Abstract
Enzyme replacement therapy has been used successfully in many lysosomal storage diseases. However, correction of brain storage has been limited by the inability of infused enzyme to cross the blood–brain barrier (BBB). We recently reported that PerT-GUS, a form of β-glucuronidase (GUS) chemically modified to eliminate its uptake and clearance by carbohydrate-dependent receptors, crossed the BBB and cleared neuronal storage in an immunotolerant model of murine mucopolysaccharidosis (MPS) type VII. In this respect, the chemically modified enzyme was superior to native β-glucuronidase. Chemically modified enzyme was also delivered more effectively to heart, kidney, and muscle. However, liver and spleen, which express high levels of carbohydrate receptors, received nearly fourfold lower levels of PerT-GUS compared with native GUS. A recent report on PerT-treated sulfamidase in murine MPS IIIA confirmed enhanced delivery to other tissues but failed to observe clearance of storage in neurons. To confirm and extend our original observations, we compared the efficacy of 12 weekly i.v. infusions of PerT-GUS versus native GUS on (i) delivery of enzyme to brain; (ii) improvement in histopathology; and (iii) correction of secondary elevations of other lysosomal enzymes. Such correction is a recognized biomarker for correction of neuronal storage. PerT-GUS was superior to native GUS in all three categories. These results provide additional evidence that long-circulating enzyme, chemically modified to escape carbohydrate-mediated clearance, may offer advantages in treating MPS VII. The relevance of this approach to treat other lysosomal storage diseases that affect brain awaits confirmation.
Keywords: beta-glucuronidase deficiency, glycosaminoglycans, mannose 6-phosphate receptor, mannose receptor
Mucopolysaccharidosis type VII (MPS VII or Sly syndrome) belongs to a group of lysosomal storage disorders (LSDs), each caused by deficiency of a lysosomal enzyme. In this case, the missing enzyme is β-glucuronidase (GUS), which catalyzes the degradation of the glycosaminoglycans (GAGs) dermatan sulfate, heparan sulfate, and chondroitin sulfate (1). In MPS VII cells, the undegraded substrates gradually accumulate in lysosomes, causing progressive lysosomal, cellular, and ultimately organ, dysfunction. Affected patients display delayed development, organomegaly, skeletal and cardiac abnormalities, and mental retardation (2). The age of onset and severity of disabilities are quite variable. Enzyme replacement therapy (ERT) has been successful in clearing storage materials from the viscera and in improving clinical pathologies in patients with other LSDs such as Gaucher disease (3), Fabry disease (4–6), Pompe disease (7–9), MPS I (10), MPS II (11), and MPS VI (12). However, delivery of enzyme to the brain is compromised by the selective and limited permeability of the blood–brain barrier (BBB) (13). Because many LSDs, such as MPS VII, affect the central nervous system (CNS), designing a therapy that can traverse the BBB and clear neuronal storage is a major unmet need.
Several studies have shown the potential of gene therapy in correcting neuronal pathologies in the MPS VII mouse model (14–17) and in the MPS II model (18). However, important concerns associated with gene therapy currently limit its clinical utility (19, 20). Another approach is direct administration of enzyme into the cerebrospinal fluid (CSF) (21, 22). Even though treated mice showed improvements in disease phenotypes, concerns remain whether such invasive methods are practical in humans.
We have focused our efforts on improving delivery of enzyme to brain by using GUS in the immunotolerant mouse model of MPS VII. When MPS VII mice received ERT during the newborn period, considerable amounts of enzyme were delivered to brain (23). The mice also had reduced neuronal storage, decreased skeletal dysplasia, prolonged survival, and improvements in behavior and auditory function (24, 25). However, brain storage was resistant to clearance if ERT was begun after 2 wk of age. Recent studies indicated the role of mannose-6-phosphate receptors (M6PR) in mediating the transcytosis of enzyme across the BBB during the newborn period (26, 27). Down-regulation of this receptor by 2 wk of age explained the resistance of brain to ERT in the adult. Nonetheless, long-term treatment with high doses of enzyme partially cleared storage in the brain of adult MPS VII mice (28). Similar results were obtained in the mouse model of MPS II (29), aspartylglycosaminuria (30), α-mannosidosis (31), and metachromatic leukodystrophy (32). To account for enzyme delivery to adult brain, we speculated that increasing the enzyme dose saturated the clearance receptors in visceral tissues, thus delaying clearance of the enzyme from the circulation and allowing uptake by receptors expressed on the BBB (28, 33).
To study the effect of simply prolonging the circulation time of the enzyme on ERT by eliminating carbohydrate-dependent clearance, we chemically inactivated terminal sugars on GUS by treatment with sodium metaperiodate, followed by borohydride reduction (34). This treatment produced a ligand (PerT-GUS) that is not susceptible to mannose receptor (MR)- or M6PR-mediated cellular uptake. We showed that PerT-GUS retains about 75% of the original activity and that the enzyme shows slightly greater sensitivity to heat inactivation, and slightly increased rate of turnover following uptake by fibroblasts. However, its kinetic properties were similar to those of the native enzyme (34). To our surprise, we found that PerT-GUS not only had a longer half-life in circulation, but was also more effective in clearing neuronal storage than native GUS (34). In contrast with these encouraging results, an independent study using similarly modified sulfamidase in the MPS IIIA mouse model found that high-dose ERT, admittedly over a shorter term, appeared no better than the native enzyme in delivery to brain, and failed to reduce neuronal storage (35). However, it did reach higher levels and was more effective in several other tissues.
