Summary
Fragile X syndrome (FXS) is the leading inherited cause of autism and intellectual disability. Aberrant synaptic translation has been implicated in the etiology of FXS, but most lines of research on therapeutic strategies have targeted protein synthesis indirectly, far upstream of the translation machinery. We sought to perturb p70 ribosomal S6 kinase 1 (S6K1), a key translation initiation and elongation regulator, in FXS model mice. We found that genetic reduction of S6K1 prevented elevated phosphorylation of translational control molecules, exaggerated protein synthesis, enhanced mGluR-dependent long-term depression (LTD), weight gain, and macro-orchidism in FXS model mice. In addition, S6K1 deletion prevented immature dendritic spine morphology and multiple behavioral phenotypes, including social interaction deficits, impaired novel object recognition, and behavioral inflexibility. Our results support the model that dysregulated protein synthesis is the key causal factor in FXS, and that restoration of normal translation can stabilize peripheral and neurological function in FXS.
Introduction
The past two decades has witnessed an explosion in research pertaining to fragile X syndrome (FXS) and associated disorders. FXS is a monogenic syndrome that is the leading genetic cause of inherited mental disability and autism (Penagarikano et al., 2007). FXS is caused by an unstable expansion of CGG repeats in the 5’UTR of the Fmr1 gene, causing hypermethylation and subsequent silencing of the gene (Verkerk et al., 1991). The transcriptional silencing results in the loss of expression of the fragile X mental retardation protein (FMRP), which is an RNA-binding protein responsible for regulating the translation of specific sets of mRNA (Darnell et al., 2011).
FMRP is involved in different aspects of RNA metabolism, including trafficking of RNP particles, translation of specific mRNA transcripts via regulation of translation initiation and elongation, and targeted degradation via the RISC complex (Jin et al., 2004; Kao et al., 2010; Melko and Bardoni, 2010; Park et al., 2008). In addition, exaggerated protein synthesis has been observed in multiple brain regions Fmr1 knockout mice (Fmr1 KO) (Qin et al., 2005). Activation of multiple GPCR-mediated pathways have been shown to induce protein synthesis-dependent long-term depression (LTD) via FMRP (Volk et al., 2007; Zhang and Alger, 2010), and these forms of LTD are enhanced in Fmr1 KO mice (Hou et al., 2006; Huber et al., 2002). Reduction of mGluR5 signaling by crossing Fmr1 KO mice with Grm5 heterozygotic KO mice corrected the exaggerated protein synthesis phenotype (Dolen et al., 2007), consistent with the ‘mGluR theory’ of FXS (Bear et al., 2004). Moreover, cognitive deficits in a Drosophila model of FXS can be rescued by general protein synthesis inhibitors (Bolduc et al., 2008). However, little effort has been focused on directly modulating the regulation of the translational control machinery to prevent phenotypes observed in mouse models of FXS.
The protein kinase mammalian target of rapamycin (mTOR) is a vital regulator of translation across all tissues and impacts cell growth, proliferation, and autophagy (Hoeffer and Klann, 2010). mTOR in association with Raptor forms the mTOR complex 1 (mTORC1), which is a necessary signaling component of long-lasting protein synthesis-dependent synaptic plasticity and memory (Costa-Mattioli et al., 2009; Richter and Klann, 2009). Not only is mTORC1 signaling triggered downstream of group I mGluRs activation and required for mGluR-LTD (Hou and Klann, 2004), but it also has been demonstrated to be dysregulated in Fmr1 KO mice (Sharma et al., 2010). In addition, hyper-responsive ERK signaling has been shown to directly influence the elevated translation rates observed in Fmr1 KO mice (Osterweil et al., 2010). p70 ribosomal S6 kinase 1 (S6K1) is a common downstream effector of both mTORC1 and ERK signaling and plays a direct role in regulating translation. S6K1 controls translation by phosphorylating ribosomal protein S6 and eukaryotic initiation factor 4B, facilitates eIF4A helicase activity by phosphorylating PDCD4, promotes peptide elongation via its actions on eEF2 Kinase, and regulates the exon-junction complex functions by activating SKAR (Holz et al., 2005; Ma et al., 2008; Raught et al., 2004; Wang et al., 2001). In addition, S6K1 is an FMRP kinase and regulates expression of LTD-relevant proteins such as SAPAP3 (Narayanan et al., 2008) and phosphorylation of S6K1 at the mTORC1 site is elevated in Fmr1 KO mice (Sharma et al., 2010). Finally, recent studies using lymphocytes and brain tissue derived from FXS patients showed an upregulation of S6K1 phosphorylation compared to normal controls (Hoeffer et al., 2012). Thus, it is possible that depressing S6K1 activity in FXS model mice could reverse the exaggerated protein synthesis and thereby correct multiple phenotypes displayed by FXS mice.
Herein we evaluated whether S6K1 could be a viable target for correcting phenotypes in FXS model mice. We generated mice with a genetic deletion of S6K1 in the Fmr1 KO background. We report that the genetic deletion of S6K1 prevented the enhanced phosphorylation of mTOR and downstream effectors of mTORC1 in FXS model mice. Consistent with this observation, removal of S6K1 also corrected exaggerated protein synthesis in the hippocampus of the FXS model mice. In addition, we found that enhanced mGluR-LTD was normalized in the Fmr1/S6K1 double knockout (dKO) mice. The genetic ablation of S6K1 also prevented several behavioral abnormalities exhibited by FXS model mice, including increased social anxiety, impaired novel object recognition and motor memory, and behavioral inflexibility. Morphological studies revealed a decrease in the number of immature spines in FXS model mice that lack S6K1. In summary, our data suggests that genetic reduction of S6K1 can prevent molecular, synaptic plasticity, dendritic morphology, and behavioral phenotypes associated with FXS and therefore may serve as a potential target for therapeutic intervention in humans with FXS.
Results
Elevated phosphorylation of translational control molecules and exaggerated protein synthesis in Fmr1 KO mice are prevented by deletion of S6K1
To determine whether reducing S6K1 could correct phenotypes observed in FXS model mice, Fmr1 KO mice were crossed to mice globally lacking S6K1. S6K1 KO mice have been reported to display deficits in early phase long-term potentiation (LTP) and acquisition of conditioned taste aversion (Antion et al., 2008b). These phenotypes are distinct from those displayed by Fmr1 KO mice and importantly, it was shown that mGluR-LTD is expressed and S6 phosphorylation is present in S6K1 KO mice (Antion et al, 2008a). The resultant Fmr1/S6K1 KO (dKO) mice were obtained with the expected genetic frequencies, with no observable physiological defects, and were reproductively viable.
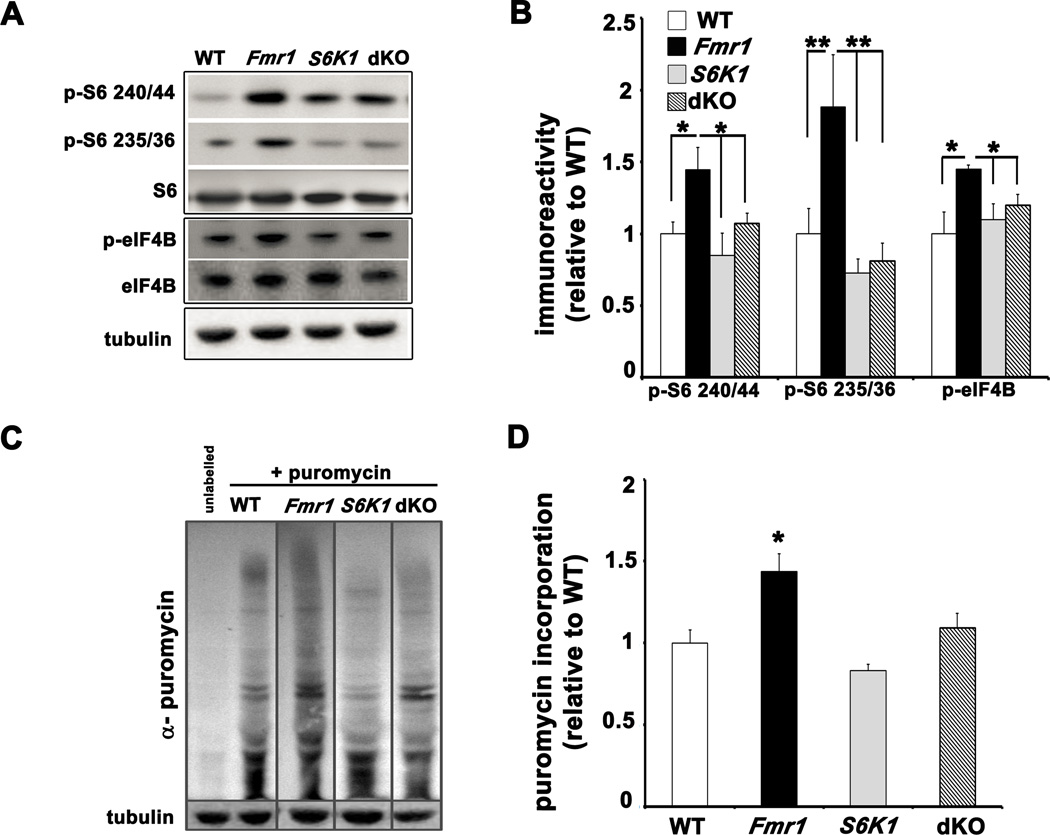
We first examined the phosphorylation state of key translational control molecules regulated by S6K1 in adult mice of all four genotypes (wild-type (WT), Fmr1 KO, S6K1 KO and dKO). In whole hippocampal lysates, Fmr1 KO mice showed increased levels of phosphorylated S6 at the 240/44 and 235/36 phosphorylation sites when compared to WT littermates (Figures 1A and 1B). In addition, phosphorylation of eIF4B was increased in Fmr1 KO mice (Figures 1A and 1B). In the dKO mice, the levels of phosphorylated S6, and eIF4B were reduced to levels similar to WT mice. Because S6K1 phosphorylates mTORC1 directly at serine 2448 and has been shown to regulate PI3K and ERK signaling via feedback regulation of IRS-1, we examined mTOR and ERK phosphorylation in hippocampal lysates from all four genotypes (Chiang and Abraham, 2005; Magnuson et al., 2012). We observed increased mTOR phosphorylation in the Fmr1 KO mice that was reduced by the genetic ablation of S6K1 (Supplementary Figures S1A and S1B). Similarly, we observed increased phosphorylation of ERK in Fmr1 KO mice that was corrected by the ablation of S6K1 (Supplementary Figures S1A and S1B). These results support the idea that removal of S6K1 in Fmr1 KO mice not only corrects the enhanced phosphorylation of downstream effectors of S6K1 involved in protein synthesis, including S6 and eIF4B, but also the feedback mechanisms that results in aberrant signaling by correcting the elevated phosphorylation of both mTOR and ERK.
