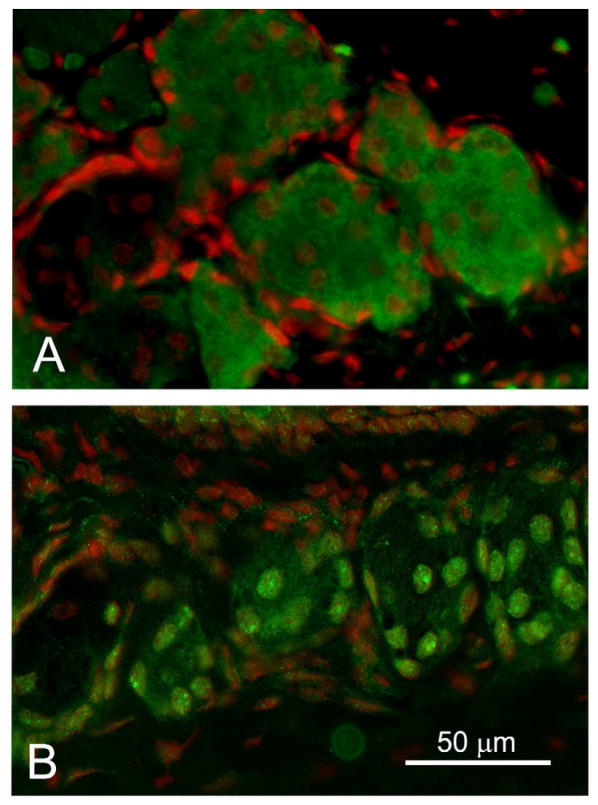
Figure 6.
PPARγ localization in tissue obtained from a 44- (A) and 72-year-old (B) subjects. Note the cytoplasmic acinar cell staining in the tissue from the younger subject that is lost in the older gland. (Adapted from Nien et al.13)
V. Discussion
MGD is a common eyelid disorder having a widespread prevalence of 39% to 50% in the U.S. population with the incidence increasing with age.14–17 MGD is also a major cause of evaporative dry eye disease,18 with loss of glands resulting in decreased tear film lipid, increased aqueous tear evaporation,19 and increased tear film osmolarity,20 leading to ocular surface changes, unstable tear film, and blepharitis.21, 22 While patients with dry eye disease and MGD comprise from 37% to 47% of the average ophthalmologists’ and optometrists’ practice, management of this disease is primarily palliative and includes warm compresses and antimicrobial and anti-inflammatory therapy.15
Currently, three forms of MGD are recognized: hypersecretory, hyposecretory, and obstructive, with the latter form generally considered to be the most common.23 Based on clinical and animal studies,24–28 obstructive MGD is thought to involve hyperkeratinization of the meibomian gland duct, leading to ductal occlusion and plugging of the meibomian gland orifice. This then causes cystic dilation of the duct and a “disuse atrophy” of the acini that is detected as gland “dropout” on transillumination infrared photography (meibography).23
While the risk of evaporative dry eye and MGD increases with age, there have been few histopathologic reports describing the effects of age on meibomian gland structure.24, 29, 30 These reports suggest that aging results in atrophy of the meibomian gland acini and decreased lipid expression from the gland.29, 30 Additional changes that have been noted include focal hyperkeratinization of the ductal epithelium, cystic dilation, and lipogranulomatoses, albeit the association with aging is less clear.24 Thus far, a major barrier to the study of MGD has been the lack of an appropriate model that parallels these findings in human age-related MGD. Specifically, models of hyperkeratinization require the systemic or topical application of materials or drugs, ie, epinephrine or polychlorinated biphenyls, that directly induce gland hyperkeratinization.25–28 Other models require the application of exogenous androgens or the castration of animals, which may have multiple organ effects.31–33
Our recent findings have identified specific age-related changes in the mouse meibomian gland, which include decreased acinar cell proliferation, decreased meibomian gland size, and increased inflammatory cell infiltration that appear to mimic those identified in human patients with MGD.12, 13 Interestingly, these changes occur concurrent with altered localization of PPARγ, which is immunocytochemically detected in cytoplasmic vesicles and nuclei of lipid synthesizing acinar cells in young mice and humans but is restricted to the acinar nuclei of older individuals having atrophic glands. This shift in the localization may have important implications regarding transcriptional regulation by PPARγ and meibocyte differentiation. Studies have shown that PPARγ functions as a “molecular switch” that recruits target gene promoters such as SRC1/CBP and TRAP/DRIP/ARC complexes when bound to ligands, but recruits co-repressors N-CoR and SMRT for target genes when not liganded.10 Additionally, PPAR γ signaling leads to serine phosphorylation and sumoylation of PPARγ and the induced nuclear export to the cytoplasm, leading to downstream effects on gene expression and cell apoptosis. While further study of PPARγ signaling in meibocytes is needed, the loss of cytoplasmic PPAR γ suggest the absence of receptor signaling associated with nuclear export and the suppression of genes associated with lipid synthesis and meibocyte differentiation.
