Abstract
The bacterial RNA polymerase holoenzyme consists of a catalytic core enzyme in complex with a σ factor that is required for promoter-specific transcription initiation. Primary, or housekeeping, σ factors are responsible for most of the gene expression that occurs during the exponential phase of growth. Primary σ factors share four regions of conserved sequence, regions 1–4, which have been further subdivided. Many primary σ factors also contain a nonconserved region (NCR) located between subregions 1.2 and 2.1, which can vary widely in length. Interactions between the NCR of the primary σ factor of Escherichia coli, σ70, and the β′ subunit of the E. coli core enzyme have been shown to influence gene expression, suggesting that the NCR of primary σ factors represents a potential target for transcription regulation. Here, we report the identification and characterization of a previously undocumented Chlamydia trachomatis transcription factor, designated GrgA (general regulator of genes A). We demonstrate in vitro that GrgA is a DNA-binding protein that can stimulate transcription from a range of σ66-dependent promoters. We further show that GrgA activates transcription by contacting the NCR of the primary σ factor of C. trachomatis, σ66. Our findings suggest GrgA serves as an important regulator of σ66-dependent transcription in C. trachomatis. Furthermore, because GrgA is present only in chlamydiae, our findings highlight how nonconserved regions of the bacterial RNA polymerase can be targets of regulatory factors that are unique to particular organisms.
Chlamydiae are obligate intracellular bacterial parasites, and their hosts range from single cellular eukaryotes to humans (1). In humans, Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen (2). C. trachomatis is known for its ability to efficiently ascend from the lower genital tract to the upper genital tract, where chlamydial replication can have many devastating consequences, including infertility and pelvic inflammatory disease in women. In addition, some C. trachomatis serovars cause eye infection, which is still a major cause of blindness in underdeveloped countries (2).
Chlamydiae have a unique developmental cycle with two alternating cellular forms. The metabolically inactive, infectious elementary body (EB) enters a vacuole through host cell endocytosis (3). Inside the vacuole (termed inclusion), the EB develops into the proliferating but noninfectious reticulate body (RB). As RBs accumulate, they reorganize, in an asynchronous manner, back to EBs, which exit the host cell at the end of the developmental cycle (4). Whereas a typical developmental cycle ranges from 2 to 4 d, the infection may enter a latent state, which is characterized by accumulation of aberrant reticulate bodies and a lack of EB production (5).
The C. trachomatis genome is ∼1 Mb in size and encodes ∼1,000 genes (6, 7). Genome-wide microarray analyses have revealed that ∼80% of all genes are expressed a few hours after infection through the remaining developmental cycle. For the remaining genes, some are expressed immediately after chlamydial entry into the cell, and others are not transcribed until a middle or late stage. (8, 9). Furthermore, certain gene transcripts exhibit increases or decreases in abundance as chlamydiae enter latency (10). These observations suggest that alterations in gene expression manifest at the level of transcription contribute to the development of latency. Nevertheless, due to the limited number of genetic tools available in chlamydiae, only a few transcription regulators have been identified.
Here we describe the identification and characterization of a previously undocumented transcription factor that we call GrgA (general regulator of genes A). GrgA was identified based upon its ability to activate transcription in vitro from the promoter that controls the expression of defA, which encodes the peptide deformylase (PDF). We demonstrate that efficient transcription activation of the defA promoter by GrgA in vitro requires contact with both DNA and a portion of a nonconserved region (NCR) of the primary σ factor of C. trachomatis, σ66. We further show that GrgA can stimulate σ66-dependent transcription in vitro of three other genes that are expressed in vivo during different stages of the chlamydial developmental cycle. Our findings suggest that GrgA is not only a regulator of defA expression in vivo, but also a general transcription activator of many σ66-dependent genes. Furthermore, because GrgA is present only in chlamydiae, our findings highlight how regulatory factors that are unique to particular organisms can target nonconserved regions of the bacterial RNA polymerase (RNAP).
Results
Identification of GrgA as a defA Promoter-Binding Protein with Transcription Activation Activity.
PDF catalyzes the removal of the N-formyl group from the leading methionine of newly synthesized proteins. Most bacterial proteins require the deformylation and subsequent removal of the N-terminal methionine to function properly. Furthermore, even for the small proportion of proteins that can function while carrying the N-formyl methionine, deformylation is necessary for the initiation of regulated degradation (11). Thus, PDF is a potential therapeutic and/or prophylactic target for infectious diseases. Accordingly, small inhibitors of PDF have shown effectiveness against a variety of pathogens, including chlamydiae both in vitro and in vivo (12, 13).
Our goal was to identify additional chlamydial proteins that might serve as potential therapeutic targets. Given that small molecules targeting PDF inhibit chlamydial growth, we sought to identify factors involved in the expression of defA, which might themselves serve as potential targets. To accomplish this goal, we used a DNA pull-down assay to identify chlamydial proteins that bound to a DNA fragment containing the defA promoter. To do this, we mixed cell extracts isolated from C. trachomatis-infected mouse L cells with streptavidin beads attached to either a biotinylated DNA fragment that carried sequences extending from position –144 to +52 of the defA promoter (pdflong) or a biotinylated DNA fragment that carried sequences from position –54 to +52 (pdfshort). We then analyzed proteins associated with either the beads bound to the pdflong fragment or the beads bound to the pdfshort fragment by liquid chromatography (LC)–MS/MS. From this analysis we identified C. trachomatis proteins with no previously assigned function that were enriched when the pull-down assay was performed with the pdflong fragment compared with the pdfshort fragment.
