Abstract
Northern rivers and lakes process large quantities of organic and inorganic carbon from the surrounding terrestrial ecosystems. These external carbon inputs fuel widespread CO2 supersaturation in continental waters, and the resulting CO2 emissions from lakes and rivers are now recognized as a globally significant loss of terrestrial production to the atmosphere. Whereas the magnitude of emissions has received much attention, the pathways of C delivery and processing that generate these emissions are still not well-understood. CO2 outgassing in aquatic systems has been unequivocally linked to microbial degradation and respiration of terrestrial organic carbon (OC), but the nature (i.e., age and source) of this OC respired in surface waters is largely unknown. We present direct radiocarbon measurements of OC respired by bacteria in freshwater aquatic systems, specifically temperate lakes and streams in Québec. Terrestrial OC fuels much of the respiration in these systems, and our results show that a significant fraction of the respired terrestrial OC is old (in the range of 1,000–3,000 y B.P.). Because the bulk OC pools in these lakes is relatively young, our results also suggest selective removal of an old but highly bioreactive terrestrial OC pool and its conversion to CO2 by bacteria. The respiration of ancient 14C-depleted terrestrial C in northern lakes and rivers provides a biological link between contemporary aquatic carbon biogeochemistry and paleo-conditions in the watershed, and it implies the aquatic-mediated return to the atmosphere of C putatively considered permanently stored, thus challenging current models of long-term C storage in terrestrial reservoirs.
Keywords: aquatic respiration, priming effect, source and age of labile OC, aquatic CO2 emissions, aquatic carbon cycle
Historically, the exchange of C between land and atmosphere was considered to primarily occur through the removal of atmospheric CO2 by photosynthesis and its subsequent evasion from the terrestrial biosphere through plant and soil respiration and biomass burning. Lately, an unrecognized pathway gaining quantitative significance in the exchange of C between land and atmosphere, particularly in northern landscapes, is the emission of terrestrially derived C from inland aquatic systems (1). In this regard, lakes and rivers serve a dual biogeochemical role, acting as both vents for the degassing of soil-generated CO2 and reactors for transforming and mineralizing organic carbon (OC) that leaches out of terrestrial systems (1–6). These processes lead to widespread supersaturation of CO2 in surface waters of northern rivers and lakes (7–9) and significant fluxes of CO2 to the atmosphere (9–11).
Current evidence suggests that aquatic bacterial decomposition of terrestrial OC is one of the major pathways that fuels these CO2 emissions from lakes to the atmosphere (2, 3, 12). This latter process is quantitatively important at the regional scale (1, 9–11), and it represents a net loss of terrestrial OC that is generally unaccounted for in current terrestrial models (for example, not considered by the Intergovernmental Panel on Climate Change) (1, 5, 9–11). Despite its relevance to terrestrial C budgets, little is known of the nature of the terrestrial OC decomposed and ultimately respired in aquatic systems (13). In particular, we do not know if this pathway returns OC that was recently fixed to the atmosphere or if it involves older OC stored in deeper soil layers. The biogeochemical implications of these two scenarios are fundamentally different, because the former represents a contemporary C loop, whereas the latter would imply an uncoupling between contemporary primary production and the emission of C from the landscape (13, 14).
The terrestrial C cycling community has long recognized the significance of determining not only the amount of C returned from soils to the atmosphere but also its age (15). To date, there has been no comparable direct empirical determination of the age of C respired in any aquatic or marine environment. Furthermore, the paradigm of age as an a priori proxy for its microbial bioavailability has been recently challenged in both terrestrial and aquatic systems (13–15). The data presented herein provide fundamental evidence that a paradigm shift is essential to accurately depict the uncoupling of OC stability and age and its chemical recalcitrance.
Results and Discussion
Δ14C of OC Respired by Aquatic Bacterioplankton.
We present here direct determinations of the age of the OC respired in aquatic systems. We measured the Δ14C of bacterial respiratory CO2 recovered from short-term incubations of natural lake and river water to determine the age of OC respired in five temperate lakes and two streams in southern Québec (Canada). The Δ14C of lake respiratory CO2 varied widely, ranging from +94‰ to −172‰ (Table 1), showing that the consumption of OC can be in excess of 1,000 y of age. Aquatic bacteria consume and respire, at any given time, a wide range of available organic substrates, and the respiratory Δ14CO2 integrates the signature of OC substrates respired across a continuum of molecular composition, source, and radiocarbon ages (15). The respiratory CO2 was more depleted in 14C than the bulk particulate C (POC) and dissolved organic C (DOC) pools in the same lakes (Table 1). Given that the ambient OC pools are dominated by relatively modern C, it is likely that respiration is also fueled by these younger substrates, and therefore, the ancient endmember respired must be considerably older than the average values recorded for the bulk respiratory CO2.
Table 1.