More recently, Meng et al. (36) reported that similarly modified tripeptidyl peptidase 1 (TPP1) did indeed display prolonged circulatory half-life, but it did not show improved targeting to brain when examined 24 h after injection in the late infantile neuronal ceroid lipofuscinosis (LINCL) mouse model. On the other hand, an extremely high dose of native TPP1 (80 mg/kg) led to 10% of WT levels 24 h after injection.
To confirm and extend our previously published results showing that PerT-GUS was superior to native GUS in crossing the BBB and clearing neuronal storage in the immunotolerant murine MPS VII model, we replicated the prior experiments in adult MPS VII mice. In addition to measuring enzyme levels in brain and reduction in the number of storage vacuoles by morphometry, we measured correction of secondary elevations of α-galactosidase and β-hexosaminidase, recognized biomarkers for correction of neuronal storage. The additional data support the original observations on PerT-GUS in the MPS VII mouse model.
Results
In all experiments reported here, we used the immunotolerant MPS VII mouse, which carries a transgene expressing the inactive E540A human GUS transgene in all tissues (37). This mutant transgene conferred tolerance to infused human GUS, allowing long-term treatment with either native or PerT-GUS without raising an immune response that might lead to an adverse reaction or raising neutralizing antibodies that could abrogate the therapeutic response to administered enzyme.
Four- to six-week-old MPS VII mice were treated with weekly i.v. injections of 4 mg/kg native or PerT-GUS for a total of 12 wk. One week after the last infusion, we measured residual levels of GUS in the plasma, brain, and liver, and analyzed the effects of treatment on neuronal storage and correction of secondary elevations of other lysosomal enzymes.
Residual Levels of PerT-GUS and Native GUS in Plasma.
The data presented in Table 1 show that at 1 wk after the last infusion, PerT-GUS levels in the plasma were still elevated (38-fold higher than in mice treated with native GUS and 19 times the level in WT mice). This finding is consistent with previous data showing that infused PerT-GUS has a remarkably long half-life in plasma (t1/2 = 18.5 h) compared with that of native GUS (t1/2 = 11.7 min) (34). The difference in the rate of clearance from plasma may be important for the enhanced therapeutic efficacy of PerT-GUS in clearing neuronal storage, but the mechanism by which PerT-GUS reaches neurons is still unclear.
Table 1.
Plasma GUS activity
| Plasma GUS levels (U/mL, mean ± SD) 7 d after final infusion | No. of mice | |
| No enzyme | 0.1 ± 0 | n = 2 |
| Native GUS | 14.5 ± 12 | n = 4 |
| PerT-GUS | 561 ± 106 | n = 4 |
Seven days after the final of 12 weekly infusions at 4 mg/kg, blood samples were collected from the right atrium into heparinized capillary tubes before perfusion. After centrifugation, plasma was collected and assayed for GUS activity. Residual plasma PerT-GUS was 38-fold higher than native GUS. Plasma GUS levels of noninfused, wild-type B6 mice were 29.7 ± 19 (U/mL, mean ± SD) (n = 8). Thus, 7 d after the final infusion, plasma GUS levels of native GUS-treated animals were below the wild-type level. However, PerT-GUS remained in the plasma at levels significantly higher than that of B6 controls (P < 0.0001).
Biochemical Evidence of Greater Delivery of PerT-GUS to Brain.
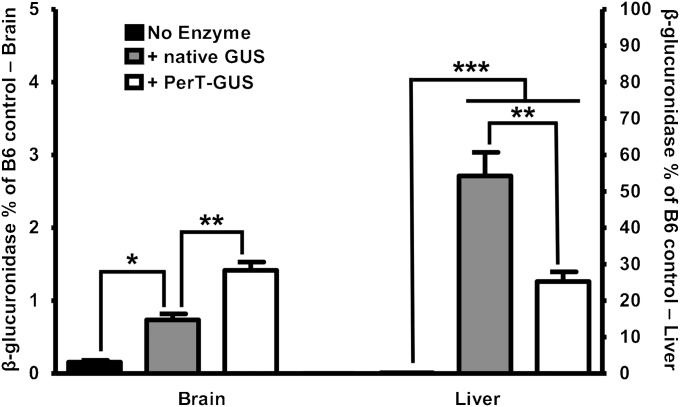
Enzyme assays on tissue extracts 1 wk after the last of 12 weekly injections showed a significantly higher level of β-glucuronidase activity in brains of both native GUS- and PerT-GUS–treated mice compared with untreated controls (P = 0.01 and P = 0.001, respectively) (Fig. 1). However, the increase following treatment with PerT-GUS was 50% greater than that seen in the brain of mice treated with native GUS (P = 0.003). This pattern is reversed in the liver, where GUS activity was found to be 50% higher in the mice treated with native GUS than in the PerT-GUS-treated mice (P < 0.005). This reduced delivery of PerT-GUS to the liver is consistent with the elimination of uptake of PerT-GUS by the MR and M6PR receptors in liver that account for the rapid clearance of infused native enzyme from plasma.