Figure 1. Increased phosphorylation of translation control molecules and exaggerated protein synthesis in Fmr1 KO mice are corrected in dKO mice.
A) Representative Western blots of whole hippocampal lysates from WT, Fmr1 KO (Fmr1), S6K1 KO (S6K1) and dKO mice that were probed for basal levels of phosphorylated S6 and eIF4B. B) All ratios shown in cumulative graph were normalized first to levels of total S6 and eIF4B and then expressed relative to WT. n=9 mice for each genotype. *p<0.05 and **p<0.01 with a two-way ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. C) Representative Western blots of lysates from hippocampal slices incubated with puromycin to measure basal rates of protein synthesis. Sensitivity of the SUnSET methods to detect changes in rates of protein synthesis is demonstrated in Supplementary Figure S1C. D) Cumulative graph with ratios expressed relative to WT. n=4 mice per genotype, 2–3 slices per condition; *p<0.05 by Kruskal-Wallis followed by Dunn's multiple comparisons post-hoc tests (genotypes compared to WT). Error bars denote S.E.M. Linked to Supplementary Figures S1 and S2.
We also examined whether the heterozygous deletion of S6K1 could correct the molecular signaling phenotypes observed in Fmr1 KO mice. Compared to WT littermates, Fmr1 KO mice lacking one copy of S6K1 exhibited S6K1 phosphorylation that was significantly increased as compared to WT littermates, which was similar to that observed in Fmr1 KO mice (Supplementary Figures S2A and S2B). Therefore, we concluded that the deletion of one copy of S6K1 results in hyperactive S6K1 activity, perhaps due to overcompensation, and that a complete abrogation of S6K1 would be necessary to correct phenotypes exhibited by Fmr1 KO mice.
To examine protein synthesis, we used SUnSET (Schmidt et al., 2009), a non-radioactive puromycin end-labeling assay. This relatively new technique has been utilized to measure protein synthesis during long-term associative memory consolidation (Hoeffer et al., 2011). Consistent with previous studies (Dolen et al., 2007; Osterweil et al., 2010), basal levels of protein synthesis were elevated in hippocampal slices from Fmr1 KO mice (Figures 1C and 1D). In contrast, slices from dKO mice displayed levels of puromycin labeling similar to WT mice (Figures 1C and 1D).
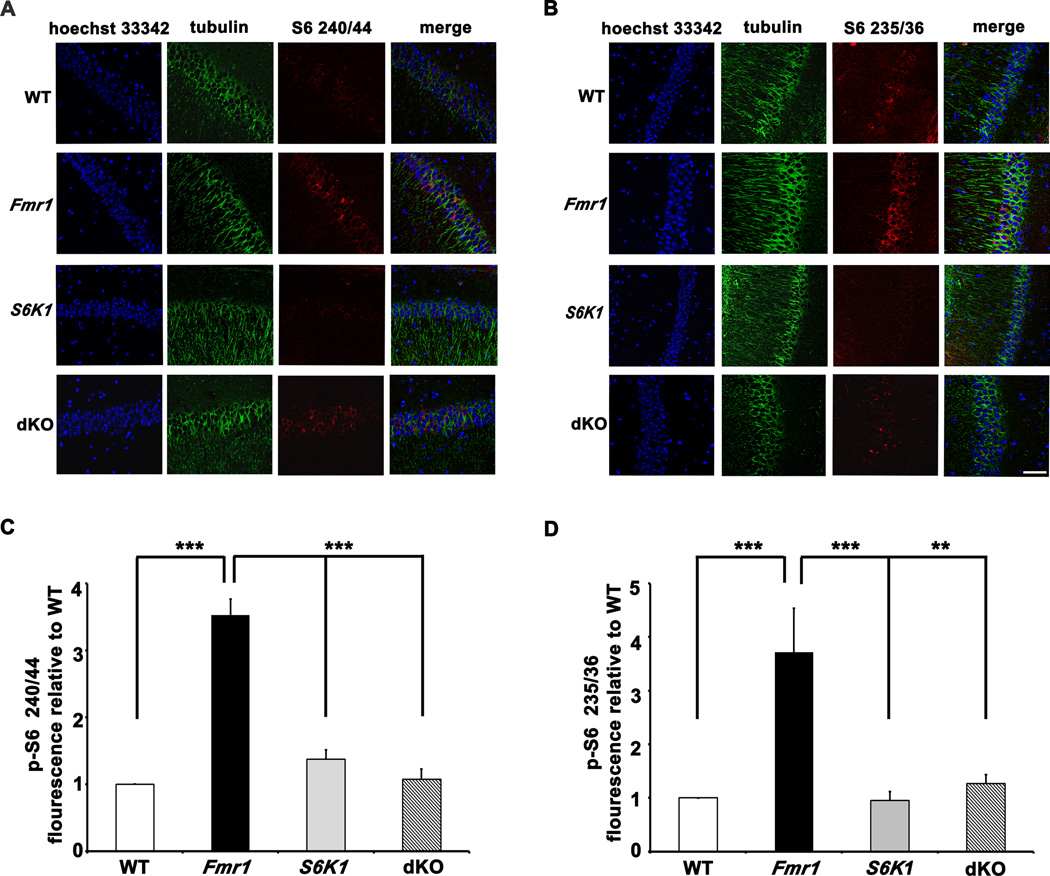
We also assessed the phosphorylation levels of S6 at both phosphorylation sites in hippocampal area CA1 using immunohistochemical methods. Stained sections from the Fmr1 KO mice exhibited increased levels of phosphorylated S6 compared to sections from WT mice, with a subtle shift in the localization of phospho-S6 to somato-dendritic compartments of the pyramidal neurons (Figures 2A and 2B). The increased S6 phosphorylation was reduced in sections from the dKO mice, with phospho-S6 largely localized to the cell bodies. Taken together, these findings suggest that FXS mice have aberrant S6K1-dependent protein synthesis and that genetic reduction of S6K1 in these mice successfully restores signaling pathways important for translational control and hence basal protein synthesis.
Figure 2. Elevated phosphorylation of S6 in pyramidal neurons of area CA1 in Fmr1 KO mice is corrected in dKO mice.
A and B) Representative images of hippocampal sections stained for phosphorylated S6 (240/44 and 235/36) in pyramidal neurons of area CA1. Scale bar denotes 20 µm. n=3 mice per genotype. C and D) Integrated intensity of phospho-S6- Alexa 568 signal measured across 60 neurons (10 neurons per slice, two slices per mouse) for each genotype. **p<0.01 and ***p<0.001 with a two-way ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. Error bars denote S.E.M.