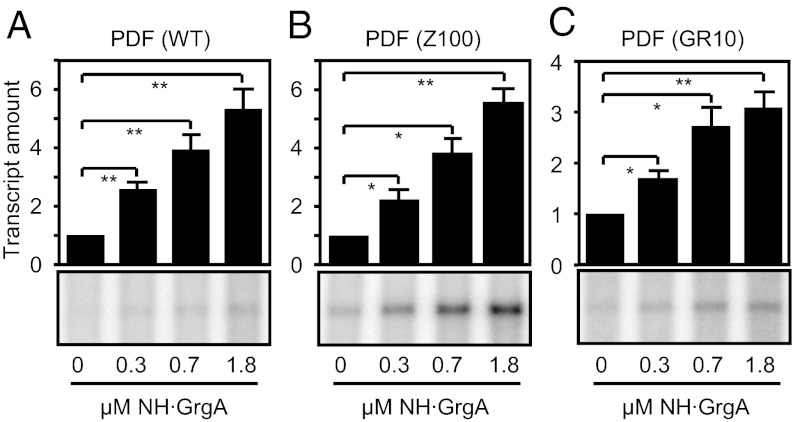
We focused our attention on the Chlamydia-specific hypothetical protein that showed the highest enrichment in binding the pdflong fragment compared with the pdfshort. This protein was encoded by an ORF designated CTL0766 in the genome of C. trachomatis L2. BLAST analysis did not detect significant homology between the CTL0766 protein and any nonchlamydial proteins, and no bacterial protein motif was identified in a motif search. We expressed and purified a His-tagged derivative of CTL0766 in Escherichia coli (Table S1 and Fig. S1 A and B) and tested its potential role in the regulation of defA gene transcription using an in vitro transcription assay (14) (Fig. 1 and Fig. S2). Addition of CTL0766 stimulated transcription of the defA promoter by chlamydial RNA polymerase (cRNAP) in a dose-dependent manner from the wild-type promoter (Fig. 1A) as well as two mutant derivatives (Z100 and GR10) (14), which contain single base-pair substitutions that increase basal transcription (Fig. 1 B and C and Fig. S2). These results demonstrate that the gene product of CTL0766 can activate transcription of the defA gene in vitro. As shown below, CTL0766 also acts as an activator of three other chlamydial genes tested. Accordingly, we rename the C. trachomatis gene CTL0766 grgA (general regulator of genes A).
Fig. 1.
GrgA stimulates transcription of the defA promoter in vitro. Shown are in vitro transcription assays performed using cRNAP, a DNA template carrying the indicated defA promoter variant, and the indicated concentration of NH⋅GrgA. The Z100 promoter derivative (used in B) carries a base pair substitution in the promoter –35 element, whereas the GR10 derivative (used in C) carries a substitution in DNA upstream of the –35 element (14). Graphs show the averages and SDs for three independent measurements. We note that C-terminally His-tagged GrgA also demonstrated a dose-dependent stimulatory effect on defA promoter activity (Fig. S2). Single and double asterisks denote that the difference between control and GrgA-containing reactions were statistically significant (P < 0.05 and P < 0.01, respectively).
GrgA Binds to DNA in a Sequence-Nonspecific Manner.
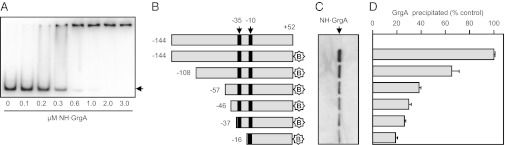
To characterize the nature of the interaction between GrgA and the defA promoter, we first performed an EMSA using purified GrgA and a 32P-labeled promoter fragment extending from position –144 to +52 of the defA promoter. Surprisingly, initial experiments detected no clear shifted band (Fig. S3A), even though the same sequence of the defA promoter was used for initial pull-down experiment that led to the identification of GrgA (see above). We reasoned that the inclusion of poly(deoxyinosinic-deoxycytidylic) acid [poly(dI-dC)] in the reaction might have prevented GrgA from binding the defA promoter fragment. Consistent with this hypothesis, dose-dependent retardation of the defA promoter fragment by GrgA was readily detectable in the absence of poly(dI-dC) (Fig. 2A). However, GrgA-bound DNA did not migrate as a clear band in the resolving gel. At lower concentrations (0.2 and 0.3 μM) of GrgA the DNA appeared as smear in the gel, whereas at higher concentrations of GrgA (≥0.6 μM) the protein–DNA complex largely or completely remained in the loading well (Fig. 2A). We further performed GrgA pull-down assays to determine what effect shortening the DNA fragment carrying the defA promoter had on the binding of GrgA. Accordingly, we generated biotinylated defA promoter fragments of different lengths (Fig. 2B) that were immobilized to streptavidin-conjugated agarose beads and determined the amount of GrgA that precipitated with equimolar amounts of each DNA fragment. Progressive removal from the 5′-end of the promoter resulted in a steady decrease in the amount of GrgA that was precipitated (Fig. 2 C and D), revealing a clear correlation between the length of the promoter DNA fragment and the amount of precipitated GrgA. These findings, which are consistent with the enrichment of GrgA association with the pdflong fragment compared with the pdfshort fragment as detected by MS (see above), suggest that GrgA does not bind preferentially to defA promoter sequences, but rather binds DNA in a sequence-nonspecific manner. Consistent with this hypothesis, replacement of the sequences extending from –144 to +5 of the defA promoter fragment with unrelated DNA sequence had no effect on the amount of GrgA precipitated (Fig. S3 B and C). Furthermore, we found that GrgA could bind to DNA located in the 5′-untranslated region of the defA gene and to two fragments containing portions of the GrgA ORF (Fig. S3 D and E). Taken together, data presented in Fig. 2 and Fig. S3 indicate that GrgA binds DNA in a sequence-nonspecific manner.