Fig. 1.
Delivery of native GUS and PerT-GUS into the brain and liver of MPS VII mice. β-Glucuronidase-specific activities (expressed as percent of WT control) are shown in the brain (left y axis) and liver (right y axis) after 12 wk of weekly injection with either native GUS or PerT-GUS. Mice were perfused before removal of tissues, as described in Materials and Methods. In the brain, a significant increase in GUS activity is produced with both types of treatment (P = 0.01 with native GUS and P = 0.0001 with PerT-GUS). The effects were more dramatic with PerT-GUS. In the liver, however, the pattern is reversed, with more activity of native GUS than modified GUS. WT control mice have GUS-specific activities of 20.5 μ/mg tissue protein in the brain and 178 μ/mg in the liver (n > 8).
Histopathology Confirms the Superiority of PerT-GUS in Clearing Neuronal Storage.
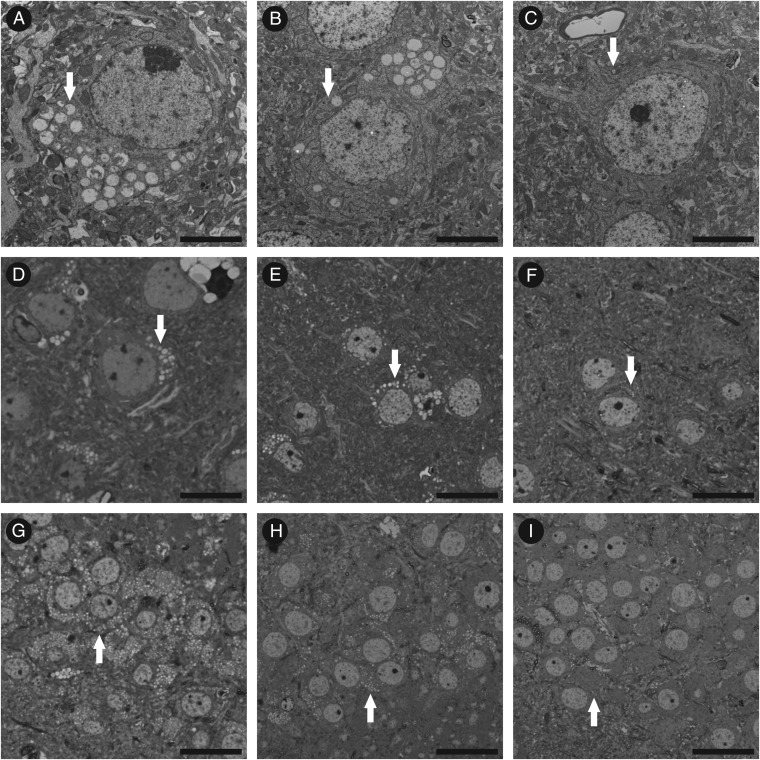
The morphology of neocortical neurons is compared in Fig. 2 in untreated (Fig. 2 A–D), GUS-treated (Fig. 2 B and E), and PerT-GUS-treated (Fig. 2 C and F) MPS VII mice from electron microscopic and light microscopic images. There appeared to be a slight reduction in neocortical storage in the GUS-treated mice and a marked reduction in storage in neocortical neurons of the PerT-GUS-treated mice. Images of the hippocampal neurons showed a similarly greater response to ERT with PerT-GUS than with native GUS (34). On the other hand, not all regions showed correction. Cerebellar Purkinje cells, which are known to be resistant to correction in this MPS VII model (38), appeared to show no reduction in storage from treatment with either enzyme.
Fig. 2.
Reduction in neuronal storage in MPS VII mice treated with GUS and PerT-GUS. (A–C) EM images of neocortical neurons from untreated, GUS-treated, and PerT-GUS-treated mice, respectively. Although they appear reduced in number, there are still easily identifiable distended lysosomal vesicles in the neurons of GUS-treated mice. The reduction in the amount of storage appears markedly greater in the neurons from PerT-GUS-treated mice. EM, electron microscopy. (D–F) Toluidine blue-stained sections from the corresponding mice visualized by light microscopy. (G–I) Toluidine blue sections showing hippocampal neurons from the same set of mice, visualized by light microscopy. Responses to ERT are similar to those seen in neocortical neurons. (A–C) Uranyl acetate–lead citrate: scale bar, 5 μm; (D–I) Toluidine blue: bar, 30 μm.