S6K1 deletion differentially influences the expression levels of proteins regulated by FMRP
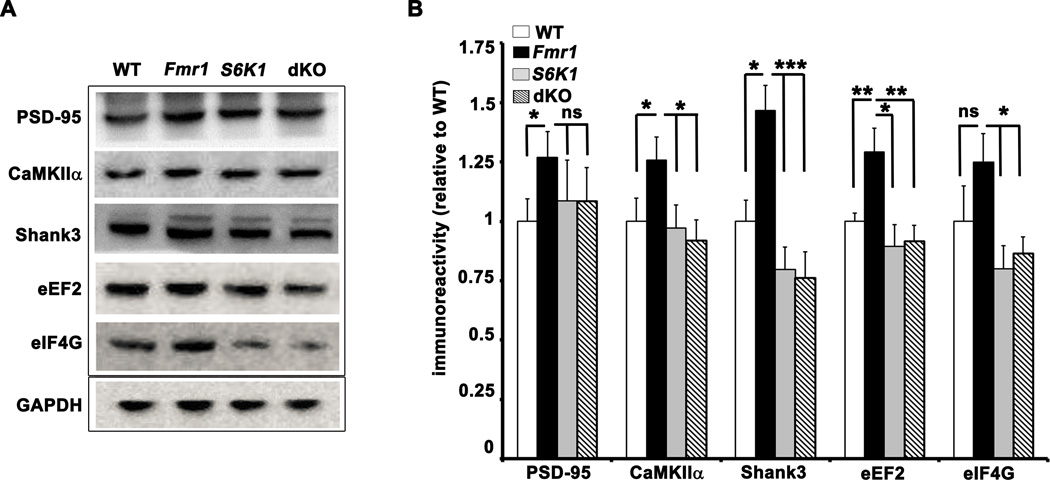
FMRP is a key translation regulator known to play a major role in several forms of synaptic plasticity. Previous reports indicate that the expression of several synaptic proteins are regulated by FMRP, including PSD-95, CaMKIIα, and MAP1B (Hou et al., 2006; Kao et al., 2010; Lu et al., 2004; Zalfa et al., 2007) and a recent HITS-CLIP screen by Darnell et al., (2011) reported a large number of mRNAs that interact with FMRP, including those that encode proteins important for synaptic plasticity. We determined whether the expression of several of these proteins was elevated in Fmr1 KO mice, and whether abnormal expression of the proteins could be corrected by deletion of S6K1. We observed increased levels of synaptic proteins PSD-95, CaMKIIα, and Shank3. In parallel we also examined the levels of eIF4G and eEF2, translation factors that are putative FMRP targets (Darnell et al., 2011). Although the deletion of S6K1 failed to normalize the elevated PSD-95 expression in Fmr1 KO mice, the expression of CaMKIIα, and Shank3 was restored to levels comparable to WT mice (Figures 3A and 3B). We also observed increased levels of eEF2 in Fmr1 KO mice and that was normalized to WT levels in dKO mice (Figures 3A and 3B). Finally, we observed a trend of increased eIF4G levels in Fmr1 KO mice that was significantly reduced in the S6K1 and dKO mice. We did not observe significant differences in basal expression of Arc/Arg 3.1 levels in whole hippocampal lysates from the Fmr1 KO mice (Supplementary Figures S3A and S3B). Because eEF2 is encoded by a 5’ TOP mRNA we further explored whether the deletion of S6K1 affected the expression of other 5’ TOP mRNA protein products. We examined the levels of S6 and polyA-binding protein (PABP), which are encoded by 5’TOP mRNAs (Meyuhaus and Dreazen, 2009) but are not likely regulated by FMRP (Darnell et al., 2011). We found no differences in levels of S6 and PABP between the four genotypes (Supplementary Figures S3A and S3B), suggesting that the regulation of eEF2 by FMRP was not related to its mRNA containing a 5'TOP. In summary, these findings suggest that there is excessive translation of FMRP targets in FXS model mice, and that most, but not all, are normalized to WT levels by decreasing the levels of S6K1.
Figure 3. Effect of S6K1 deletion in Fmr1 KO mice on the protein expression levels of FMRP targets involved in synaptic plasticity and protein synthesis.
A) Representative Western blots of whole hippocampal lysates from adult mice of all four genotypes showing the protein expression levels of the FMRP targets PSD-95, CaMKIIα, Shank3, eEF2, and eIF4G identified by a recent HITS-CLIP screen (Darnell et al., 2011). B) Cumulative graph with ratios expressed relative to WT. n=8–9 per protein per genotype. *p<0.05, ** p<0.01, and ***p<0.001 with a two-way ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. Error bars denote S.E.M. Linked to Supplementary Figure S3.
Enhanced mGluR-LTD in Fmr1 KO mice is prevented by deletion of S6K1I
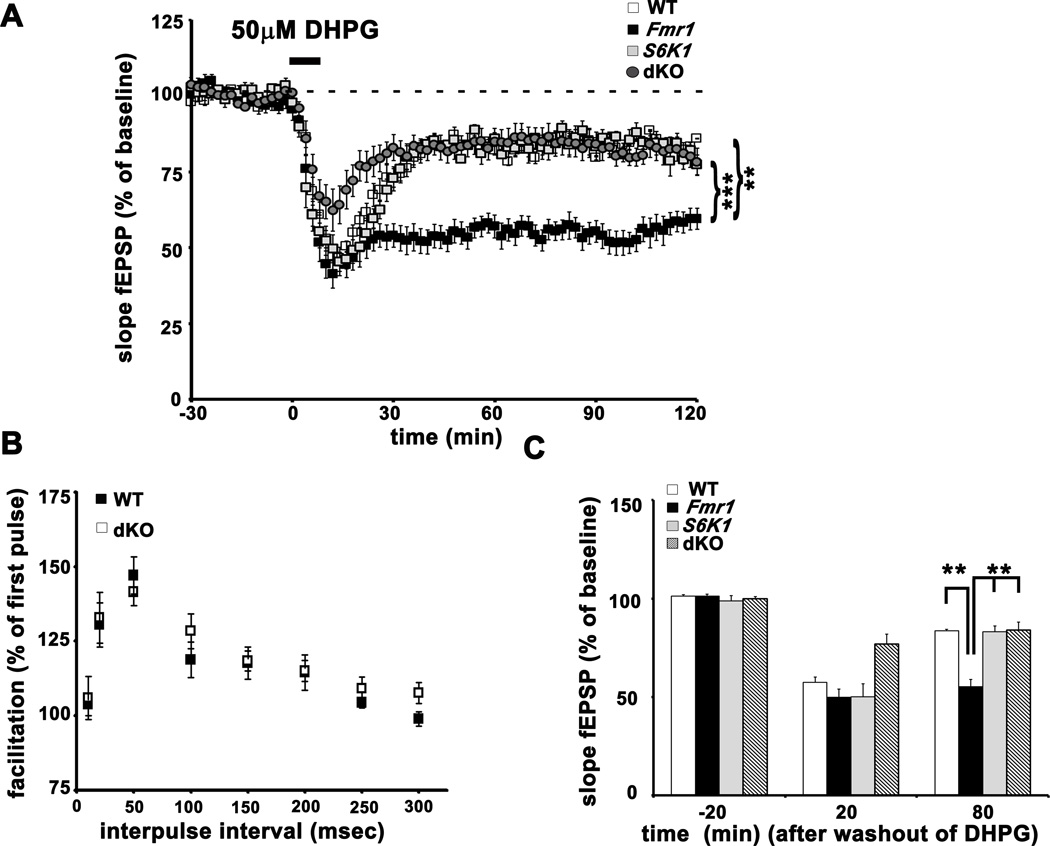
Fmr1 KO mice display enhanced hippocampal LTD in response to stimulation of group1 metabotropic glutamate receptors with the agonist DHPG (Huber et al., 2002). Because genetic reduction of S6K1 corrected exaggerated protein synthesis in the Fmr1 KO mice, we asked whether this manipulation also could correct the enhanced mGluR-LTD. We induced mGluR-LTD in hippocampal slices from all four genotypes with an application of 50 µM DHPG for 10 min. Consistent with previous findings, we observed enhanced LTD in slices from Fmr1 KO mice (Figures 4A and 4C). The enhanced mGluR-LTD was markedly reduced in slices from the dKO mice and was similar to LTD observed in slices from WT and S6K1 KO mice. No differences in paired-pulse facilitation between dKO and WT slices were found (Figure 4B). Fmr1 KO mice with a heterozygous deletion of S6K1 still exhibited enhanced mGluR-LTD (Supplementary Figures S4A and S4B). Thus, the complete genetic ablation of S6K1 is required to normalize the enhanced mGluR-LTD exhibited by FXS model mice.
Figure 4. Exaggerated mGluR-LTD in Fmr1 KO mice is reversed in dKO slices.