Biogeochemical characteristics of sampled water bodies
| Water body (sampling date) | Chl a (μg L−1) | Total phosphorus (μg L−1) | DOC (mg L−1) | Respiration CO2 Δ14C (‰) | Respiration CO2 14C-age* (y B.P.) | POC Δ14C (‰) | DOC Δ14C (‰) | DIC Δ14C (‰) | BRTotal(μg C L−1d−1) | BRTerr(μg C L−1d−1) | Residence time (y) |
| Bran de Scie (9/1) | 5.1 | 16.7 | 7.5 | 87 ± 3 | >Modern | 115 ± 4 | 14 ± 5 | 37 ± 3 | 113 | 16 | 0.03 |
| Bran de Scie (9/1) | 3 ± 4 | >Modern | |||||||||
| Des Monts (9/8) | 2.5 | 12.3 | 7.4 | 94 ± 3 | >Modern | 111 ± 4 | 73 ± 4 | 52 ± 3 | 77 | 40 | 0.01 |
| Des Monts (9/8) | 74 ± 6 | >Modern | |||||||||
| Stukely (9/15) | 2.5 | 6.5 | 5.0 | −94 ± 3 | 740 | 179 ± 4 | 93 ± 4 | 37 ± 4 | 66 | 28 | 4.00 |
| Bowker (9/15) | 2.0 | 5.8 | 2.8 | −48 ± 4 | 345 | 73 ± 3 | 76 ± 4 | −0.4 ± 3 | 48 | 16 | 8.96 |
| Fraser (9/28) | 1.5 | 11.2 | 6.9 | −172 ± 4 | 1,470 | 106 ± 4 | 101 ± 4 | −91 ± 3 | 45 | 25 | 0.36 |
| Bran de Scie (9/22) | — | 10.6 | 4.9 | −116 ± 3 | 935 | 126 ± 4 | 50 ± 4 | 19 ± 5 | 48 | 42 | — |
| Fraser (9/22) | — | 21.1 | 6.1 | −21 ± 3 | 115 | —† | 110 ± 6 | −36 ± 4 | 65 | 54 | — |
*Determined with ReCReS (SI Methods).
†Lost at graphitization.
Respiration of Ancient Terrestrial OC.
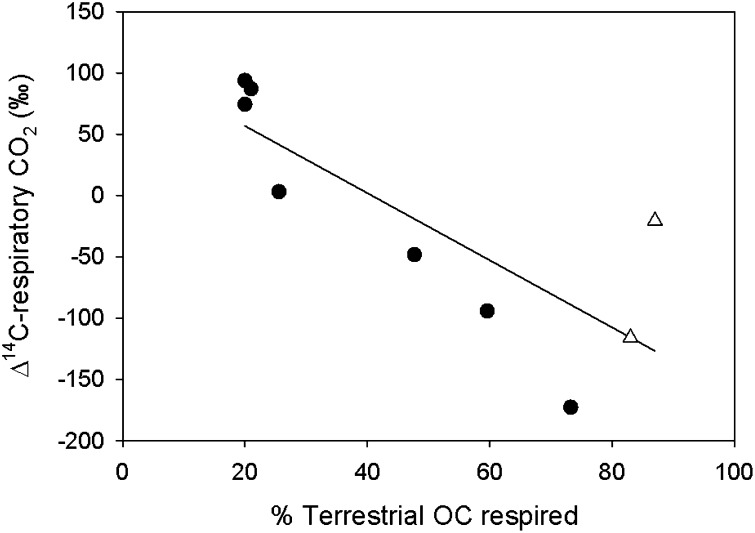
The fact that the old component of respired C is of terrestrial origin is suggested by the strong negative relationship between the radiocarbon age of the respiratory CO2 and the proportion of this C that is of terrestrial origin (Fig. 1), previously determined on the basis of the δ13C signature of this respiratory CO2 (3). Extrapolation of our own results shown in Fig. 1 (100% respired terrestrial OC) places the old endmember at approximately −300‰ Δ14C, well within the range of signatures of ancient bioreactive OC determined in a variety of soil types (−200‰ to −400‰ Δ14C or ∼1,800–4,100 y B.P.) (15–18) and streams and riparian zones (∼4,000–16,663 y B.P.) (13, 19).
Fig. 1.
The Δ14C of bacterial respiratory CO2 as a function of the relative contribution of terrigenous DOC to bacterial respiration (r2 = 0.65; P < 0.005; y = 111.5–2.7 × x). Open triangles denote the two streams sampled.