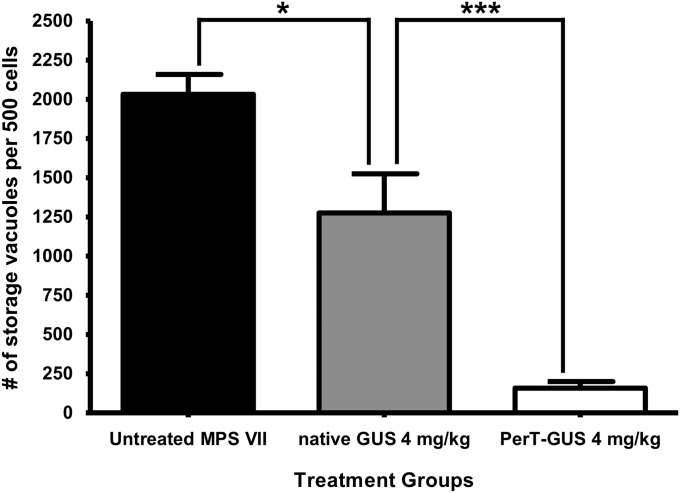
To obtain a quantitative measure of the reduction in neuronal storage in response to treatment with the two types of GUS, we used a morphometric approach, as described in Materials and Methods. Fig. 3 summarizes the results of this assessment of storage in neocortical neurons of untreated MPS VII mice, and those treated with either PerT-GUS or native GUS. The data show that ERT with 4 mg/kg native GUS administered weekly over 12 wk produced a moderate reduction in storage in the neocortical neurons compared with untreated controls (P = 0.02). The same dose of PerT-GUS produced a reduction in the number of storage vesicles from the number seen in untreated MPS VII mice (P < 0.0001) that was even more dramatic. The magnitude of the treatment effect of PerT-GUS was much greater than that of native GUS (P < 0.005). These results confirm that PerT-GUS is more effective than native phosphorylated GUS in clearing storage vesicles from neocortical neurons of MPS VII mice.
Fig. 3.
Lysosomal storage levels in neocortical neurons of MPS VII mice. Mice untreated or treated with 12 weekly infusions of the respective enzyme were killed by perfusion 1 wk after the last infusion. Brain tissues were fixed in 2% paraformaldehyde and 4% glutaraldehyde for sectioning. The number of storage vacuoles was determined using an established morphometric method. Significant reduction in storage was obtained for treatments with native GUS and PerT-GUS (P = 0.02 and P < 0.0001, respectively). However, PerT-GUS produced a more complete correction of storage compared with native GUS (P = 0.005).
PerT-GUS is Superior to Native GUS in Correcting Secondary Elevations of Other Lysosomal Enzymes.
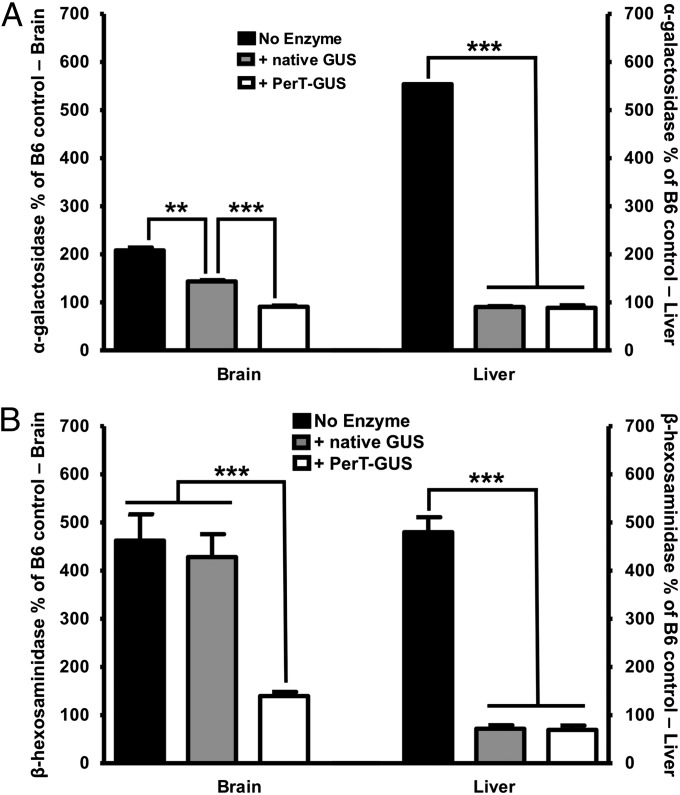
In lysosomal storage diseases, such as MPS VII, deficiency of the missing enzyme (in this case, β-glucuronidase) often leads to an increase in the levels of other lysosomal enzymes such as α-galactosidase and β-hexosaminidase (39). The phenomenon, which is termed secondary elevation, provides a useful biomarker for the effectiveness of ERT in clearing lysosomal storage (38, 40, 41). Fig. 4A shows the results of α-galactosidase assays that demonstrated significant reduction in secondary elevation of this enzyme in brain after the 12-wk treatment with either native GUS (P = 0.0003) or PerT-GUS (P < 0.0001). The greater reduction of α-galactosidase activity in brain following PerT-GUS treatment over that seen with native GUS (P < 0.0001) correlated with the greater amount of GUS delivered to brain (Fig. 1). In the liver, however, correction of secondary elevations of α-galactosidase was complete for both PerT-GUS and native GUS, indicating delivery of both enzymes to liver exceeded the threshold required for complete correction of secondary elevation in this tissue.
Fig. 4.