Fig. 2.
GrgA binds DNA. (A) EMSA assays performed in the absence of poly(dI-dC) using a DNA fragment carrying sequences extending from position –144 to +52 of the defA promoter. (B) Schematic of the DNA fragments used to precipitate NH·GrgA (star indicates the presence of a 3′ biotin moiety). (C) Western blot analysis of the amount of NH·GrgA precipitated by the corresponding DNA fragments in B. (D) Graph shows the amount of GrgA precipitated as a percentage of that precipitated by the biotinylated –144 to +52 fragment. Plotted are the averages and SDs for three independent measurements.
GrgA-Dependent Transcription Activation Requires DNA Binding.
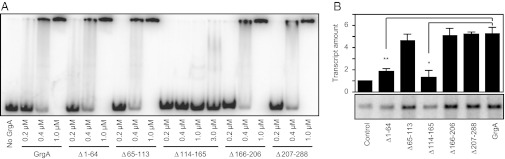
We next pursued the identification of amino acid residues in GrgA that were required for sequence-nonspecific DNA binding. We constructed a series of GrgA mutants with deletions of 50–82 amino acids (Table S1 and Fig. S4 A and B) and assessed these mutants’ abilities to associate with defA promoter DNA using both a pull-down assay (Fig. S4C) and an EMSA assay (Fig. 3A). GrgA mutants lacking amino acids 1–64 (Δ1–64), 65–113 (Δ65–113), 166–206 (Δ166–206), or 207–288 (Δ207–288) all exhibited DNA binding activities that were identical to that of the wild-type GrgA protein. In contrast, the GrgA mutant lacking amino acid residues 114–165 (Δ114–165) was severely compromised in its ability to bind DNA (Fig. 3A and Fig. S4C), suggesting that residues 114–165 comprise a portion of GrgA that is essential for sequence-nonspecific DNA binding. Noticeably, this region is rich in positively charged lysine and arginine residues (Fig. S4D), which may mediate the binding of GrgA to the negatively charged DNA. Furthermore, the lysine/arginine-rich sequence and a following region appeared to form a helix-turn-helix (Fig. S4D), a structural motif characteristic of DNA binding proteins.
Fig. 3.
DNA binding by GrgA is essential but not sufficient for full transcription activation. (A) EMSA assays performed using a radiolabeled DNA fragment carrying sequences extending from position –144 to +52 of the defA promoter in the presence of the indicated concentrations of wild-type GrgA or the indicated GrgA mutant. (B) In vitro transcription assays performed in the presence or absence of 1.8 μM wild-type GrgA or the indicated GrgA mutant. Assays were performed using cRNAP and a DNA template carrying the Z100 defA promoter variant. Graph shows the averages and SDs for three independent measurements.
We next assessed the effects of the various deletions in GrgA on the ability of GrgA to activate transcription from the defA promoter. Transcription activation was essentially that of the wild-type GrgA for the Δ65–113, Δ166–206, and Δ207–288 mutants. In contrast, the Δ114–165 mutant, which could not bind DNA, suffered a significant (∼75%) loss of transcription activation activity compared with wild-type GrgA (Fig. 3B), suggesting that the ability of GrgA to bind DNA is important for GrgA-dependent transcription activation. In addition, the Δ1–64 mutant, which retained a strong DNA binding activity (Fig. 3A and Fig. S4C), also displayed a significant (∼65%) loss of transcription activation activity (Fig. 3B). These results indicate that DNA binding is necessary but not sufficient for GrgA to efficiently activate transcription.
GrgA-Dependent Transcription Activation Requires Interaction with the Nonconserved Region of σ66.