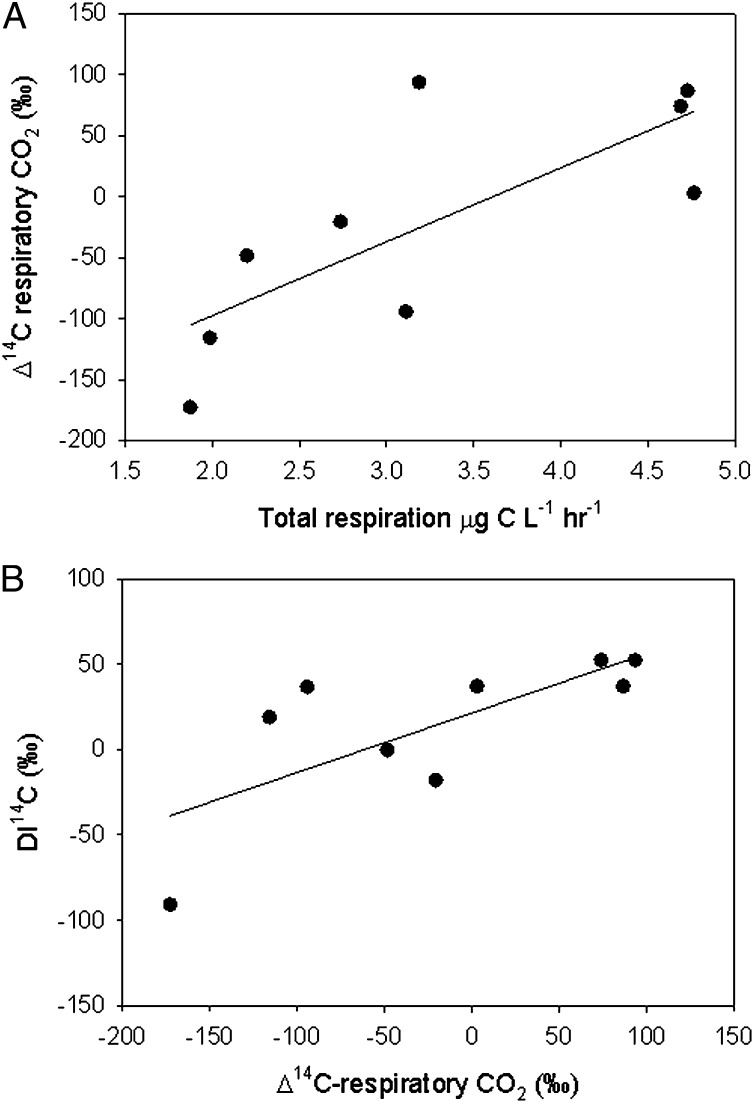
The respiratory CO2 becomes less 14C-depleted (more modern) as total planktonic respiration increases (Fig. 2A), the latter having been determined in parallel for the same samples (3). Because algal OC mirrors the Δ14C of the bulk dissolved inorganic carbon (DIC) pool (3, 20), the Δ14C of algal production in these lakes should be in the same range as the Δ14C of DIC (+2‰ to ±50‰) (Table 1), considerably more enriched in 14C than the respiratory CO2. Because total plankton respiration is a function of primary production in these lakes (3), this pattern suggests a dilution of an ancient terrestrial respiratory OC component with CO2 derived from the respiration of younger OC, most likely of authochthonous origin. Interestingly, there was a significant positive relationship between the Δ14C of the respiratory CO2 and the Δ14C of the bulk DIC pool (Fig. 2B), suggesting that CO2 generated through bacterial respiration of ancient terrestrial OC influences the bulk lake DIC signature, which had been previously hypothesized but to date, never empirically ascertained (21). Our results also suggest a strong selective removal of specific age fractions of the ambient OC pool, which has been reported for other systems (18, 19), and therefore, the age of the C respired cannot a priori be derived from the age of the bulk OC (Table 1).
Fig. 2.
(A) The relationship between the Δ14C signature of respiratory CO2 and the total planktonic respiration measured independently as O2 consumption in parallel incubations (r2 = 0.58; P < 0.01; y = −219.1 + 60.7 × x) and (B) the influence of the Δ14C of respiratory CO2 on the Δ14C values of DIC (r2 = 0.48; P < 0.05; y = −19.5 + 0.3 × x) in lakes.
Uncoupling of Age and Lability in Terrestrial and Aquatic Systems.
Our results agree with recent studies that have shown that age and reactivity of OC are uncoupled in both aquatic (13, 14, 22) and terrestrial (15, 16, 18, 23) environments. The traditional view that the long-term persistence of OM in soils is the direct result of its recalcitrance has been recently challenged (15, 16, 18, 23–25) and replaced by the notion that OC persistence in soils is regulated by a combination of intrinsic factors (related to substrate quality and chemical composition) and extrinsic factors (such as soil physical structure, formation of organo-mineral complexes, and bioenergetic constraints of the soil microbiota) (18, 23). An important corollary of this changing paradigm is that OC persistence in soils is not necessarily a function of its recalcitrance but rather, its stabilization in what has been termed partial refuges (23). Our results clearly suggest that, when this old, mineral-associated OC is transported from soils to the aquatic system, these refugia are lost, and the OC can be readily mineralized, which was reported in the work by Gurwick et al. (13) for the riparian zone. Furthermore, after desorption, the physical movement of OC from nutrient-poor soils to aquatic systems with fresh inputs of nutrients and labile OC in addition to light and various chemical factors may prime the metabolism of previously unreactive OC through the microbial priming effect and/or photosensitization (14, 16, 18, 23–25). It has previously been suggested that bacterial incorporation of preaged terrestrial OC may be facilitated, in part, by the cleavage of photoreactive DOC and the subsequent release and metabolism of low-molecular weight compounds (22, 26, 27). Moreover, exposure of old (>6,000 y B.P.) presumably recalcitrant oceanic DOC to UV light has been shown to enhance its bioavailability to the microbial consortium (28, 29). We suggest an analogous process operating at the aquatic–terrestrial interface, where soil organic matter that has been sequestered from UV light for decades to millennia may be phototransformed and metabolized by aquatic bacteria.