(A) α-Galactosidase levels after 12 weekly injections with either native or modified GUS. Both enzymes significantly decreased α-galactosidase levels in the brain (P = 0.0003 for native GUS and P < 0.0001 for PerT-GUS). However, PerT-GUS restored α-galactosidase levels to those of wild-type control mice. In the liver, both treatments produced a complete correction of secondary elevations and no significant difference between native GUS and PerT-GUS was observed. Enzyme activities are expressed as percentage of wild-type controls. Wild-type control mice have α-galactosidase-specific activities of 12.2 μ/mg in the brain and 24.9 μ/mg in the liver (n > 8). (B) Reduction in secondary elevation of β-hexosaminidase after 12 weekly infusions. In brain, only PerT-GUS showed efficacy in reducing β-hexosaminidase activity (P = 0.0008). In liver, both enzymes were equally effective and produced complete correction of secondary elevation of β-hexosaminidase. Enzyme activities are expressed as percentage of wild-type controls. Wild-type control mice have β-hexosaminidase-specific activities of 2,551 μ/mg in the brain and 1,773 μ/mg in the liver (n > 8).
Fig. 4B presents data on the correction of secondary elevations of β-hexosaminidase. In brain, PerT-GUS treatment produced a dramatic reduction in β-hexosaminidase activity (P < 0.001). No significant correction of β-hexosaminidase levels by native GUS was observed in the brain (P > 0.65). In the liver, however, β-hexosaminidase levels were completely corrected by both enzymes.
These data indicate that PerT-GUS is superior to the native enzyme in crossing the BBB and correcting neuronal storage, as judged by correction of secondary elevations. However, at this dose, both native GUS and PerT-GUS delivery to liver exceeded the threshold required for complete correction of secondary elevations in this organ.
Discussion
Despite the success of ERT in improving many clinical aspects of several LSDs, delivery of recombinant human enzyme to brain and correction of neuronal storage remain major challenges in the treatment of LSDs that affect the CNS. Prior results from this laboratory showed that, surprisingly, chemically modified β-glucuronidase administered over 12 wk crossed the BBB and cleared neuronal storage in the immunotolerant MPS VII mouse model. This study also reported higher levels of PerT-GUS than native GUS in several other tissues, including heart, kidney, lung, muscle, and eye (34). In contrast, native GUS was more effective than PerT-GUS at reaching liver and spleen, two tissues rich in MR (Kupffer cells in liver and macrophages in spleen) and M6PR (both hepatocytes and Kupffer cells) (33, 42, 43). MR and M6PR are largely responsible for uptake of the carbohydrate-bearing native enzyme in liver and spleen.
Since that report, Rozaklis et al. reported a similar study with chemically modified sulfamidase (modSGSH) in the MPS IIIA mouse model (35). Although they found that higher levels of modified enzyme were delivered to visceral tissues and corrected storage at these sites, they found no evidence that modSGSH crossed the BBB. Furthermore, there was no evidence that the modified sulfamidase was superior to native enzyme in correcting storage in the CNS. There are several possible explanations for this difference from the results in MPS VII. Modified sulfamidase had a significantly shorter half-life in plasma (<4 h) compared with PerT-GUS (18.5 h). It also had a shorter half-life in tissues following uptake. Furthermore, the MPS IIIA treatment was conducted over a shorter period, with mice receiving weekly infusions over 3 wk rather than 12 wk. These differences in the nature of the enzyme and the length of treatment could help explain why modified sulfamidase was less effective in crossing the BBB and correcting neuronal storage.
Meng et al. reported related findings (i.e., that chemically modified TPP1) was not better than native enzyme at targeting brain in the LINCL mouse model (36). The discrepancy between results in the MPS VII and the MPS IIIA and LINCL mouse models motivated us to perform additional experiments to confirm and extend the original observations showing the superiority of PerT-GUS over native GUS in clearing neuronal storage. The data presented confirmed that PerT-GUS indeed was delivered to brain more effectively than native GUS. In addition, the modified enzyme reduced the number of storage vesicles in neocortical neurons more effectively, as is evident both at the light and electron microscopic levels, and was also superior in correcting secondary elevations of α-galactosidase and β-hexosaminidase in brain.
Correction of secondary elevations provides important additional evidence that PerT-GUS has been delivered to brain lysosomes. One disadvantage of the immunotolerant mouse model, which carries a transgene that expresses an inactive form of GUS, is that it eliminates the opportunity to detect the infused enzyme in brain immunochemically, for example, to show immunolocalization with other lysosomal markers. The inactive enzyme produced by the transgene (GUS in our case) cross-reacts with antihuman GUS antibodies and prevents detection of the infused enzyme by immunostaining. Perhaps prelabeling the infused PerT-GUS with low molecular weight fluorescent labels might circumvent this limitation, as was shown recently in vitro for iduronidase (44).
The success obtained with PerT-GUS raises interesting questions about the mechanisms of delivery of this enzyme across the highly impermeable BBB. A possible route of entry of PerT-GUS to brain capillary endothelial cells is fluid-phase pinocytosis. The uptake of a soluble protein by fluid-phase pinocytosis is strictly concentration dependent and the rate of uptake is considerably slower than the rate of uptake of most proteins by receptor-mediated endocytosis. Nonetheless, the slow clearance of PerT-GUS from plasma, combined with its being regularly infused into the bloodstream over a 12-wk period, might allow for some enzyme to be slowly and continuously taken up by fluid-phase pinocytosis by endothelial cells. On the other hand, native GUS was cleared by the liver within a few minutes after each injection, leaving it much less time to be exposed to the brain endothelial surface over the 12-wk treatment.