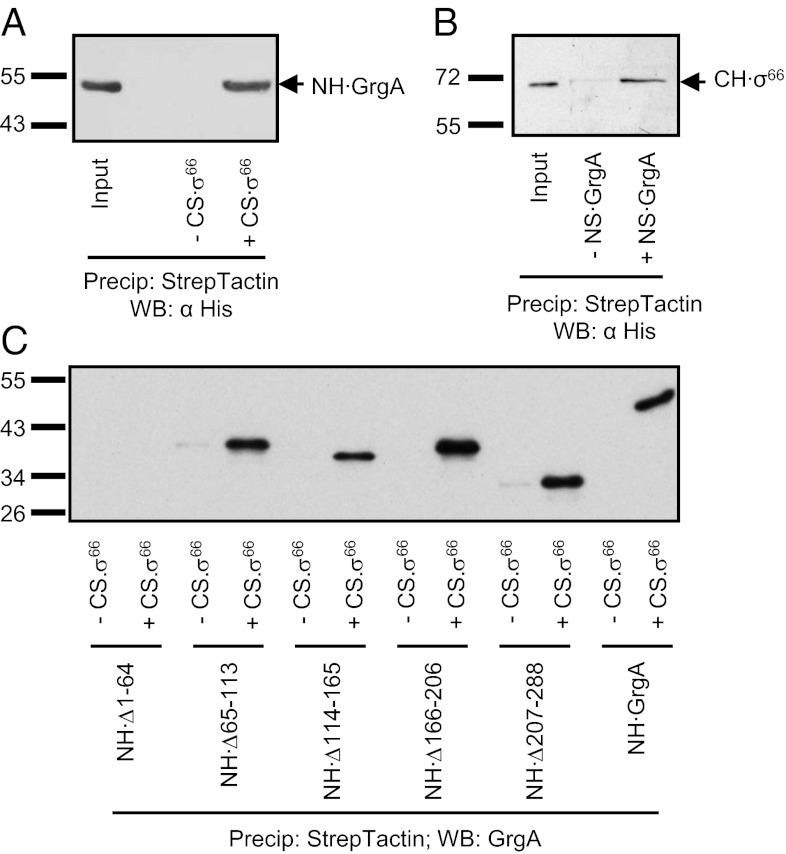
We have previously demonstrated that a hybrid RNAP holoenzyme consisting of the E. coli RNAP core enzyme and chlamydial σ66 can transcribe from the defA promoter (14). We therefore tested whether or not GrgA could activate transcription of the defA promoter in reactions performed using this hybrid holoenzyme. We found that addition of GrgA to transcription reactions performed using the hybrid holoenzyme stimulated transcription (Fig. S5A) in a manner similar to the stimulatory effect observed in reactions performed using cRNAP (Fig. 1 and Fig. S2). In contrast, GrgA was unable to activate transcription in reactions performed with E. coli RNAP core enzyme reconstituted with E. coli σ70 (Fig. S5B) or C. trachomatis σ28 (Fig. S5C). These findings raised the possibility that GrgA may stimulate transcription through direct interaction with σ66. Consistent with this hypothesis, Strep-Tactin–immobilized, C-terminally Strep-tagged σ66 (CS·σ66) was able to precipitate GrgA (Fig. 4A); furthermore, N-terminally Strep-tagged GrgA (NS·GrgA) also precipitated C-terminally His-tagged σ66 (CH·σ66; Fig. 4B).
Fig. 4.
GrgA binds σ66. (A) Precipitation of NH·GrgA by Strep-Tactin–immobilized CS·σ66. Shown is a Western blot detecting His-tagged GrgA. (B) Precipitation of CH·σ66 by NS·GrgA. Shown is a Western blot detecting σ66. (C) Precipitation of wild-type GrgA or the indicated mutant GrgA derivatives by Strep-Tactin–immobilized CS·σ66. Shown is a Western blot detecting GrgA variants. Anti-GrgA instead of anti-His was used for detection because the anti-His recognized GrgA variants with greatly varying efficiency, as demonstrated in Fig. S8.
We next used the GrgA mutants constructed for mapping the DNA binding region (Fig. S4A) to determine what residues of GrgA were required for the interaction with σ66. The Δ65–113, Δ114–165, Δ166–206, and Δ207–288 GrgA mutants all retained the ability to interact with σ66 (Fig. 4C). Importantly, the finding that the Δ114–165 mutant retained the ability to bind σ66 indicates that this mutant is stable, providing further support that the reduction in transcription activation observed with this mutant (Fig. 3B) is a consequence of its inability to bind DNA. In contrast to the other GrgA mutants, the Δ1–64 mutant, which could efficiently bind DNA (Fig. 3A and Fig. S4C), did not detectably interact with σ66 (Fig. 4C). This finding suggests that the inability of the Δ1–64 mutant to efficiently stimulate transcription from the defA promoter (Fig. 3B) is due to the inability of the mutant to contact σ66.
To further explore the hypothesis that GrgA-dependent transcription activation requires contact with σ66, we sought to identify regions of σ66 that were required for the interaction with GrgA. Primary σ factors share four regions of conserved sequence, regions 1–4 (15–18). Furthermore, many primary σ factors carry a NCR between regions 1 and 2 that can widely vary in sequence and length (15–17). We therefore constructed individual C. trachomatis σ66 fragments (Table S1 and Fig. S6A) that comprised σ region 1 (residues 1–146), region 2 (residues 303–408), region 3 (residues 386–490), region 4 (residues 473–571), or the NCR (residues 121–322), and attempted to express them in E. coli. All of the fragments except the one comprising region 3 were successfully expressed and purified (Fig. S6B). We next determined whether or not we could precipitate these fragments with GrgA, and found that only the fragment encompassing the NCR could precipitate GrgA (Fig. 5A), suggesting that GrgA contacts the σ66 NCR.
Fig. 5.