Ancient Terrestrial OC Supports Contemporary Aquatic Respiration.
Presumably, this ancient bioreactive OC is delivered to lakes through ground and surface water inputs. Although the former is most likely the carrier of old OC leached from deep soil horizons (30), our own results as well as the results in the work by Tipping et al. (31) show that inflowing streams may also transfer old bioreactive OC. Our measurements on samples from inflowing headwater streams taken close to the inlet of two of the study lakes showed that the OC respired was also, on average, old (up to 1,000 y B.P.) (Table 1). This stream OC is overwhelmingly terrestrial (3), suggesting that streams in this region deliver to lakes, among other pools, a fraction of ancient but highly reactive terrestrial OC, which is in agreement with previous studies (13, 19, 30, 32).
There are only a handful of studies to date that have attempted to estimate the age of terrestrial OC degraded or emitted from aquatic systems, and these studies have yielded widely contrasting results (SI Results and Discussion and Table S1). For example, the work by Mayorga et al. (20) showed that CO2 efflux in the Amazon basin is supported primarily by modern OC, and the work by Raymond et al. (21) reported a similar result for the temperate York River estuary. At the other end of the spectrum, there is evidence for varying degrees of consumption of ancient terrestrial OC in northern temperate rivers and streams (13, 14, 19, 22). These results highlight the inherent complexity of the pathways of delivery and processing of OC to aquatic systems (33) and the fact that there are major landscape and climatic differences that preclude direct extrapolation between regions. Nevertheless, our results, together with these limited published radiocarbon data examining the age of metabolically bioreactive OC (Table S1), would suggest a pattern of increasing age of C processed by aquatic systems along a latitudinal gradient from the tropics to the boreal (tundra) and subarctic zones.
Significance of Aquatic Respiration Relative to Terrestrial Net Primary Production.
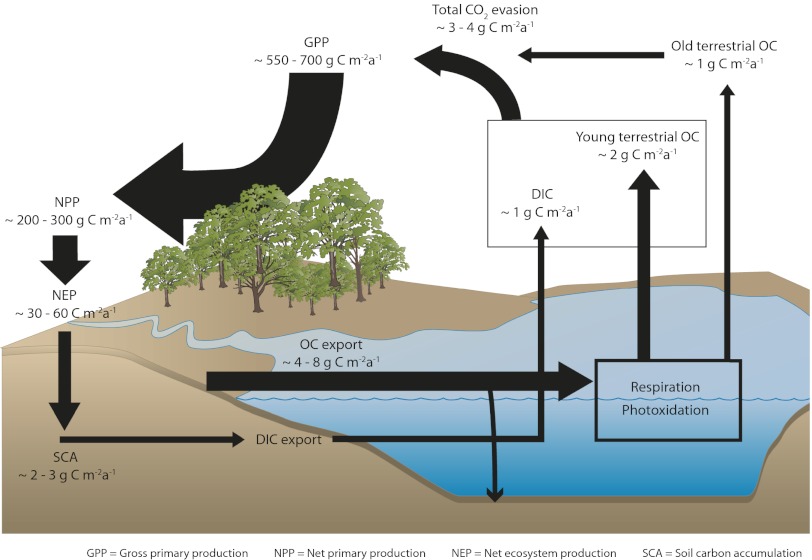
Are the potential fluxes of ancient CO2 generated by this pathway significant relative to other components of the watershed C budget? Our target watershed has been the focus of extensive studies in the past, and in Fig. 3, we have combined the average estimates for the major C fluxes to place our results within a broader context. We combined estimates of ambient bacterial respiration rates carried out in parallel on the same samples with the radiocarbon endmember signatures to partition this flux to estimate the potential CO2 flux from lakes to the atmosphere based on old and modern terrestrial OC. This first-order calculation yields total loss rates of terrestrial OC through lake respiration in the order of 2–4 g C expressed per m−2 of drainage basin during the open water season. By combining this total bacterially mediated loss of terrestrial OC with the results of Eqs. 3, 4, and 5, we estimate that up to 30% (or around 1 g C a−1 per m−2 of drainage basin) may correspond to ancient OC; these estimates are minimum estimates, because they are entirely pelagic and include neither winter nor benthic metabolism, which may further contribute to the respiration of preaged terrestrial OC.
Fig. 3.