Another possibility is that PerT-GUS is delivered by receptor-mediated endocytosis via a cryptic receptor on the endothelial surface that has yet to be characterized. A related explanation for the successful entry of PerT-GUS might be adsorptive endocytosis of enzymes bound to heparan sulfate proteoglycans on the endothelial cell surface (45–47). Heparan sulfate is one of the natural substrates of GUS (and, presumably, PerT-GUS). PerT-GUS likely binds to heparan sulfate on the membrane of endothelial cells, and this binding may mediate its gradual uptake via adsorptive endocytosis. The long-circulating property of PerT-GUS would allow continuous exposure of the capillary endothelial membrane to the enzyme compared with the short exposure to the rapidly cleared native GUS. The longer exposure might allow gradual uptake of bound enzyme in the course of membrane internalization and recycling. Such binding to heparan sulfate on the cell surface could also explain the observed correction of CNS storage by long-term infusion of iduronidase sulfatase (even at low doses) in the MPS II mouse model (28).
Even if PerT-GUS is taken up by endothelial cells by fluid-phase pinocytosis, endocytosis mediated by cryptic receptors, or adsorptive endocytosis through binding to heparan sulfate, the mechanism by which the enzyme then crosses the BBB to enter cortical and hippopcampal neurons and reduce storage would still be a puzzle. Most enzyme delivered to neurons is thought to be mediated by the M6PRs, and the recognition marker for this uptake system has been destroyed in PerT-GUS by the chemical modification. An alternate mechanism, which was described in the early 1980s, is cell-mediated delivery of lysosomal enzymes from macrophages to neuronal cells in an M6P-independent transfer by direct cell–cell contact (48, 49). In those studies, β-glucuronidase was shown to be transferred from macrophages into fibroblasts, neuroblastoma cells, and glial cells in vitro. In some LSDs with a CNS component, inflammatory cytokines recruit bone-marrow–derived macrophages across the BBB to sites of inflammation in the brain (50). If the long-circulating PerT-GUS is taken up by some cells in the macrophage lineage, which are then recruited to brain by inflammatory cytokines, enzymes could be delivered to neurons by cell–cell contact. Such a scenario might solve the challenge of crossing several membranes, without receptor-mediated targeting, to enter neurons and correct neuronal storage. Whatever the mechanism by which PerT-GUS crosses the BBB in murine MPS VII, its superiority to native enzyme in correcting neuronal storage makes it an attractive candidate for treating MPS VII.
The usefulness of this approach to other LSDs that affect the CNS deserves further study. Extending this approach to other disease models has two caveats. First, duration of treatment seemed important for clearing neuronal storage. Long-term treatment in the MPS VII model was possible because we created an immunotolerant mouse model that could receive long-term ERT without immune complications (37). Inducing tolerance in other mouse models is likely to be required to test whether this approach can be generalized to other LSDs. Secondly, not all enzymes will be suitable to this approach. Those unstable to the conditions of periodate oxidation or to the subsequent borohydride reduction, which can reduce essential disulfide bonds, would not be candidates. However, other means of creating long-circulating lysosomal enzymes might be found that could address these limitations and allow this approach to benefit other LSDs that affect the brain (51). In future experiments with the MPS VII model, it will be important to determine whether the morphological and biochemical evidence of delivery of PerT-GUS to brain translates into prevention and/or correction of cognitive defects.
Materials and Methods
Animals.
Saint Louis University School of Medicine.
All experiments and procedures involving live animals were conducted in compliance with approved Institutional Animal Care and Use Committee protocols of the Saint Louis University School of Medicine. For this study, homozygous mutant (MPS VII) mice that were tolerant to human β-glucuronidase were used (37). Mice were maintained in a C57/BL6 strain background and genotyped by PCR of tail DNA as described (37).
Enzymes.
GUS enzymes used in this study were purified by a multistep procedure with conventional column chromatography as described previously (34). To inactivate the mannose and M6P on GUS, the enzyme was treated with sodium metaperiodate followed by sodium borohydride reduction, as described in ref. 34.
Treatment of MPS VII Mice with Native GUS vs. PerT-GUS.
MPS VII mice were treated with 12 weekly infusions of native GUS or PerT-GUS at a dose of 4 mg/kg body weight. One week after the last infusion, brain and liver from untreated MPS VII mice (n = 2) and from animals that received either 4 mg/kg native GUS (n = 4) or 4 mg/kg PerT-GUS (n = 4) were obtained at necropsy after normal saline perfusion. The tissues were kept in dry ice for biochemical analysis or fixed in 2% (vol/vol) paraformaldehyde and 4% (wt/vol) glutaraldehyde for histopathology. For evaluation of lysosomal storage by light microscopy, tissues were postfixed in osmium tetroxide, embedded in Spurr’s resin, cut into 0.5-μm-thick sections, and stained with toluidine blue. To evaluate storage in cortical neurons, 500 contiguous parietal neocortical neurons were scored for the number of lucent cytoplasmic vacuoles, indicating lysosomal storage. A maximum of seven vacuoles were counted per cell, and results were evaluated by Student t test.