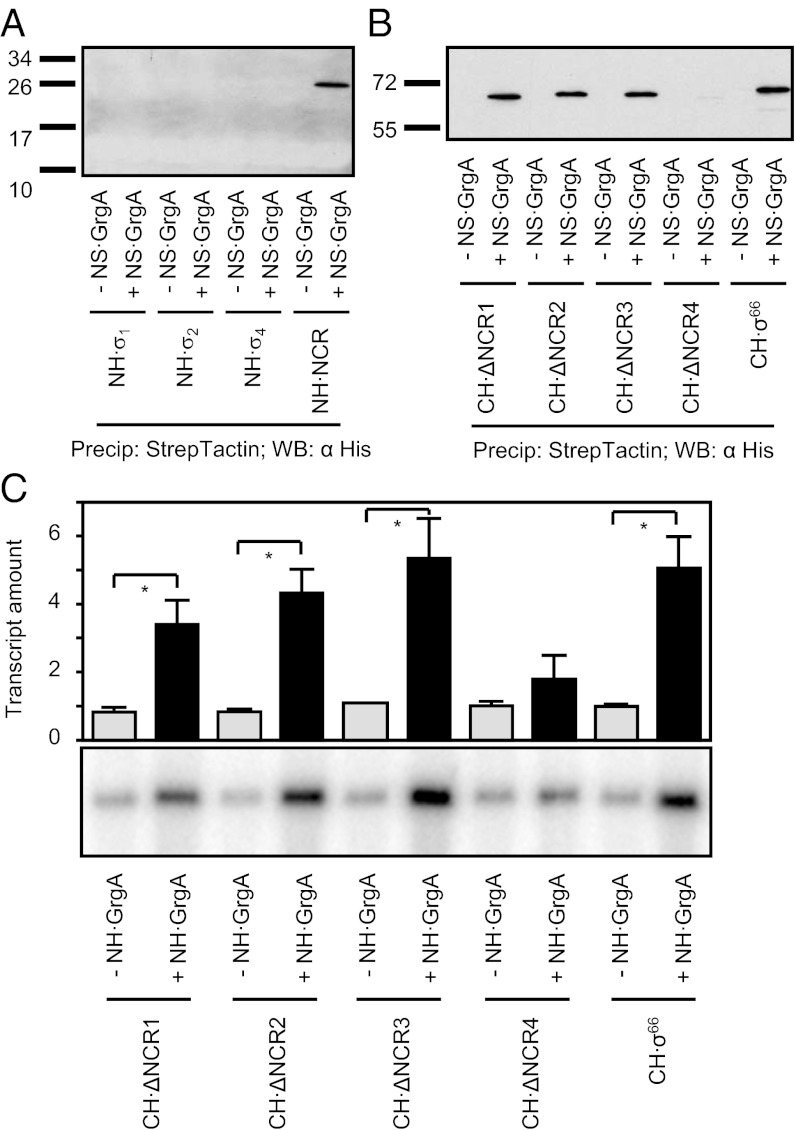
Interaction between GrgA and the nonconserved region of σ66 is required for efficient transcription activation. (A) Precipitation of His-tagged fragments of σ66 by Strep-Tactin–immobilized GrgA (NS·GrgA). Shown is a Western blot detecting the His-tagged σ66 fragments recovered after precipitation. (B) Precipitation of His-tagged derivatives of σ66 by Strep-Tactin–immobilized GrgA (NS·GrgA). Shown is a Western blot detecting the His-tagged σ66 derivatives recovered after precipitation. ΔNCR1 lacks residues 132–183; ΔNCR2 lacks residues 184–223; ΔNCR3, lacks residues 224–268; and ΔNCR4 lacks residues 270–316 (Fig. S6). (C) In vitro transcription assays performed in the presence or absence of 1.8 μM wild-type GrgA using a hybrid holoenzyme consisting of E. coli core and the indicated σ66 derivative. The DNA template used for these assays was the Z100 defA promoter variant. Graph shows the averages and SDs for three independent measurements.
To further define the residues of the NCR that were important for GrgA binding, we constructed a series of σ66 mutants that each contained 40–52 amino acid deletions in the NCR (ΔNCR1, which lacked residues 132–183; ΔNCR2, which lacked residues 184–224; ΔNCR3, which lacked residues 224–268; and ΔNCR4, which lacked residues 269–316; Fig. S6 C and D). Among these mutants, only ΔNCR4 lost the ability to bind to GrgA (Fig. 5B), indicating that residues 269–316 of the NCR of σ66 are required for interaction with GrgA.
We next tested the effect of deleting residues 269–316 of σ66 on the ability of GrgA to activate transcription of the defA promoter. To do this, we performed in vitro transcription assays using a hybrid RNAP holoenzyme consisting of the E. coli RNAP core enzyme and either wild-type or mutant σ66. Removal of residues 269–316 in σ66 had no effect on basal transcription but severely reduced the ability of GrgA to stimulate transcription (Fig. 5C). In contrast, removal of other NCR residues that did not affect the ability of GrgA to bind σ66 did not significantly affect GrgA-dependent activation (Fig. 5C). Thus, disrupting the interaction between GrgA and the NCR of σ66 by either removing residues 1–64 of GrgA or residues 269–316 of σ66 severely impairs GrgA-dependent transcription activation (Figs. 3, 4, and 5C). Taken together, these results establish that the interaction between GrgA and the NCR of σ66 is required for efficient transcription activation by GrgA.
GrgA Is a General Activator of σ66-Dependent Genes in Vitro.