A) DHPG-induced mGluR-LTD in hippocampal slices from WT, Fmr1, S6K1 and dKO. n=13 slices for WT, 12 slices for Fmr1 KO, S6K1 KO and dKO mice. **p<0.01 and ***p<0.001 with a repeated measures ANOVA (genotype×time) followed by Bonferroni post-hoc tests. B) Paired-pulse facilitation in slices from dKO mice was similar WT mice. Percent facilitation was calculated as a ratio of the second fEPSP to the first fEPSP with an interpulse interval ranging from 10 to 300 msec. n=10 slices WT and dKO mice. C) Mean values of the slope of the fEPSP before and after washout of DHPG. n=11 slices per genotype. ***p<0.001 with a two-way ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. Error bars denote S.E.M. Linked to Supplementary Figure S4.
Abnormal dendritic spine morphology in Fmr1 KO mice is corrected by deletion of S6K1
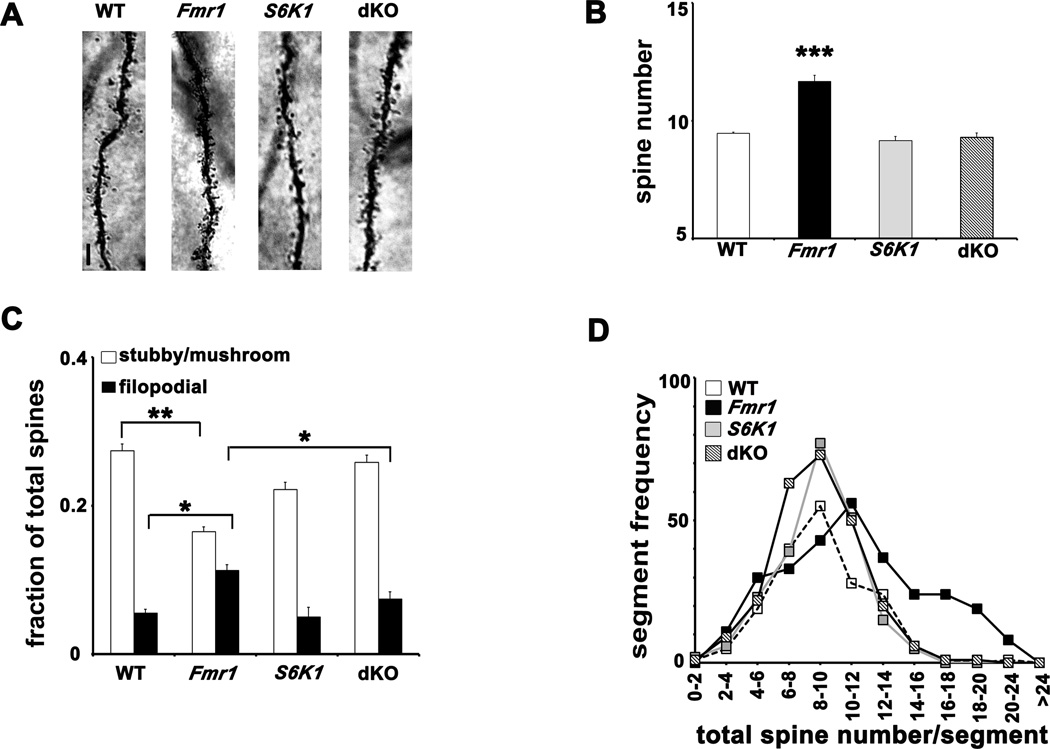
Abnormal dendritic morphology has been reported in several brain areas in Fmr1 KO mice and humans with FXS, as indicated by an excessive number of spines and an increase in immature, filopodia-like spines (He and Portera-Cailleau, 2012). Because deleting S6K1 prevented altered translational control, exaggerated protein synthesis, and enhanced mGluR-LTD displayed by Fmr1 KO mice, we measured spine density and morphology in apical dendrites of pyramidal neurons in hippocampal area CA1 in mice from all four genotypes. Using the rapid Golgi staining procedure, we counted spine protrusions in the apical dendritic branches, 50 µm from the soma at intervals of 10 µm, and spine morphology type based on previously published guidelines (Grossman et al., 2006). We found a small but significant increase in spine density in Fmr1 KO neurons, which was corrected to WT levels in the dKO neurons (Figures 5A and 5B). Frequency histograms indicated a greater number of 10 µm segments with high spine density in Fmr1 KO neurons compared to the other genotypes (Figure 5D). Spine classification analysis revealed a higher fraction of filopodia-like and thin spines compared to stubby and mushroom spines in Fmr1 KO neurons. In contrast, the fraction of filopodia-like spines was significantly decreased with a concomitant elevation of mature stubby/mushroom spines in dKO neurons, comparable to levels in WT and S6K1 KO neurons (Figure 5C). No appreciable differences were found in spines of other types (Supplementary Figure S5). These findings demonstrate that removing S6K1 can prevent dendritic morphology abnormalities associated with loss of FMRP.
Figure 5. Aberrant dendritic morphology in area CA1 of Fmr1 KO mice is corrected in dKO mice.
A) Representative images of Golgi-Cox stained CA1 apical dendrites from all four genotypes. Scale bar denotes 3 µm. B) Spine counts on apical dendrites per 10 µm. n=20–22 neurons (5 neurons per mouse, 4 mice and 180, 10 µm segments per genotype). *** p<0.001 by Kruskal-Wallis followed by Dunn’s multiple comparisons post-hoc tests (genotypes compared to WT). C) Spine morphology studies showing the fraction of filopodial and stubby/mushroom spines. n=20–22 neurons per genotype. *p<0.05, **p<0.01 with a group-wise Student’s t-test. In addition, a two-way ANOVA was used as shown in Figure S5. D) Frequency histograms representing distribution of 10 µm segments as a function of the number of spines contained in each dendrite. Error bars denote S.E.M. Linked to Supplementary Figure S5.
Multiple behavioral phenotypes in Fmr1 KO mice are corrected by deletion of S6K1
To assess whether the genetic reduction of S6K1 in Fmr1 KO mice could alleviate autistic-like behaviors, we performed an array of tests on all four genotypes. It has been reported that the behavior of Fmr1 KO mice in these tests is sensitive to genetic background, age, and the experimental paradigms used (Dobkin et al., 2000; Spencer et al., 2011). Therefore, we designed our behavioral battery to include tests that have reported consistent differences between WT and Fmr1 KO mice and have been conducted previously in our laboratory (Hoeffer et al., 2008).
To examine motor coordination and skill acquisition, we tested performance on the rota-rod using parameters similar to those described previously (Spencer et al., 2011). Fmr1 KO mice were significantly impaired in learning and motor coordination across eight trials over two days as compared to their WT littermates (Figure 6A). In contrast, the dKO mice displayed markedly higher motor coordination and acquisition, performing consistently better than the other three genotypes on both days of the test (Figure 6A). Motor coordination and acquisition of S6K1 KO mice was diminished, which differs from previous findings (Antion et al., 2008b), perhaps due to background differences. Taken together, the rota-rod results indicate that removal of S6K1 corrects impaired motor coordination and motor skill acquisition in FXS model mice.
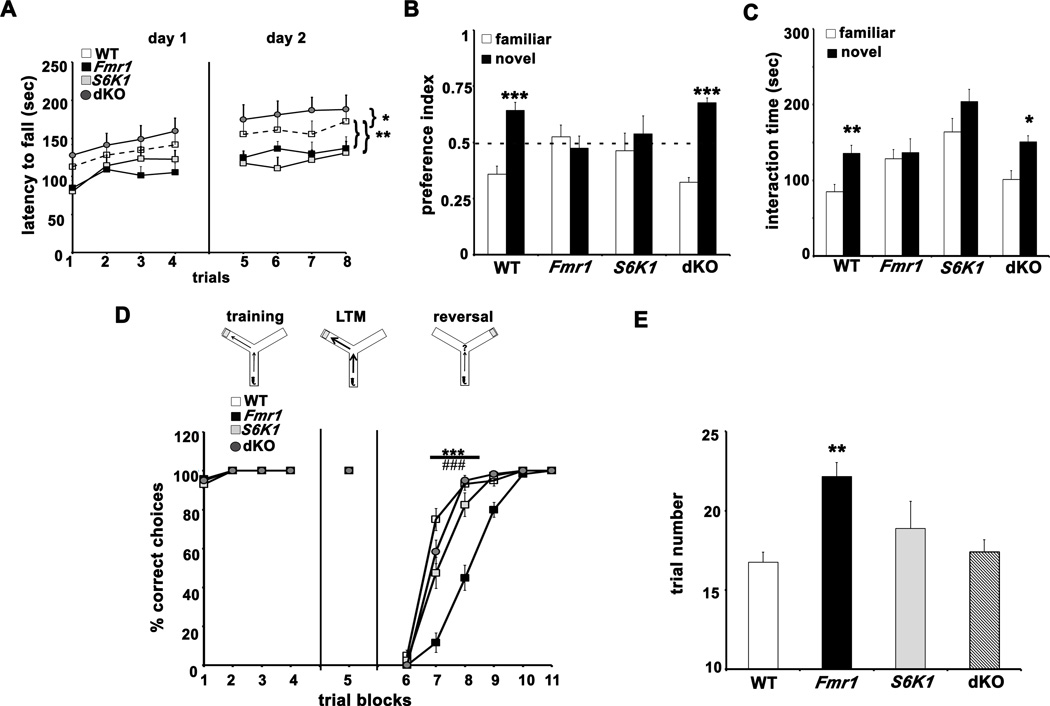
Figure 6. ASD-like behaviors in Fmr1 KO mice are corrected in dKO mice.