Clearance of Native GUS vs. PerT-GUS from the Circulation After Infusion into Mice.
MPS VII mice were infused via tail vein with GUS or PerT-GUS at a dose of 4 mg/kg in a total volume of 125 µl. One week after the final infusion, blood samples were taken from the right atrium immediately before perfusion into heparinized capillary tubes. Plasma was collected after centrifugation and assayed for GUS activity. Values were expressed as units of enzyme per milliliter of plasma volume.
Tissue Processing for Biochemical Analysis.
MPS VII mice were infused via tail vein with GUS or PerT-GUS at a dose of 4 mg/kg weekly for a total of 12 wk. One week after the final infusion, mice were perfused with 30 mL of 25 mM Tris (pH 7.2), 140 mM NaCl. Perfused tissues were collected, flash frozen in liquid nitrogen, and stored in dry ice for further processing. Tissues were thawed, weighed, and homogenized for 30 s with a Polytron homogenizer in 10–20 vol of 25 mM Tris (pH 7.2), 140 mM NaCl, 1 mM phenylmethylsulfonyl fluoride. Total homogenates were frozen at −80 °C overnight, thawed, and then sonicated for 20 s to produce a homogeneous extract. Extracts were assayed for GUS, α-galactosidase (GAL), and β-hexosaminidase (HEX) activities, and protein. The results were expressed as units per milligram of tissue protein.
Measurement of GUS, GAL, and HEX Activities and Protein.
GUS, GAL, and HEX activities were measured fluorometrically using the substrates 4-methyl-umbelliferyl (MU) β-d-glucuronide, 4-MU-α-d-galactopyranoside, and 4-MU-N-acetyl-β-d-glucosaminide (Sigma), respectively (52). Protein levels were assayed by the method of Lowry (53).
Acknowledgments
The authors wish to thank Tracey Baird for editorial assistance in the preparation of this manuscript. This work was supported by National Institutes of Health Grant GM034182 (to W.S.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Neufeld EF, Muenzer J. 2001. The Metabolic and Molecular Bases of Inherited Disease, eds Scriver CR, Beaudet AL, Sly WS, Valle D. (McGraw-Hill, New York), pp 3421–3451.
- 2.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: Report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 3.Beutler E, et al. Enzyme replacement therapy for Gaucher disease. Blood. 1991;78:1183–1189. [PubMed] [Google Scholar]
- 4.Thurberg BL, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Eng CM, et al. A phase 1/2 clinical trial of enzyme replacement in Fabry disease: Pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet. 2001;68:711–722. doi: 10.1086/318809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox WR, et al. International Fabry Disease Study Group Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004;75:65–74. doi: 10.1086/422366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurberg BL, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, et al. Carbohydrate-remodelled acid alpha-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem J. 2005;389:619–628. doi: 10.1042/BJ20050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, et al. Conjugation of mannose 6-phosphate-containing oligosaccharides to acid alpha-glucosidase improves the clearance of glycogen in Pompe mice. J Biol Chem. 2004;279:50336–50341. doi: 10.1074/jbc.M409676200. [DOI] [PubMed] [Google Scholar]
- 10.Kakkis ED, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J, et al. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 12.Harmatz P, et al. MPS VI Phase 3 Study Group Enzyme replacement therapy for mucopolysaccharidosis VI: A phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–539. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 14.Frisella WA, et al. Intracranial injection of recombinant adeno-associated virus improves cognitive function in a murine model of mucopolysaccharidosis type VII. Mol Ther. 2001;3:351–358. doi: 10.1006/mthe.2001.0274. [DOI] [PubMed] [Google Scholar]
- 15.Liu G, et al. Adeno-associated virus type 5 reduces learning deficits and restores glutamate receptor subunit levels in MPS VII mice CNS. Mol Ther. 2007;15:242–247. doi: 10.1038/sj.mt.6300016. [DOI] [PubMed] [Google Scholar]
- 16.Daly TM, Vogler C, Levy B, Haskins ME, Sands MS. Neonatal gene transfer leads to widespread correction of pathology in a murine model of lysosomal storage disease. Proc Natl Acad Sci USA. 1999;96:2296–2300. doi: 10.1073/pnas.96.5.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sferra TJ, et al. Widespread correction of lysosomal storage following intrahepatic injection of a recombinant adeno-associated virus in the adult MPS VII mouse. Mol Ther. 2004;10:478–491. doi: 10.1016/j.ymthe.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Cardone M, et al. Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum Mol Genet. 2006;15:1225–1236. doi: 10.1093/hmg/ddl038. [DOI] [PubMed] [Google Scholar]