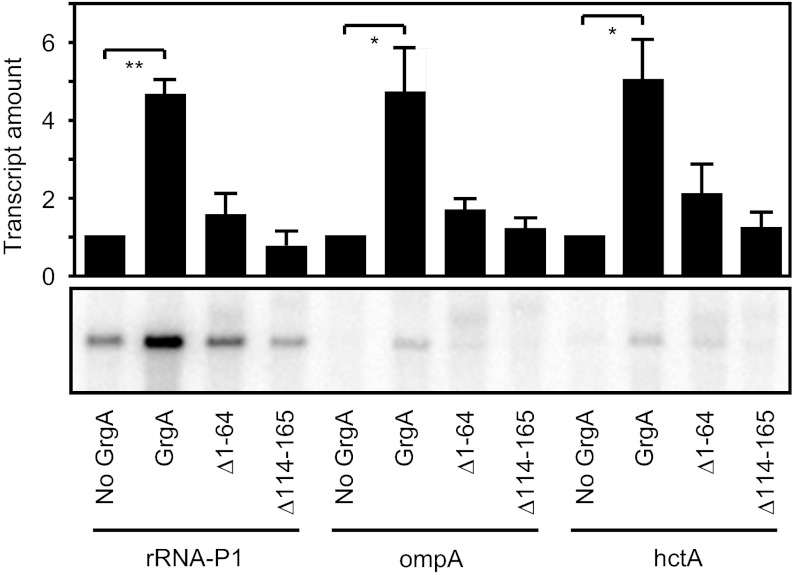
Given that GrgA bound DNA in a sequence-nonspecific manner and could activate transcription from the defA promoter, we next determined if the transcription activation activity of GrgA was limited to the defA gene. Accordingly, we assessed the effects of GrgA in vitro on the activities of three additional σ66-dependent promoters that are active at different stages of growth: ribosomal RNA promotor P1 (rRNA P1; early), major outer membrane protein A (ompA; middle), and histone-like protein A (hctA; late) (8, 9, 19, 20). GrgA demonstrated significant stimulatory effects on all of the three promoters (Fig. 6). Furthermore, both the Δ1–64 GrgA mutant (that does not interact with σ66) and the Δ114–165 GrgA mutant (that does not bind DNA) exhibited significantly reduced levels of activation at each promoter compared with that observed with wild-type GrgA (Fig. 6). Thus, the GrgA-dependent activation observed in the context of the rRNA P1, ompA, and hctA promoters, like that observed in the context of the defA promoter, requires GrgA to contact the DNA and the NCR of σ66.
Fig. 6.
GrgA is a general activator of σ66-dependent transcription in vitro. In vitro transcription assays performed in the presence or absence of 1.8 μM wild-type GrgA or the indicated GrgA mutant. Assays were done using cRNAP and a DNA template carrying the indicated promoter. Graph shows the averages and SDs for three independent measurements.
Discussion
Here we identify a transcription factor from C. trachomatis, GrgA, which activates transcription of several σ66-dependent promoters in vitro by binding DNA and contacting the σ66 NCR. These findings strongly suggest GrgA serves as an important regulator of σ66-dependent transcription in C. trachomatis.
GrgA Is a General Regulator of σ66-Dependent Transcription.
Chlamydia is an important pathogen that has a unique developmental cycle with two alternating cellular forms. Knowledge of the extent to which the regulation of transcription contributes to the chlamydial life cycle has been limited, in part, by the lack of suitable experimental tools. In this regard, only a handful of transcription inhibitors (21–25) and three transcription activators (26–28) had been identified in chlamydiae before our study.
Our work identifies GrgA as a previously undocumented transcription factor in C. trachomatis. Although we originally identified GrgA on the basis of its ability to stimulate transcription from the σ66-dependent defA promoter (Fig. 1), we found that GrgA stimulates transcription from several σ66-dependent promoters that are active at different stages of the chlamydial developmental cycle (Fig. 6). Based upon these in vitro findings, we propose that GrgA functions as a general transcription activator in C. trachomatis that up-regulates the expression of a broad spectrum of genes during all developmental phases. Consistent with this proposal, Western blot analysis indicates that GrgA is present in both chlamydial cellular forms, the EB and RB (Fig. S7). Nevertheless, although our in vitro data provide strong evidence that GrgA functions in vivo as an important regulator of σ66-dependent gene expression, a direct test of the functional role that GrgA plays in vivo awaits the development of methods to perform targeted mutagenesis in Chlamydia.
GrgA-Dependent Transcription Activation Requires Contact with both DNA and the σ66 NCR.
We found that efficient GrgA-dependent activation requires GrgA to retain the ability to contact both DNA and the σ66 NCR. In particular, we found that a GrgA mutant lacking amino acid residues required for DNA binding (Δ114–165) and a GrgA mutant lacking amino acid residues required for contact with the σ66 NCR (Δ1–64) were significantly impaired for transcription activation (Figs. 3, 4, and 6). The importance of the GrgA–σ66 NCR interaction is further indicated by the inability of wild-type GrgA to efficiently activate transcription when reactions were performed with RNAP reconstituted with a σ66 mutant lacking amino acids required for the interaction with GrgA (Fig. 5).