A) Motor coordination and memory tested using the rota-rod test. *p<0.05, **p<0.01 with a repeated measures ANOVA (genotype×trial) followed by a Dunnett’s post-hoc test (WT as control group). B) Preference indices of mice towards novel object introduced in the novel object recognition test. *** p<0.001 with a paired Student's t-test (familiar vs novel for each genotype). C) Interaction time of mice with familiar and novel mice in the social novelty test. *p<0.05, ** p<0.01 with a paired Student's t-test (familiar vs novel for each genotype). D) Behavioral flexibility in the Y-maze test. *** p<0.001, WT vs. Fmr1; ### p<0.001, Fmr1 vs. dKO with a repeated measures ANOVA (genotype×trial) followed by Bonferronni post-hoc tests. E) Number of trails required by each genotype to achieve criterion in Y-maze test. **p< 0.01 with a two-way ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. Error bars denote S.E.M. n=12 WT, 12 Fmr1, 8 S6K1 and 12 dKO for all tests. Linked to Supplementary Figure S6.
Deficits in appropriate social interaction and perspective-taking are one of the core features of autism spectrum disorders (ASDs). Therefore, we employed the 3-chambered social interaction test to determine whether removal of S6K1 could prevent impaired/inappropriate social behavior shown by Fmr1 KO mice. In the social approach test, experimental mice interacted more with the stimulus mouse compared to the object across all genotypes (Supplementary Figure S6D). When the object was replaced by a novel mouse in the next phase of the test, clear genotype-specific differences emerged (Figure 6C). Fmr1 KO mice failed to distinguish between the familiar and novel mice, whereas WT and dKO mice spent significantly more time interacting with the novel mouse. S6K1 KO mice showed a trend towards increased interaction with novel stimuli. These findings indicate that removing S6K1 can correct deficits in social interactions displayed by FXS model mice.
As a further test of whether removing S6K1 can prevent the inability of Fmr1 KO mice to recognize and/or remember new environmental stimuli and cues, we examined the behavior of all four genotypes in the novel object recognition test (Figure 6B). After being habituated with two objects for two consecutive days in the arena, Fmr1 KO mice failed to recognize the novel object introduced by replacing one of the familiar objects on the third day of the test. In contrast, WT and dKO mice were able to distinguish the new object with high fidelity when tested under same conditions. Interestingly, S6K1 KO mice were impaired in their ability to discriminate between familiar and novel objects. These findings indicate that removal of S6K1 in Fmr1 KO mice can restore appropriate novel object recognition memory.
We also examined whether the four genotypes showed differences in their ability to learn and recall a task, and their flexibility in modifying their responses when the task was changed using a water-based Y-maze test. All four genotypes responded to training and learned the test with comparable efficiency on the first day (Figure 6D). Memory recall for the arm location of the hidden platform also was robust on the day after training for all four genotypes, indicating that there were no deficits in long-term memory (Figure 6D). When the platform location was reversed, Fmr1 KO mice displayed impairments in reversal learning, requiring an additional 15 trials to meet criterion as compared to WT, S6K1 KO, and dKO mice (Figure 6 D and E). These findings suggest that upregulation of S6K1 signaling plays a key role in behavioral inflexibility in FXS model mice.
Fmr1 KO mice also have been reported to show increased ambulatory behavior in the open field test (Spencer et al., 2005), which we reproduced in our behavioral cohort (Supplementary Figures S6A and S6B). In contrast, S6K1 KO littermates exhibited significantly decreased exploration and preferred the peripheral areas vs. the center of the arena. Interestingly, dKO mice displayed open field exploration indistinguishable from the Fmr1 KO mice, indicating that the deletion of S6K1 did not correct increased ambulatory behavior in FXS model mice. In addition, marble burying behavior, a phenotype that has been used to model obsessive-compulsive behavior in mice, was enhanced in Fmr1 KO mice compared to WT littermates. We found that Fmr1 KO, S6K1 KO and dKO mice buried a higher number of marbles compared to WT littermates (Supplementary Figure S6C). These findings indicate that the enhanced repetitive behavior of Fmr1 KO mice is not rescued by the abrogation of S6K1.
Excessive weight gain and macro-orchidism in Fmr1 KO mice are corrected by deletion of S6K1
FXS patients display neuroendocrine dysfunction that is reflected in a generalized increase in total body weight, macrocephaly, and enhanced stature (Penagarikano et al., 2007). Another frequently associated feature is macro-orchidism (enlarged testicles), first observed in male patients immediately after attaining puberty (Hagerman et al., 1983). The weight gain phenotype in Fmr1 KO mice has been successfully rescued by reducing mGluR5 signaling (Dolen et al., 2007). In contrast, the macro-orchidism phenotype in FXS mice has yet to be fully corrected. Therefore, we proceeded to determine whether removing S6K1 could prevent weight gain and macro-orchidism phenotypes displayed by FXS model mice.
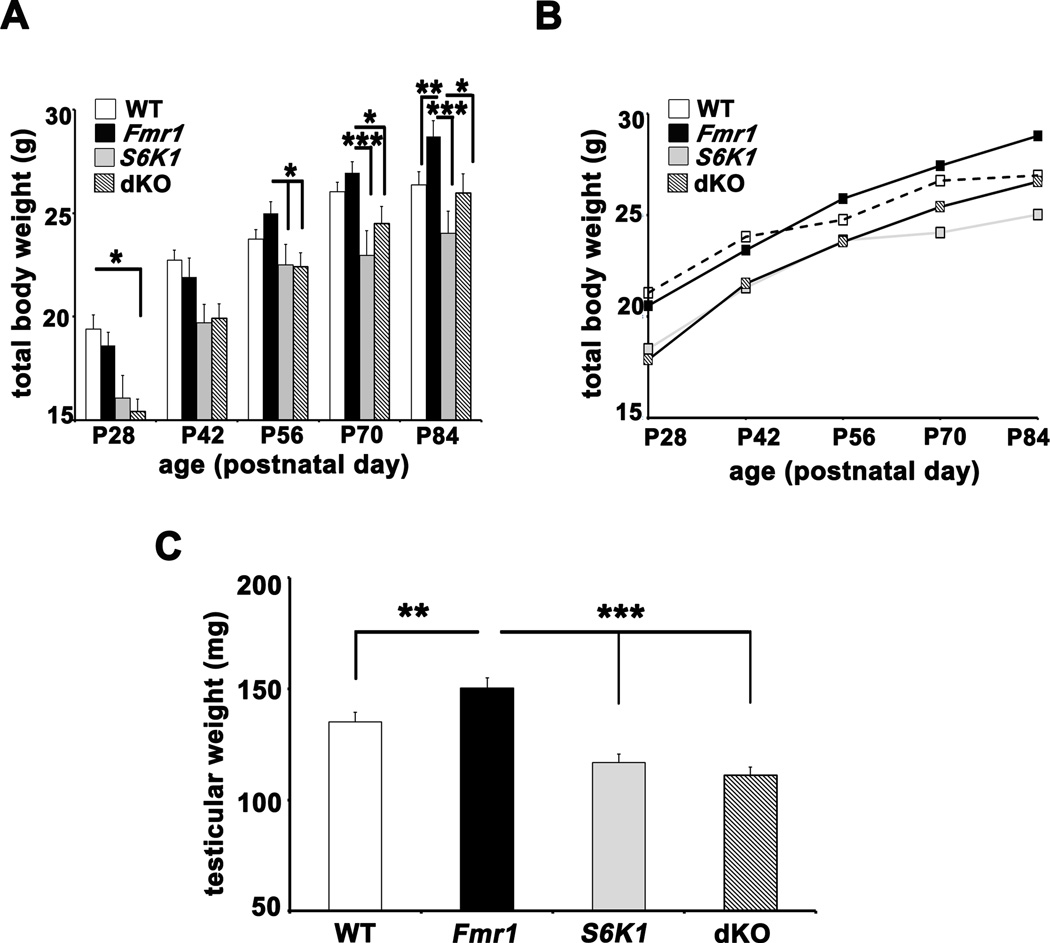
Male mice from six litters were monitored over eight weeks from the time of weaning (postnatal day 23–25) to twelve weeks of age. We consistently observed that pups were exceedingly small and weak if they were weaned at P20. Male littermates were weighed once weekly at the same time. Although juvenile Fmr1 KO mice weighed slightly less than wild-type littermates, by six weeks the rate of weight gain of the Fmr1 KO mice exceeded that of the other three genotypes (Figures 7A and 7B) In contrast, S6K1 KO and dKO mice were significantly smaller at four weeks of age and continued to gain weight slowly. They were also distinctly smaller in body size at four to six weeks. The dKO mice consistently gained weight and stature to become indistinguishable from WT mice by twelve weeks of age and were significantly smaller than their Fmr1 KO counterparts. Consistent with previous reports (Pende et al., 2004), S6K1 KO mice remained smaller in stature than all other genotypes even at 12 weeks of age. Thus, the absence of both Fmr1 and S6K1 in the dKO mice act in concert to restore normal body weight comparable to that observed in wild-type mice.