Overall, these findings suggest that the aging mouse may be a novel model for studying age-related changes in the meibomian gland and overcome a major barrier to the study of age-related MGD. Additionally, our findings in this aging model suggest that age-related MGD may be associated with an alternative hyposecretory pathway that fundamentally differs from conventional, obstructive MGD.23, 34 In obstructive MGD, hyperkeratinization of the meibomian gland orifice is thought to lead to cystic ductal dilation and downstream “disuse atrophy” of the meibomian gland acini. This hypothesis is based on findings in animal models showing that chemically induced gland hyperkeratinization leads to initial blockage of the orifice.25, 26, 28 However, it should be noted that a hallmark of MGD in patients is gland dropout, as seen clinically by slit lamp examination and meibography.35, 36 By comparison, hyperkeratinized animal eyelids show swelling of the gland and distinct transillumination defects starting at the gland orifice, and not meibomian gland dropout.25, 27
As an alternative to obstructive MGD, we therefore hypothesize that age-related MGD involves altered PPARγ gene regulation that directly affects meibocyte differentiation and lipid synthesis, leading to acinar atrophy, gland dropout, and downstream hyposecretory MGD. This alternative mechanism is consistent with the clinical finding that meibomian gland dropout significantly correlates with increased ocular surface water evaporation.37 Importantly, this mechanism suggests that the quantity of the meibomian gland secretions, as defined by the rate of meibocyte differentiation, may be the primary cause of age-related MGD and the development of evaporative dry eye. In this regard, it should be noted that recent studies suggest that age is associated with increased rather than decreased lipid on the eyelid margin, suggesting that changes in lipid quality may also occur.38, 39 Clearly, additional study into the role of PPARγ in regulating meibomian gland function is needed to provide a better understanding of the underlying mechanism of age-related MGD, as well as suggest novel therapeutic approaches to the treatment of evaporative dry eye.
Acknowledgments
Supported by NEI EY021510, Discovery Eye Foundation, The Skirball Program in Molecular Ophthalmology, and Research to Prevent Blindness.
Footnotes
The authors have no commercial or proprietary interest any concept or product discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 4.Findlater GS, McDougall RD, Kaufman MH. Eyelid development, fusion and subsequent reopening in the mouse. J Anat. 1993;183 (Pt 1):121–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Teraishi T, Yoshioka M. Electron-microscopic and immunohistochemical studies of eyelid reopening in the mouse. Anat Embryol (Berl) 2001;204:101–7. doi: 10.1007/s004290100189. [DOI] [PubMed] [Google Scholar]
- 6.Andersen H, Ehlers N, Matthiessen ME. Histochemistry and development of the human eyelids. Acta Ophthalmol. 1965;43:642–8. doi: 10.1111/j.1755-3768.1965.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 7.Knop N, Knop E. [Meibomian glands. Part I: anatomy, embryology and histology of the Meibomian glands] Ophthalmologe. 2009;106:872–83. doi: 10.1007/s00347-009-2006-1. German. [DOI] [PubMed] [Google Scholar]
- 8.Nien CJ, Massei S, Lin G, et al. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–42. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Beekum O, Fleskens V, Kalkhoven E. Posttranslational modifications of PPAR-gamma: fine-tuning the metabolic master regulator. Obesity (Silver Spring) 2009;17:213–9. doi: 10.1038/oby.2008.473. [DOI] [PubMed] [Google Scholar]
- 11.Jester BE, Nien CJ, Winkler M, et al. Volumetric reconstruction of the mouse meibomian gland using high-resolution nonlinear optical imaging. Anat Rec (Hoboken) 2011;294:185–92. doi: 10.1002/ar.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nien CJ, Paugh JR, Massei S, et al. Age-related changes in the meibomian gland. Exp Eye Res. 2009;89:1021–7. doi: 10.1016/j.exer.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nien CJ, Massei SR, Lin G, et al. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129:462–9. doi: 10.1001/archophthalmol.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci. 1990;67:710–2. doi: 10.1097/00006324-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7:S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 16.Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci. 1996;73:208–10. doi: 10.1097/00006324-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10:144–8. doi: 10.1111/j.1475-1313.1990.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 18.Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–60. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- 19.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 20.Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–6. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 21.McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- 22.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–70. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 23.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–26. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 24.Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94:383–7. doi: 10.1016/0002-9394(82)90365-8. [DOI] [PubMed] [Google Scholar]
- 25.Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30:936–45. [PubMed] [Google Scholar]
- 26.Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988;29:1190–4. [PubMed] [Google Scholar]
- 27.Jester JV, Rife L, Nii D, et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–7. [PubMed] [Google Scholar]
- 28.Ohnishi Y, Kohno T. Polychlorinated biphenyls poisoning in monkey eye. Invest Ophthalmol Vis Sci. 1979;18:981–4. [PubMed] [Google Scholar]
- 29.Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–42. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70–S74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- 31.Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp Eye Res. 2006;83:291–6. doi: 10.1016/j.exer.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Schirra F, Suzuki T, Richards SM, et al. Androgen control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2005;46:3666–75. doi: 10.1167/iovs.05-0426. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan DA, Sullivan BD, Ullman MD, et al. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41:3732–42. [PubMed] [Google Scholar]
- 34.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 35.Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–85. doi: 10.1097/00003226-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Robin JB, Jester JV, Nobe J, et al. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology. 1985;92:1423–6. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- 37.Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–51. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- 38.Chew CK, Hykin PG, Jansweijer C, et al. The casual level of meibomian lipids in humans. Curr Eye Res. 1993;12:255–9. doi: 10.3109/02713689308999471. [DOI] [PubMed] [Google Scholar]
- 39.Ashraf Z, Pasha U, Greenstone V, et al. Quantification of human sebum on skin and human meibum on the eye lid margin using Sebutape(R), spectroscopy and chemical analysis. Curr Eye Res. 2011;36:553–62. doi: 10.3109/02713683.2011.574331. [DOI] [PubMed] [Google Scholar]