Many bacterial proteins have been identified that bind DNA and activate transcription through direct contact with conserved region 4 of the primary σ factor (for review, see ref. 29). To our knowledge, GrgA represents the first DNA binding protein that activates transcription through direct contact with the NCR region of a primary σ factor. Prior studies have identified a role for the NCR of the primary σ factor of E. coli, σ70, in modulating both promoter escape and early elongation pausing (30). In particular, interaction between the σ70 NCR and the β′ subunit of the E. coli core enzyme has been shown to facilitate escape from promoter DNA during initial transcription as well as escape from σ70-dependent pausing during early elongation. We found that GrgA can stimulate transcription with both chlamydial RNAP and with a hybrid RNAP consisting of chlamydial σ66 and E. coli core (Figs. 1 and 6 and Fig. S5). Furthermore, the NCR of σ66 and the NCR of σ70 lack significant sequence similarity, suggesting that the NCR of σ66 is unlikely to interact with the β′ subunit of the E. coli core enzyme. Thus, we consider it unlikely that GrgA activates transcription by influencing interactions between the σ66 NCR and the RNAP core enzyme. We propose instead that GrgA activates transcription by stabilizing the binding of RNAP to promoter DNA. Nevertheless, defining the precise mechanism by which GrgA activates transcription awaits further investigation.
In conclusion, we have identified GrgA as a Chlamydia-specific transcription activator that exerts its stimulatory effect through interactions with the NCR of σ66 and sequence-nonspecific interactions with DNA. Furthermore, because GrgA can stimulate transcription in vitro from several promoters that control the expression of genes that are critical for chlamydial growth, GrgA likely represents a promising antichlamydial target.
Materials and Methods
Purification and Identification of PDF Promoter-Binding Proteins.
RBs were partially purified from 12 L of suspension culture and disrupted by sonication. The lysate was clarified by centrifugation. The clarified RB lysate was mixed with biotinylated PDF promoter DNA immobilized to streptavidin-conjugated agarose beads, which were then packed into a column and washed with buffer I (SI Materials and Methods) supplemented with 200 mM NaCl. Bound proteins were eluted using buffer I containing 600 mM NaCl. Proteins were mixed with SDS/PAGE sample buffer, resolved by electrophoresis, and digested by trypsin. Tryptic peptides were identified by nanoLC-MS/MS as detailed in SI Materials and Methods.
Purification of Recombinant GrgA.
For transcription assays and protein–protein interaction assays, N- or C-terminally His-tagged GrgA (Table S1) was purified from E. coli extracts prepared in guanidine hydrochloride. Denatured proteins were purified with metal TALON affinity resin and renatured as detailed in SI Materials and Methods. Strep-tagged GrgA or σ66 were purified using Strep-Tactin beads following manufacturer’s instruction.
In Vitro Transcription Assay.
The ability of GrgA to regulate transcription from chlamydial promoters was determined using a previously reported in vitro transcription assay (14, 31) with modifications, as detailed in SI Materials and Methods.
GrgA–DNA Interaction.
EMSA was performed with or without poly(dI-dC). Streptavidin-immobilized biotinylated DNA fragments were used to precipitate GrgA. Alternatively, antibody immobilized GrgA was used to precipitate DNA. Experimental conditions for each of these assays are provided in SI Materials and Methods.
Protein–Protein Interaction.
A lysate of E. coli expressing either NS·GrgA or CS·σ66 (Table S1), which contained 50 mM Hepes and 300 mM NaCl, was diluted with equal volume of H2O. A total of 200 μL of the diluted lysate was mixed with 10 μL of Strep-Tactin beads (20 μL suspension) on a nutator for 1 h at 4 °C. The beads were washed 4× with 25 mM Hepes (pH 7.0) containing 150 mM NaCl and 1% Nonidet P-40 (HeNN buffer), and mixed with 5 μg of CH·σ66, a CH·σ66 mutant, NH·GrgA, or a NH·GrgA mutant for 1 h at 4 °C. After four additional washes with HeNN, His-tagged proteins were resolved by SDS/PAGE and visualized by Western blotting using anti-His or anti-GrgA.
Supplementary Material
Acknowledgments
We thank Dr. Peter Lobel and his colleagues, Drs. Haiyan Zheng and Meiqian Qian, at the Robert Wood Johnson Medical School (RWJMS) Proteomics Core for advice and help with mass spectrometry; Dr. Marc Gartenberg (RWJMS) for access to his phosphorimager; Dr. Masayori Inouye for access to his French press used for the preparation of non-denatured E. coli extracts; Dr. Guangming Zhong (University of Texas Health Sciences Center at San Antonio) for producing mouse anti-GrgA; Dr. Ming Tan (University of California, Irvine) for transcriptional reporter plasmids; Christopher Oey for assistance in the purification of recombinant proteins; and Dr. John Kerrigan for in silico sequence analyses of GrgA. This work was supported in part by National Institutes of Health Grants AI071954 (to H.F.) and GM088343 (to B.E.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207300109/-/DCSupplemental.