The inclusion of bacterially mediated CO2 emission from lakes and rivers in a simplified watershed C budget. The conceptual depiction distinguishes lake C emissions that originate from direct DIC injection to lakes from the bacterial respiration of both recent and ancient terrestrial OC. The model combines published ranges for the main fluxes for northern temperate landscapes (34–38), our own previous estimates of C emissions for these lakes (3), and average estimates of CO2 fluxes originating from bacterial respiration of recent and old terrestrial OC as described in Methods. All rates are expressed per meter2 of total landscape and thus, are comparable.
Net ecosystem production (NEP) in this watershed has been estimated to range between 30 and 60 g C m−2 a−1 (Methods and Fig. 3), which is in agreement with previous estimates for this general region (34). However, long-term rates of soil OC accumulation in this region are considerably lower than NEP (in the order of 2–3 g C m−2 a−1) (35) and the same magnitude as the DOC export from land to lakes that has been measured in similar catchments (between 4 and 8 g C m−2 a−1) (36–38). Our calculations show that the emission of CO2 derived from the respiration of ancient terrestrial OC is small (<5%) relative to contemporary NEP, but it is of the same magnitude as both the DOC export from the drainage basin and the long-term soil C accumulation (Fig. 3). Large discrepancies between NEP, net ecosystem exchange, and net accumulation of OC in soils have been noted before (39, 40), and several explanations have been proposed, including higher soil OC decay rates than generally assumed, larger fire-related losses of surface OC, and biases in the actual estimates of NEP (39). The microbially mediated breakdown of ancient terrestrial OC in lakes and rivers that we report here provides yet another process that results in lower long-term C accumulation rates in the landscape than predicted from contemporary measurements of ecosystem C exchange.
At the regional scale, the mobilization of preaged terrestrial C and its biological degradation in freshwater systems suggest connections and lags between the fixation of C by the terrestrial vegetation and its return to the atmosphere that are more complex than generally accepted. The respiration of modern terrestrial OC in soils (15) and rivers (20) or the movement of OC from one reservoir to another with similar residence times (for example, the transfer from soils to aquatic sediments) (41) are examples of processes that do not result in a net increase in atmospheric CO2. However, if, as posited, the transfer of ancient OC across the terrestrial–aquatic interface enables its consumption and subsequent respiration by bacteria (24, 25), then this C has moved from a dormant into a dynamic and actively exchanging component of the global C cycle, and it signifies a positive feedback on atmospheric CO2 concentrations. In this context, it becomes critical to understand how this key shunt of terrestrial C through the aquatic interface to the atmosphere will be affected by future climate change by alterations to both the pathways of delivery and the nature of the OC reaching lakes (24, 25, 33).
Global Implications of Lake Emissions of Ancient C or Global C Cycle Consequences of Ancient OC Respiration.
The component of lake emissions that involves the degradation and subsequent emission of ancient C represents a net C transfer to the atmosphere analogous to other human-induced and natural processes that contribute to the atmospheric C pool. Among the latter, the emission of ancient C in the form of CO2 and methane from thawing permafrost and thaw lakes in subarctic landscapes has been recently identified as a major natural net C source, and it is currently estimated that the resulting C emissions of old C are in the order of 0.5–1.0 pg C a−1, rendering this pathway globally significant (42). For comparative purposes, the current estimates of global lake CO2 emissions are in the order of 1.4–1.6 pg a−1 (1, 9), and a large fraction of these global emissions originate from northern temperate and boreal lakes (7, 9), similar to those emissions that we have studied here. It is premature to extrapolate our results at the regional or global scale, because we still do not know how the delivery and subsequent degradation in lakes of ancient terrestrial OC vary across the major northern landscape types; however, we can safely assume that our results are not simply a localized feature. The study watershed is representative in many ways of large portions of the temperate and subboreal landscapes of North America and Euroasia. If, indeed, our results were to apply to northern lakes and rivers in general, this finding would imply a flux of ancient C to the atmosphere of potentially the same order of magnitude as the loss of C from thawing permafrost (42) and thus, global significance.
Currently, we do not know how this microbially mediated flux of ancient CO2 from temperate/subboreal lakes has varied over time. Conceivably, the export of old terrestrial OC from soils and its respiration in the aquatic system may have been occurring for millennia, and as such, it is not necessarily a consequence of anthropogenic climate change. In this regard, there is evidence that both the DOC-induced color of water and the actual DOC concentrations have been increasing in many northern landscapes (43–45), including boreal Québec (46). The magnitude and regulation of this browning phenomenon are the focus of intense research and debate but likely involve shifts in the pathways of delivery of terrestrial C to rivers and lakes (43). Whether this browning of inland waters results in positive or negative feedbacks on the mobilization and efflux of ancient C to the atmosphere has not been established.