- 19.Porteus MH, Connelly JP, Pruett SM. A look to future directions in gene therapy research for monogenic diseases. PLoS Genet. 2006;2:e133. doi: 10.1371/journal.pgen.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macauley SL, Sands MS. Promising CNS-directed enzyme replacement therapy for lysosomal storage diseases. Exp Neurol. 2009;218:5–8. doi: 10.1016/j.expneurol.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang M, et al. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, et al. Large-volume intrathecal enzyme delivery increases survival of a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2011;19:1842–1848. doi: 10.1038/mt.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogler C, et al. Enzyme replacement with recombinant beta-glucuronidase in the newborn mucopolysaccharidosis type VII mouse. Pediatr Res. 1993;34:837–840. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Vogler C, et al. Murine mucopolysaccharidosis type VII: The impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis. 1998;21:575–586. doi: 10.1023/a:1005423222927. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor LH, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII leads to improvements in behavior and auditory function. J Clin Invest. 1998;101:1394–1400. doi: 10.1172/JCI1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urayama A, Grubb JH, Sly WS, Banks WA. Mannose 6-phosphate receptor-mediated transport of sulfamidase across the blood-brain barrier in the newborn mouse. Mol Ther. 2008;16:1261–1266. doi: 10.1038/mt.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogler C, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polito VA, et al. Correction of CNS defects in the MPSII mouse model via systemic enzyme replacement therapy. Hum Mol Genet. 2010;19:4871–4885. doi: 10.1093/hmg/ddq420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunder U, et al. Enzyme replacement therapy in a mouse model of aspartylglycosaminuria. FASEB J. 2000;14:361–367. doi: 10.1096/fasebj.14.2.361. [DOI] [PubMed] [Google Scholar]
- 31.Roces DP, et al. Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: A preclinical animal study. Hum Mol Genet. 2004;13:1979–1988. doi: 10.1093/hmg/ddh220. [DOI] [PubMed] [Google Scholar]
- 32.Matzner U, et al. Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet. 2005;14:1139–1152. doi: 10.1093/hmg/ddi126. [DOI] [PubMed] [Google Scholar]
- 33.Sly WS, et al. Enzyme therapy in mannose receptor-null mucopolysaccharidosis VII mice defines roles for the mannose 6-phosphate and mannose receptors. Proc Natl Acad Sci USA. 2006;103:15172–15177. doi: 10.1073/pnas.0607053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grubb JH, et al. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozaklis T, et al. Impact of high-dose, chemically modified sulfamidase on pathology in a murine model of MPS IIIA. Exp Neurol. 2011;230:123–130. doi: 10.1016/j.expneurol.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Meng Y, Sohar I, Wang L, Sleat DE, Lobel P. Systemic administration of tripeptidyl peptidase I in a mouse model of late infantile neuronal ceroid lipofuscinosis: Effect of glycan modification. PLoS ONE. 2012;7:e40509. doi: 10.1371/journal.pone.0040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sly WS, et al. Active site mutant transgene confers tolerance to human beta-glucuronidase without affecting the phenotype of MPS VII mice. Proc Natl Acad Sci USA. 2001;98:2205–2210. doi: 10.1073/pnas.051623698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sands MS, et al. Murine mucopolysaccharidosis type VII: Long term therapeutic effects of enzyme replacement and enzyme replacement followed by bone marrow transplantation. J Clin Invest. 1997;99:1596–1605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birkenmeier EH, et al. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991;78:3081–3092. [PubMed] [Google Scholar]
- 40.Sands MS, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman BJ, et al. Behavior and therapeutic efficacy of beta-glucuronidase-positive mononuclear phagocytes in a murine model of mucopolysaccharidosis type VII. Blood. 1999;94:2142–2150. [PubMed] [Google Scholar]
- 42.Achord DT, Brot FE, Bell CE, Sly WS. Human beta-glucuronidase: In vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978;15:269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- 43.Achord D, Brot F, Gonzalez-Noriega A, Sly W, Stahl P. Human beta-glucuronidase. II. Fate of infused human placental beta-glucuronidase in the rat. Pediatr Res. 1977;11:816–822. doi: 10.1203/00006450-197707000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Tippin BL, et al. Biochemical characterization of fluorescent-labeled recombinant human alpha-L-iduronidase in vitro. Biotechnol Appl Biochem. 2011;58:391–396. doi: 10.1002/bab.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima M, Irimura T, Nicolson GL. Heparanases and tumor metastasis. J Cell Biochem. 1988;36:157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- 46.Bao X, et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenaerts L, van Dam W, Persoons L, Naesens L. Interaction between mouse adenovirus type 1 and cell surface heparan sulfate proteoglycans. PLoS ONE. 2012;7:e31454. doi: 10.1371/journal.pone.0031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen I, Dean MF, Muir H, Harris G. Acquisition of beta-glucuronidase activity by deficient fibroblasts during direct contact with lymphoid cells. J Cell Sci. 1982;55:211–231. doi: 10.1242/jcs.55.1.211. [DOI] [PubMed] [Google Scholar]
- 49.Dean MF, McNamara A, Jenne BM. Direct transfer of beta-glucuronidase from mouse macrophages to other types of cell. J Cell Sci. 1985;79:137–149. doi: 10.1242/jcs.79.1.137. [DOI] [PubMed] [Google Scholar]
- 50.Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci USA. 2004;101:8425–8430. doi: 10.1073/pnas.0400625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montaño AM, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Glaser JH, Sly WS. Beta-glucuronidase deficiency mucopolysaccharidosis: Methods for enzymatic diagnosis. J Lab Clin Med. 1973;82:969–977. [PubMed] [Google Scholar]
- 53.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]