References
- 1.Stephens RS, Myers G, Eppinger M, Bavoil PM. Divergence without difference: Phylogenetics and taxonomy of Chlamydia resolved. FEMS Immunol Med Microbiol. 2009;55:115–119. doi: 10.1111/j.1574-695X.2008.00516.x. [DOI] [PubMed] [Google Scholar]
- 2.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia Intracellular Biology, Pathogenesis. Washington, DC: ASM Press; 1999. pp. 139–169. [Google Scholar]
- 3.Hybiske K, Stephens RS. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun. 2007;75:3925–3934. doi: 10.1128/IAI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci USA. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todd WJ, Caldwell HD. The interaction of Chlamydia trachomatis with host cells: Ultrastructural studies of the mechanism of release of a biovar II strain from HeLa 229 cells. J Infect Dis. 1985;151:1037–1044. doi: 10.1093/infdis/151.6.1037. [DOI] [PubMed] [Google Scholar]
- 6.Stephens RS, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 7.Thomson NR, et al. Chlamydia trachomatis: Genome sequence analysis of lymphogranuloma venereum isolates. Genome Res. 2008;18:161–171. doi: 10.1101/gr.7020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson TL, Olinger L, Chong K, Schoolnik G, Stephens RS. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J Bacteriol. 2003;185:3179–3189. doi: 10.1128/JB.185.10.3179-3189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belland RJ, et al. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci USA. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haranaga S, Ikejima H, Yamaguchi H, Friedman H, Yamamoto Y. Analysis of Chlamydia pneumoniae growth in cells by reverse transcription-PCR targeted to bacterial gene transcripts. Clin Diagn Lab Immunol. 2002;9:313–319. doi: 10.1128/CDLI.9.2.313-319.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capecchi MR. Initiation of E. coli proteins. Proc Natl Acad Sci USA. 1966;55:1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen D, Yuan Z. Therapeutic potential of peptide deformylase inhibitors. Expert Opin Investig Drugs. 2005;14:1107–1116. doi: 10.1517/13543784.14.9.1107. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan A, et al. Inhibition of chlamydial infection in the genital tract of female mice by topical application of a peptide deformylase inhibitor. Microbiol Res. 2009;164:338–346. doi: 10.1016/j.micres.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao X, et al. Non-coding nucleotides and amino acids near the active site regulate peptide deformylase expression and inhibitor susceptibility in Chlamydia trachomatis. Microbiology. 2011;157:2569–2581. doi: 10.1099/mic.0.049668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross CA, et al. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harb Symp Quant Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- 16.Paget MS, Helmann JD. The sigma70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lonetto M, Gribskov M, Gross CA. The sigma 70 family: Sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami KS, Darst SA. Bacterial RNA polymerases: The whole story. Curr Opin Struct Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 19.Hackstadt T, Baehr W, Ying Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc Natl Acad Sci USA. 1991;88:3937–3941. doi: 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perara E, Ganem D, Engel JN. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc Natl Acad Sci USA. 1992;89:2125–2129. doi: 10.1073/pnas.89.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyllie S, Raulston JE. Identifying regulators of transcription in an obligate intracellular pathogen: A metal-dependent repressor in Chlamydia trachomatis. Mol Microbiol. 2001;40:1027–1036. doi: 10.1046/j.1365-2958.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- 22.Akers JC, Tan M. Molecular mechanism of tryptophan-dependent transcriptional regulation in Chlamydia trachomatis. J Bacteriol. 2006;188:4236–4243. doi: 10.1128/JB.01660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao X, et al. A regulator from Chlamydia trachomatis modulates the activity of RNA polymerase through direct interaction with the beta subunit and the primary sigma subunit. Genes Dev. 2009;23:1818–1829. doi: 10.1101/gad.1784009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akers JC, HoDac H, Lathrop RH, Tan M. Identification and functional analysis of CT069 as a novel transcriptional regulator in Chlamydia. J Bacteriol. 2011;193:6123–6131. doi: 10.1128/JB.05976-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson AC, Tan M. Functional analysis of the heat shock regulator HrcA of Chlamydia trachomatis. J Bacteriol. 2002;184:6566–6571. doi: 10.1128/JB.184.23.6566-6571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo IC, Walthers D, Hefty PS, Kenney LJ, Stephens RS. ChxR is a transcriptional activator in Chlamydia. Proc Natl Acad Sci USA. 2006;103:750–755. doi: 10.1073/pnas.0509690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo IC, Stephens RS. A developmentally regulated two-component signal transduction system in Chlamydia. J Biol Chem. 2003;278:17314–17319. doi: 10.1074/jbc.M212170200. [DOI] [PubMed] [Google Scholar]
- 28.Zhong J, Douglas AL, Hatch TP. Characterization of integration host factor (IHF) binding upstream of the cysteine-rich protein operon (omcAB) promoter of Chlamydia trachomatis LGV serovar L2. Mol Microbiol. 2001;41:451–462. doi: 10.1046/j.1365-2958.2001.02531.x. [DOI] [PubMed] [Google Scholar]
- 29.Dove SL, Darst SA, Hochschild A. Region 4 of sigma as a target for transcription regulation. Mol Microbiol. 2003;48:863–874. doi: 10.1046/j.1365-2958.2003.03467.x. [DOI] [PubMed] [Google Scholar]
- 30.Leibman M, Hochschild A. A sigma-core interaction of the RNA polymerase holoenzyme that enhances promoter escape. EMBO J. 2007;26:1579–1590. doi: 10.1038/sj.emboj.7601612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan M, Engel JN. Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J Bacteriol. 1996;178:6975–6982. doi: 10.1128/jb.178.23.6975-6982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.