To summarize, our results suggest an unforeseen role of northern aquatic systems in the landscape: the role of reaction sites for the mineralization of ancient terrestrial OC. This function is critical; whereas the respiration of young terrestrial carbon reinforces a tight land–atmosphere C loop, the release of old OC represents loss of C that was considered removed from atmospheric circulation. There is clearly still much to be learned concerning the interactions between terrestrial and continental aquatic systems in northern landscapes, but our results suggest that lakes must be viewed as an integral part of the landscape C storage/decomposition system, because key reactions in the OC cycle may only occur in surface waters. Our understanding of the terrestrial C cycle and regional OC sources and sinks is, thus, incomplete without the inclusion of aquatic processes, particularly in water-rich northern landscapes.
Methods
Study Site and Sample Collection.
Five lakes and two streams from the Eastern Townships region of southern Québec, Canada (45.50° N, 73.58° W) were sampled during the summer of 2004. The lakes span a large range in morphometry (mean depth = 5–27 m), residence time (0.9–8 y), and trophic status (Table 1 and Table S2). Land cover data obtained from geographic information systems using ArcView were used to characterize the watersheds of the Eastern Township lakes into three primary land use classifications: forested, wetland, and pasture. Overall, the watersheds were dominated primarily by forested land cover, ranging from a maximum of 96% for both Stukely and Bowker to a minimum of 49% in Bran de Scie. In contrast, pastureland was greatest for Bran de Scie and lowest for Stukely and Bowker (3.4% and 4.3%, respectively). Des Monts was characterized by a mix of forest (76%) and pastureland (18%). Only Bran de Scie and Fraser contained a wetland component (0.7% and 0.5%, respectively).
Sample water was collected with a diaphragm pump connected to an acid-rinsed (10% HCl) plastic hose at a depth of 1.0 m to avoid any potential contamination with the surface film and stored in cooled acid-washed containers until processing in the laboratory within 3 h of collection. In the laboratory, a portion of the water was set aside for chlorophyll and nutrient analyses as well as total plankton respiration measurements (see below). The remainder of the water sample was pumped using a peristaltic pump through combusted (525 °C for 4 h) Millipore AE glass fiber filter (1.0-mm nominal pore size) to concentrate particles for radiocarbon analysis. The 1.0-mm filtrate was subsequently passed through an inline (0.2 mm) Gelman filter capsule to remove bacteria, and the filtrate was collected for DOC, DIC (poisoned with HgCl2) radiocarbon analysis, and respiratory CO2 incubations (SI Methods). Samples for POC, DOC, DIC, and respiratory CO2 were subsequently analyzed for natural abundance (δ13C and Δ14C) isotopic analysis (SI Methods). All sample manipulations and incubations were carried out in an ultraclean dedicated laboratory, and we performed regular wipe checks to assess background 13C and 14C levels.
Determination of Total and Bacterial Respiration.
Short-term measurements of total planktonic respiration were carried out in parallel to the Respiratory Carbon Recovery System (ReCReS) experiments and have been reported elsewhere (3). In brief, plankton respiration was determined as O2 consumption in short-term, dark incubations of unfiltered water; O2 concentrations were measured at several time points during the 24 h incubations using membrane inlet spectrometry, and rates were converted to C production using a respiratory quotient of one. We have estimated bacterial respiration from the measured total planktonic rates by calculating the contribution of nonbacterial components and subtracting it from the total. Algal respiration was first calculated by estimating the average rates of primary production for each lake based on the average Chl a concentrations using the empirical model provided in the work by del Giorgio et al. (47) for these same northern temperate lakes and assuming an average algal respiration 25% of primary production. Algal respiration generally ranges from 5% to 10% of photosynthetic biomass maximum (the maximum rate of biomass-specific photosynthesis) (48), and therefore, the above assumption yields a generous estimate of algal respiration (R). We also assumed that metazoan and microzooplankton respiration was equivalent to the respiration of algae, and therefore, the total nonbacterial respiration was estimated as 2× algal respiration. The nonbacterial respiration was subtracted from the total measured R to derive total bacterial respiration (BR). The work by del Giorgio et al. (48) showed that this approach yields realistic estimates of BR based on independent approaches
Proportion of Modern and Preaged Terrigenous and Algal OC Supporting Bacterial Respiration.
The proportion of terrestrial DOC respired by bacteria in these samples had been previously determined in the work by McCallister and del Giorgio (3). In brief, we used the algal endpoint derived from the zooplankton δ13C signature with a terrigenous OC endmember of −27‰ to determine the relative contributions of algal and terrigenous-derived OC sources to BR using a δ13C isotopic, two-source mixing model. The series of two equations and two unknowns is (Eq. 1)
and (Eq. 2)
where CO2 δ 13C values are measured from the ReCReS and f1 and f2 are the relative contributions of terrigenous (CTerr) and algal (CAlgal) sources to the CO2 signature, respectively. We note one exception to this two-endmember model. Des Mont is a shallow lake dominated by submersed aquatic macrophytes, which release significant amounts of highly labile DOC (49) of similar isotopic signature as terrigenous sources (−27.2‰) (3). In this instance, our mass balance most likely overestimates the proportion of terrigenous OC respired in this lake because of the overlap between the terrigenous and aquatic macrophytes and the greater relative lability of macrophyte-derived OC (SI Methods). To correct for this potential overestimate of terrestrially derived OC respired in Des Monts, we calculated the algal contribution to the respiratory 13C–CO2 signature (detailed above in Eqs. 1 and 2). We then partitioned the balance of respiratory CO2 between terrestrially (soil and litter) and macrophyte-derived OC based on their respective labilities (SI Methods).