Figure 7. Excessive weight-gain and macro-orchidism in Fmr1 KO mice are corrected in dKO mice.
A) Body weight data across four genotypes represented as histograms over 12 weeks. n=14–18 at P28, n=10–14 at P42, n= 10 at P56, P70 and P84. *p<0.05, **p<0.01 and ***p<0.001 with a two-way ANOVA (Fmr1×S6K1, at each time point) followed by Bonferroni post-hoc tests. B) Averaged body weight (mean only) of the four genotypes as a curve over time. C) Mean testicular weights of male mice (P90–100) of the four genotypes. n=11 for WT and Fmr1 mice; n=8 for S6K1 mice; n=10 for dKO mice. **p<0.01 and ***p<0.001 with a twoway ANOVA (Fmr1×S6K1) followed by Bonferroni post-hoc tests. Error bars denote S.E.M.
We also examined testes harvested from adult male mice of all four genotypes at P90–100. Testicular weights of Fmr1 KO mice exceeded those of wild-type littermates by an average 20 mg, which was corrected with the deletion of S6K1 (Figure 7C) and were over-corrected in the dKO. These findings suggest that the suppression of protein synthesis by removal of S6K1 in the Fmr1 KO mice corrects not only the excessive body weight phenotype, but macro-orchidism as well.
Discussion
Dysregulated protein synthesis is widely accepted as a core molecular abnormality associated with FXS. Because neuronal protein synthesis is critical for learning and memory, altered synaptic translation is considered a major contributor to the intellectual deficits seen in FXS. In this study we genetically manipulated a translational control molecule, S6K1, in an attempt to correct phenotypes displayed by Fmr1 KO mice. We found that this S6K1 deletion corrected a wide range of phenotypes associated with FXS, including abnormal translational control, exaggerated basal protein synthesis, abnormal protein expression of mRNA targets of FMRP, enhanced mGluR-LTD, aberrant dendritic morphology, ASD-like behaviors, and the peripheral features of weight gain and macro-orchidism. Since our biochemical assays have focused entirely on monitoring basal rates of signaling and translation we believe that our findings indicate that these effects are due to a conserved change in molecular machinery independent of a cell-surface signal transducer. This is particularly relevant since multiple studies have reported dysregulation of neuronal signaling pathways apart from group 1 metabotropic glutamate receptors in FXS, including dopamine receptor D1/5 signaling mediated by GRK-2, BDNF-Trk B signaling, and other Gq-mediated G-protein coupled receptors such as muscarinic acetylcholine receptors (Castren et al., 2002; Veeraragavan et al., 2011; Wang et al., 2008).
Restoration of proper translational control and protein synthesis in FXS mice by removal of S6K1
Rescuing dysregulated translation in FXS by manipulating initiation and elongation factors probably is not realistic given their critical importance for protein synthesis-dependent growth and proliferation in other parts the body (Gandin et al., 2008). Equally problematic are strategies targeting major signaling hub kinases such as mTOR and ERK due to their other substrates and their involvement in other forms of synaptic plasticity and memory function (Costa-Mattioli et al., 2009; Richter and Klann, 2009). An alternative approach for dampening protein synthesis in FXS is to target signaling molecules downstream of the hub kinases that modulate, but are not vital for translation. Of the handful of candidates fulfilling these criteria, S6K1 emerges because it integrates signals from both mTORC1 and Ras-ERK pathways, bothof which have been suggested to be involved in FXS phenotypes (Wang et al., 2010). In addition, S6K1 modulates translation at both initiation and elongation steps via phosphorylation of multiple effectors. In addition, S6K1 has been postulated to be an FMRP kinase (Narayanan et al., 2008) that antagonizes FMRP function to inhibit translation of its mRNA targets. Thus, we hypothesized that targeting S6K1 would ameliorate exaggerated protein synthesis in FXS at multiple levels. In addition to correcting the FXS-associated elevation in phosphorylation of translation control molecules and exaggerated protein synthesis (Figure 1), the reduction of S6K1 also corrected the elevated basal phosphorylation of both mTOR and ERK (Supplementary Figures S1A and S1B) in the FXS mice, which suggests that S6K1 is involved in regulatory feedback of upstream signaling pathways in FXS as well. Although there is evidence for S6K1-mediated phosphorylation of mTOR at serine 2448 (Chiang and Abraham, 2005), the site we examined in Supplementary Figures S1A and S1B, there is not an obvious regulatory mechanism to explain the correction of the elevated ERK phosphorylation in FXS mice by the removal of S6K1 (Figures S1A and S1B). Previous studies showed that ablation of S6K1 in mice did not result in dramatic deficits that would exacerbate the phenotypes displayed by Fmr1 KO mice (Antion et al., 2008a and b). Although a wide range of FXS phenotypes were corrected in the dKO mice, it would be pertinent to examine additional candidate molecules that modulate protein synthesis at other translational control steps to attempt to restore the remaining ASD-like characteristics that were not corrected in dKO mice (Figure S6). This is especially important in light of other studies in which targeting receptors and kinases upstream of translation control can rectify specific subsets of features associated with FXS (Auerbach et al., 2011; Dolen et al., 2007; Hayashi et al., 2007).
Our examination of the protein levels of FMRP targets in the four genotypes has provided novel insights into altered translational control in FXS mice (Figures 3A and 3B). In a simple model one could assume that loss of FMRP causes increased translation of its target mRNAs and that the application of a generalized brake on protein synthesis, such as removing S6K1 should reset the protein levels of the FMRP targets. We found increased protein levels of all of the FMRP target mRNAs we examined in the Fmr1 KO mice (CaMKIIα, Shank3, eEF2, eIF4G), all of which were reduced to WT levels in the dKO mice except for PSD-95. We also found that basal protein expression levels of Arc/Arg 3.1 were similar in all four genotypes, which is consistent with previous studies demonstrating that differences in Arc/Arg 3.1 in FXS mice are sensitive to changes in neuronal activity (Park et al., 2008). Our results suggest that 1) most FMRP-regulated mRNAs require S6K1 activity for their translation, 2) certain mRNAs like PSD-95 may have adapted to route their translation in an S6K1-independent manner, and 3) the translation control of FMRP targets by S6K1 are independent of the presence of 5’ TOP motif in the target mRNAs. The latter is supported by results showing that even though the levels of eEF2, whose mRNA contains a 5’ TOP motif, are elevated in Fmr1 KO mice, the expression of other proteins such as S6 and PABP whose mRNA have 5’TOP motifs (Hornstein et al., 1999; Antion et al., 2008a) but are not targets of FMRP (Darnell et al., 2011), showed no changes in total protein levels (Supplementary Figures S3A and S3B). These findings are consistent with recent reports showing that 5’ TOP-mediated translation is independent of S6K1 (Magnuson et al., 2012; Meyuhaus and Dreazen, 2009). The tonic brake on general protein synthesis exerted by S6K1 deletion likely impacts both translation initiation and elongation because we observed decreased levels of eEF2 and eIF4G in S6K1 KO and dKO mice (Figure 3). Finally, our result showing elevated Shank3 levels in Fmr1 KO mice further supports the idea of molecular overlap of FXS and autism (Darnell et al., 2011; Herbert, 2011).
A noteworthy point is the apparent non-overlap between previous studies on whether basal levels of mTOR and ERK phosphorylation are elevated in Fmr1 KO mice (Sharma et al., 2010; Osterweil et al., 2010). As discussed thoroughly by Osterweil and colleagues (2010), these differences stem from methods of tissue preparation standardized for different experimental objectives. Recently, however, elevated levels of phosphorylated mTOR and ERK were observed in non-neuronal cells and post-mortem tissue from individuals with FXS (Hoeffer et al., 2012; Wang et al., 2012), suggesting that these molecules are relevant markers for FXS.