The radiocarbon values of respiratory CO2 derived were then apportioned between contemporary and preaged terrigenous and algal-derived radiocarbon signatures (Eqs. 3, 4, and 5) for each lake independently. As a first-order approach, the terrestrial OC respired (15, 23) was assumed to include two pools, one modern (Δ14C of 90‰; representative of recent forest litter and surficial soils) and one preaged fraction (Δ14C signature ranges from −200‰ to −400‰), which represents an ancient but nevertheless labile component. The algal signature is equivalent to DI14C (3, 20, 22), and we used the relative proportion of algal vs. total terrestrial OC respired that had been calculated previously from δ13C–respiratory CO2 (3), thus allowing the Δ14C of the total terrigenous component (Δ14Cbulk terrigenous) to be solved according to the formula (Eq. 3)
The Δ14C of the total terrigenous C respired was partitioned between a modern and preaged (−200‰ to −400‰) component with the equations (Eq. 4)
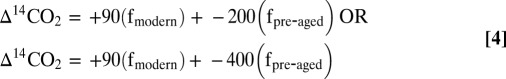 |
and (Eq. 5)
To summarize, we first calculate the radiocarbon signature (Δ14Cbulk terrigenous) of the total (modern + preaged) terrestrial C pool respired using Eq. 3 for each lake independently. This radiocarbon signature is then parsed into the percentage of preaged OC on a modern terrestrial OC signature of +90‰ and a preaged endmember of either −200‰ or −400‰. Based on these endmember signatures, we calculate that an average of 30% of the respiration of terrestrial OC is attributable to the preaged component when the younger (−200‰) endmember is selected and 20% when an older endmember (−400‰) is applied.
Determination of Water Column Bacterial Respiration Fueled by Old and Young Terrigenous DOC and Resulting Water–Air CO2 Fluxes.
We also partitioned the terrestrially derived component of water column bacterial respiration into a fraction based on old terrestrial C and a fraction based on young terrestrial C by expressing bacterial respiration rates of terrigenous C (Table 1) per meter−2 of drainage basin for each lake and combining these rates with the results of the isotopic mass balance described above (Eqs. 3, 4, and 5). The contributions of modern and old OC to the terrestrially supported component of bacterial respiration derived from the mass balance averaged 30%, and we used these average values for all five lakes (Fig. 3). We then integrated the resulting bacterial respiration rates over the depth of the epilimnia of each lake to derive the potential areal CO2 efflux rates generated by the respiration of terrigenous OC and more specifically, the respiration of old terrestrial OC, and we express these CO2 fluxes as grams C per meter−2 of drainage basin per year (assuming 210 ice-free days); therefore, the rates are comparable with other components of the regional terrestrial C budget.
Watershed C Balance.
Terrestrial primary production has been modeled in detail for our entire study watershed using an adapted version of the TRIPLEX model (50) as part of an ongoing collaborative research project. This spatially explicit model is based on vegetation type, climate, and soils, and it yielded estimates of gross and net primary production and net ecosystem production that are in good agreement with regional-scale models of primary production (34, 40). DOC export from comparable northern temperate and boreal regions has been reported to range from 4 to 8 g C m−2 a−1 (36–38). Previous studies in this watershed also reported an annual average C evasion from these lakes of ∼4 g C m−2 (of watershed) a−1 (3) and suggested that up to 75% of this total flux (or up to 3 g C m−2 a−1) could be generated by the biological or photochemical degradation of DOC, the remaining caused by direct injection of DIC (CO2) to the lake. We combined these estimates with the estimate of CO2 generated by the respiration of old terrestrial OC to derive an approximate contribution of the latter pathway to total lake CO2 evasion at the watershed scale (Fig. 3).
Supplementary Material
Acknowledgments
We thank F. Guillemette for help in the laboratory and the field, P. Middlestead of the Hatch Laboratory of the University of Ottawa for help with isotopic sample processing, and the staff at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry (LLNL CAMS) for assistance with radiocarbon analyses. We also thank J. Cole and M. Pace for insightful comments. This work was supported by National Science Foundation Grant DEB 0820725 (to S.L.M.) and grants from the National Science and Engineering Research Council of Canada, Hydro-Québec, and the Fonds québécois de la recherche sur la nature et les technologies (to P.A.d.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207305109/-/DCSupplemental.