Restoration of normal mGluR-LTD and dendritic morphology in FXS mice by removal of S6K1
Removal of S6K1 in Fmr1 KO mice prevented enhanced hippocampal mGluR-LTD (Figure 4A). The most parsimonious explanation for this result is that reducing S6K1 levels nullifies the increased phosphorylation of translational control molecules such as S6 and eIF4B (Figures 1 and 2) and exaggerated synthesis of proteins important for the expression of LTD. It previously was shown that expression of mGluR-LTD in hippocampal slices from S6K1 KO mice is blocked by anisomycin (Antion et al., 2008 a), whereas the enhanced mGluR-LTD in Fmr1 KO slices is anisomycin-insensitive (Nosyreva and Huber, 2006). It remains to be determined whether mGluR-LTD is protein synthesis-dependent and whether activation of group I mGluRs triggers protein synthesis in the dKO mice.
There have been conflicting reports of spine density changes in the hippocampus of Fmr1 KO mice that are likely due to differences in the techniques used and the age of the mice studied (He and Portera-Cailliau, 2012). Our results showing increased spine density and morphology phenotypes in CA1 pyramidal neurons from Fmr1 KO mice (Figure 5) overlap with reports from other groups that examined mice more than three months of age (Levenga et al., 2011). The correction of spine density and morphology phenotypes associated with FXS in the dKO mice suggests the possibility of an interaction of S6K1 with the Rac1/PAK pathway that has not been explored in neurons. Recently a role for S6K1 in Rac1-driven platelet activation and aggregation has been elucidated, consistent with this notion (Aslan et al., 2011). The regulation of Shank3 in dKO mice had added implications for spine morphology since this scaffolding protein has been shown to influence of spine remodeling via actin-dependent mechanisms (Durand et al., 2011).
Studies of the effects of S6K1 deletion have focused mainly on energy metabolism, caloric restriction, and insulin signaling (Selman et al., 2009); there are few reports on the role of S6K1 in the nervous system. For example, the effects of deletion of S6K1 on neuronal morphology have not been reported. Consistent with their small stature, we observed a significant decrease in neuronal cell size (Figure 2) and a trend for smaller dendritic arbors in S6K1 KO mice, although spine number and morphology were not different from WT mice (Figure 5). We also found that the enlarged testis phenotype to be overcorrected in the dKO mice (Figure 7C), further supporting the idea that S6K1 is involved in the regulation of cellular size (both neuronal and non-neuronal) and spine morphology.
Multiple ASD-like behaviors displayed by FXS mice are corrected by removal of S6K1
The behavior of Fmr1 KO mice has been extensively studied and impairments in multiple socio-emotional responses, hyperactivity, obsessive-repetitive behaviors, and susceptibility to seizures have been reported, many of which are consistent with ASD (Gross et al., 2012 and Spencer et al., 2011). We found many of these phenotypes to be reproduced in Fmr1 KO mice in our behavioral cohort, including hyperactivity, repetitive behaviors and impaired social interactions. Genetic deletion of S6K1 in Fmr1 KO mice was successful in correcting numerous behavioral abnormalities, including social interaction, novel object recognition, and behavioral flexibility (Figure 6). Our analyses also revealed that S6K1 KO mice themselves display impaired novel object recognition and (Figure 6B) and abnormal social behavior (Figure 6C). In contrast, S6K1 KO mice were adept at reversal learning in the Y-maze (Figures 6D and 6E). In addition, S6K1 KO mice were hypoactive in the open field arena (Supplemental Figures S3A and S3B) as reported earlier (Antion et al., 2008b), but were impaired in rota-rod performance (Figure 6A).
We chose to limit our biochemical, electrophysiological and morphological studies to the hippocampus due to the extensive data available in this brain area for Fmr1 KO mice. However, many of the behavioral tests we conducted have well-established cortical-, striatal-, and amygdala-dependent components. We observed no rescue of FXS-associated hyperactivity and marble burying features in dKO mice, suggesting that there is limited impact of deleting S6K1 on altered cortico-striatal circuitry in FXS model mice. However, impairments in novel social and object recognition were rectified, suggesting that the S6K1 removal may results in region-specific correction of cortical impairments in FXS mice. It will be important to examine molecular and synaptic phenotypes in other brain regions to obtain a more holistic idea of how the lack of S6K1 wields a corrective influence on the FXS brain.
Peripheral abnormalities associated with FXS are corrected by removal of S6K1
Though FXS is largely considered a disorder of the nervous system, FMRP expression is widespread during development, with postnatal only expression limited to neurons and testes (Hinds et al., 1993). This suggests a possible role for non-neuronal FMRP in peripheral phenotypes, the effects of which are felt even after the actual protein expression has abated. This may be the reason why strategies based on neuronal mediators do not rescue peripheral symptoms such as macro-orchidism and connective tissue abnormalities entirely (Dolen et al., 2007 and Michalon et al., 2012). Thus, the correction of macro-orchidism in the dKO mice was likely because S6K1 was constitutively and globally reduced across tissues and organ systems in FXS mice. It remains to be determined whether connective tissue defects in the Fmr1 KO mice also are rescued by deletion of S6K1.
Balanced protein synthesis and the interplay of FMRP and S6K1
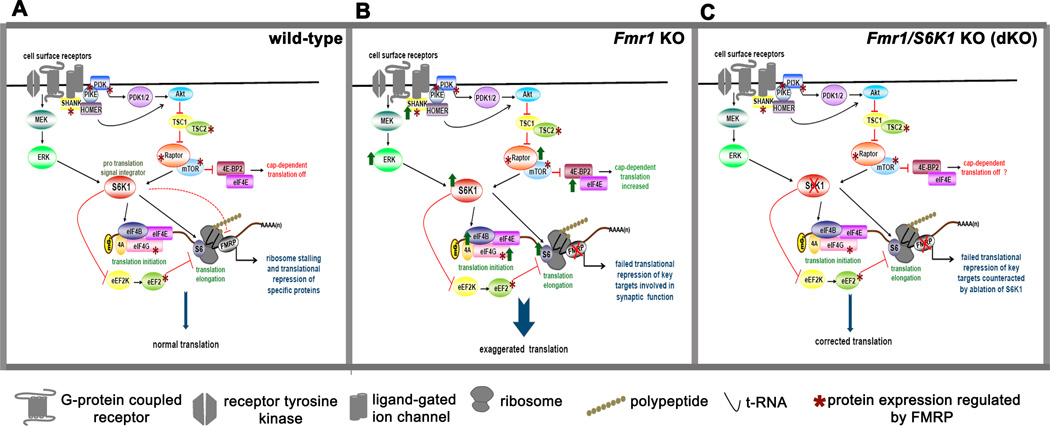
We propose that the translational control of specific mRNAs in neurons is dynamically regulated by the opposing actions of FMRP and S6K1 (Figure 8). In WT mice, signaling downstream of cell surface receptors activate mTORC1 and/or ERK that results in activation of S6K1, which promotes protein synthesis via phosphorylation of multiple downstream effectors (Figure 8A). S6K1 promotes translation, including translation of FMRP target mRNAs, via its direct and indirect actions on initiation and elongation factors, while at the same time limiting the translation of FMRP target mRNAs via its action as an FMRP kinase (Narayanan et al., 2008). In FXS, the absence of FMRP creates a perpetual translation ‘ON’ state that leads to increased protein expression of FMRP targets, which not only include key mediators of synaptic plasticity such as PSD-95, CaMKIIα, and Shank3, but also regulators of mTORC1 signaling such as PIKE, TSC2, Raptor, eIF4G and eEF2 (shown with asterix in Figure 8A). Darnell et al (2011) also identified regulators of ERK signaling that are FMRP targets, which if overexpressed also would enhance ERK signaling. Enhanced expression of regulators of mTORC1 and ERK would form a feed-forward loop that again would promote general translation. Removal of S6K1 acts as a tonic break on the exaggerated protein synthesis in FXS by normalizing the phosphorylation and/or expression levels of key translation control molecules such as ribosomal protein S6, eIF4B and eEF2 and/or by depleting the levels of critical initiation factors such as eIF4G (Figure 8C). This model is consistent with the lowered levels of protein synthesis that occur with deletion of S6K1 as shown by our SUnSET experiments (Figures 1C and 1D). The experiments we conducted examining FMRP target proteins support this model (Figures 2A and 2B), however there were some exceptions, notably PSD-95 (Figures 2A and 2B). Future studies should help to clarify the role of S6K1 in translational control and to identify classes of FMRP target mRNAs that have multiple, redundant strategies to ensure their translation. S6K1 KO mice are viable, so in the absence of S6K1 protein synthesis still occurs via other modes of translational control (Richter and Klann, 2009) and/or the compensatory actions by other related kinases such as S6K2 and RSK. Consistent with the latter possibility, S6K1 KO mice exhibit increased expression of S6K2 (Shima et al., 1998).
Figure 8. Model for regulation of translation by FMRP and S6K1.
A) In wild-type mice, FMRP suppresses translation of its target mRNAs. S6K1 integrates signals from the mTORC1 and ERK1 pathways downstream of surface receptors to promote translation via its direct and indirect regulation of initiation and elongation factors. FMRP also controls the translation and expression of key signaling molecules as shown by the asterix, introducing additional feedback controls. This antagonistic interplay of FMRP and S6K1 provides a dynamic regulation of protein synthesis. B) In Fmr1 KO mice, the absence of FMRP results in exaggerated protein synthesis via upregulation of multiple upstream signaling molecules and downstream effectors involved in translational control, including S6K1, promoting feed-forward, uncontrolled translation. C) Genetic reduction of S6K1 in the Fmr1 KO mice as modeled in the dKO mice removes the net positive drive toward exaggerated protein synthesis and applies a tonic brake on the translation of subsets of FMRP-regulated RNA, which resets de novo protein synthesis to levels similar to that in wild-type mice. It is likely that the restoration of normal translation occurs via non-canonical signaling mechanisms.
In summary, our findings indicate that the removal of S6K1 corrects multiple pathophysiologies and behavioral abnormalities in FXS model mice. Moreover, the genetic reduction of S6K1 can prevent a broad range of phenotypes, including peripheral abnormalities, associated with FXS that has not been achieved with previous genetic manipulations. With the recent identification of specific inhibitors of S6K1 (Pearce et al., 2010), we visualize opportunities for translating the results from our genetic experiments to a viable pharmacological approach to target S6K1 to reverse a broad range of phenotypes in FXS model mice. Such an approach may develop into a therapeutic option for humans with FXS in the future.
Experimental Procedures
Mice
Fmr1 KO/S6K1 KO (dKO) mice were initially generated by crossing heterozygous female mice carrying the Fmr1 mutation with heterozygous male mice carrying the S6K1 mutation. Subsequently all animals used for experimentation were derived from crossing of female XFmr1+XFmr1−/ S6K+/− with males either XFmr1+Y/S6K1+/− or XFmr1−Y/S6K1+/−. See supplemental content for detailed information.
Western blots and immunohistochemistry
Western blots were performed as described previously (Sharma et al., 2010). For immunohistochemical analyses, mice were deeply anesthetized and trans-cardially perfused with PBS followed by 4% PFA. Post-fixed brains were sectioned coronally at a thickness of 40 µm, followed by permeabilization, blocking, and incubation with primary antibodies overnight. Following incubation with fluorescently-tagged secondary antibodies, slices were mounted and imaged. For details see supplementary content.
Electrophysiology
400 µm transverse hippocampal slices were prepared from 4–6 week old mice of the four genotypes described above. Slice preparation and all LTD experiments were performed as described previously (Sharma et al., 2010). For details see supplementary content.
Measurement of de novo protein synthesis
400 µm transverse hippocampal slices were obtained from 4–6 week old mice of the four genotypes. Puromycin labeling of the slices was adapted from procedure described previously (Hoeffer et al., 2011). For details see supplementary content.
Neuroanatomy
Spine number and morphological analyses were done on rapid Golgi-Cox stained brain sections using a protocol described previously (Hayashi et al., 2007). For details see supplementary content.
Behavioral Tests
Two independent cohorts of mice of each genotype (a total of 8–12 per genotype) were used for the behavioral tests. Mice were 4–6 months of age and all mice used were male. The behavioral tests were conducted in increasing order of difficulty and stress ranging from open field analysis, marble burying, rota-rod, social interaction, novel object recognition, and Y-maze choice arm reversal. All tests were performed in conditions and in a manner as described previously (Hoeffer et al., 2008). For details see supplementary content.
Supplementary Material
Highlights.
Deletion of S6K1 corrected exaggerated protein synthesis in FXS mice
Deletion of S6K1 corrected plasticity and dendritic spine abnormalities in FXS mice
Deletion of S6K1 corrected multiple ASD-like behaviors displayed by FXS mice
Deletion of S6K1 normalized increased weight gain and macro-orchidism in FXS mice
Acknowledgements
This work was supported by NIH grants NS034007 and NS047384 (E.K.), FRAXA Research Foundation (E.K.) and a FRAXA Postdoctoral Fellowship (A.B). J.P.M. was supported by summer training grant NSF REU Site Grant in Neural Science DBI 1004172.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent longterm depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol. Cell. Biol. 2008a;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antion MD, Merhav M, Hoeffer CA, Reis G, Kozma SC, Thomas G, Schuman EM, Rosenblum K, Klann E. Removal of S6K1 and S6K2 leads to divergent alterations in learning, memory, and synaptic plasticity. Learn. Mem. 2008b;15:29–38. doi: 10.1101/lm.661908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan JE, Tormoen GW, Loren CP, Pang J, McCarty OJ. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood. 2011;118:3129–3136. doi: 10.1182/blood-2011-02-331579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren M, Lampinen KE, Miettinen R, Koponen E, Sipola I, Bakker CE, Oostra BA, Castren E. BDNF regulates the expression of fragile X mental retardation protein mRNA in the hippocampus. Neurobiol. Dis. 2002;11:221–229. doi: 10.1006/nbdi.2002.0544. [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 2005;280:25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP Stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Perroy J, Loll F, Perrais D, Fagni L, Bourgeron T, Montcouquiol M, Sans N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol. Psychiatry. 2012;17:71–84. doi: 10.1038/mp.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin V, Miluzio A, Barbieri AM, Beugnet A, Kiyokawa H, Marchisio PC, Biffo S. Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature. 2008;455:684–688. doi: 10.1038/nature07267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Berry-Kravis EM, Bassell GJ. Therapeutic Strategies in Fragile X Syndrome: Dysregulated mGluR Signaling and Beyond. Neuropsychopharmacology. 2012;37:178–195. doi: 10.1038/npp.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT .Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, McBogg P, Hagerman PJ. The fragile X syndrome: history, diagnosis, and treatment. J. Dev. Behav. Pediatr. 1983;4:122–130. doi: 10.1097/00004703-198306000-00009. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolen BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc. Natl. Acad. Sci. USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.049. in press. Epub Apr 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat. Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- Herbert MR. SHANK3, the synapse, and autism. N. Engl. J. Med. 2011;365:173–175. doi: 10.1056/NEJMcibr1104261. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTORRaptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, et al. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc. Natl. Acad. Sci. USA. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer C, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, Whelan AM, Zukin RS, Klann E, Tassone F. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Gen. Brain Behav. 2012;11:332–341. doi: 10.1111/j.1601-183X.2012.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Hornstein E, Git A, Braunstein I, Avni D, Meyuhas O. The expression of poly (A)-binding protein gene is translationally regulated in a growth dependent fashion through a 50- terminal oligopyrimidine tract motif. J. Biol. Chem. 1999;274:1708–1714. doi: 10.1074/jbc.274.3.1708. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptordependent long-term depression. J. Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein control mGluRdependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 2004;7:113–117. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Kao DI, Aldridge GM, Weiler IJ, Greenough WT. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc. Natl. Acad. Sci. USA. 2010;107:15601–15606. doi: 10.1073/pnas.1010564107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol. Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- Levenga J, de Vrij FM, Buijsen RA, Li T, Nieuwenhuizen IM, Pop A, Oostra BA, Willemsen R. Subregion-specific dendritic spine abnormalities in the hippocampus of Fmr1 KO mice. Neurobiol. Learn. Mem. 2011;95:467–472. doi: 10.1016/j.nlm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl. Acad. Sci. USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Melko M, Bardoni B. The role of G-quadruplex in RNA metabolism: involvement of FMRP and FMR2P. Biochimie. 2010;92:919–926. doi: 10.1016/j.biochi.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Meyuhas O, Dreazen A. Ribosomal protein S6 kinase from TOP mRNAs to cell size. Prog. Mol. Biol. Transl. Sci. 2009;90:109–153. doi: 10.1016/S1877-1173(09)90003-5. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J. Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Alton GR, Richter DT, Kath JC, Lingardo L, Chapman J, Hwang C, Alessi DR. Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1) Biochem. J. 2010;431:245–255. doi: 10.1042/BJ20101024. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile×syndrome. Annu. Rev. Genomics Hum. Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogenactivated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J. Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. Phosphorylation of eukaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1 KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behav. Neurosci. 2011;125:783–790. doi: 10.1037/a0025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J. Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:73–82. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Snape M, Klann E, Stone JG, Singh A, Petersen RB, Castellani RJ, Casadesus G, Smith MA, Zhu X. Activation of the extracellular signal-regulated kinase pathway contributes to the behavioral deficit of fragile x-syndrome. J. Neurochem. 2012;121:672–679. doi: 10.1111/j.1471-4159.2012.07722.x. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat. Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J. Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.