References
- 1.Cole JJ, et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems. 2007;10:171–184. [Google Scholar]
- 2.Jonsson AG, Karlsson J, Jansson M. Sources of carbon dioxide supersaturation in clearwater and humic lakes in northern Sweden. Ecosystems. 2003;6:224–235. [Google Scholar]
- 3.McCallister SL, del Giorgio PA. Direct measurement of the δ13C signature of carbon respired by bacteria in lakes: Linkages to potential carbon sources, ecosystem baseline metabolism, and CO2 fluxes. Limnol Oceanogr. 2008;53:1204–1216. [Google Scholar]
- 4.Kortelainen P, et al. Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Glob Change Biol. 2006;12:1554–1567. [Google Scholar]
- 5.Richey JE, Melack JM, Aufdenkampe AK, Ballester VM, Hess LL. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature. 2002;416:617–620. doi: 10.1038/416617a. [DOI] [PubMed] [Google Scholar]
- 6.Dillon PJ, Molot LA. Dissolved organic and inorganic carbon mass balances in central Ontario lakes. Biogeochemistry. 1997;36:29–42. [Google Scholar]
- 7.Cole JJ, Caraco NF, Kling GW, Kratz TK. Carbon dioxide supersaturation in the surface waters of lakes. Science. 1994;265:1568–1570. doi: 10.1126/science.265.5178.1568. [DOI] [PubMed] [Google Scholar]
- 8.Kling GW, Kipphut GW, Miller MC. Arctic lakes and streams as gas conduits to the atmosphere: Implications for tundra carbon budgets. Science. 1991;251:298–301. doi: 10.1126/science.251.4991.298. [DOI] [PubMed] [Google Scholar]
- 9.Tranvik LJ, et al. Lakes and impoundments as regulators of carbon cycling and climate. Limnol Oceanogr. 2009;54:2298–2314. [Google Scholar]
- 10.Algesten G, et al. Role of lakes for organic carbon cycling in the boreal zone. Glob Change Biol. 2004;10:141–147. [Google Scholar]
- 11.Hanson PC, et al. A model of carbon evasion and sedimentation in temperate lakes. Glob Change Biol. 2004;10:1285–1298. [Google Scholar]
- 12.Karlsson J, Jansson M, Jonsson A. Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol Oceanogr. 2007;52:603–608. [Google Scholar]
- 13.Gurwick NP, et al. Mineralization of ancient carbon in the subsurface of riparian forests. J Geophys Res. 2008 doi: 10.1029/2007JG000482. [DOI] [Google Scholar]
- 14.Cole JJ, Caraco NF. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar Freshw Res. 2001;52:101–110. [Google Scholar]
- 15.Trumbore S. Radiocarbon and soil carbon dynamics. Annu Rev Earth Planet Sci. 2009;37:47–66. [Google Scholar]
- 16.Heimann M, Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature. 2008;451:289–292. doi: 10.1038/nature06591. [DOI] [PubMed] [Google Scholar]
- 17.Rumpel C, Kogel-Knabner I, Bruhn F. Vertical distribution, age, and chemical composition of organic carbon in town forest soils of different pedogenesis. Org Geochem. 2002;33:1131–1142. [Google Scholar]
- 18.Fontaine S, et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature. 2007;450:277–280. doi: 10.1038/nature06275. [DOI] [PubMed] [Google Scholar]
- 19.Billett MF, Garnett MH, Harvey F. UK peatland streams release old carbon dioxide to the atmosphere and young dissolved organic carbon to rivers. Geophys Res Lett. 2007;34:L23401. [Google Scholar]
- 20.Mayorga E, et al. Young organic matter as a source of carbon dioxide outgassing from Amazonian rivers. Nature. 2005;436:538–541. doi: 10.1038/nature03880. [DOI] [PubMed] [Google Scholar]
- 21.Raymond PA, et al. Controls on the variability of organic matter and dissolved inorganic carbon ages in northeast US rivers. Mar Chem. 2004;92:353–366. [Google Scholar]
- 22.McCallister SL, Bauer JE, Cherrier J, Ducklow HW. Assessing the sources and ages of organic matter supporting estuarine bacterial production: A novel multiple isotope (δ13C, δ15N, Δ14C) approach. Limnol Oceanogr. 2004;49:1687–1702. [Google Scholar]
- 23.Ekschmitt K, Liu M, Vetter S, Fox O, Wolters V. Strategies used by soil biota to overcome soil organic matter stability—why is dead organic matter left over in the soil? Geoderma. 2005;128:167–176. [Google Scholar]
- 24.Guenet B, Danger M, Abbadie L, Lacroix G. Priming effect: Bridging the gap between terrestrial and aquatic ecology. Ecology. 2010;91:2850–2861. doi: 10.1890/09-1968.1. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi TS. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc Natl Acad Sci USA. 2011;108:19473–19481. doi: 10.1073/pnas.1017982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCallister SL, Bauer JE, Kelly J, Ducklow HW. Effects of combined photochemical and microbial decomposition on estuarine dissolved organic C, N, and P bioavailability and bacterial metabolism. Aquat Microb Ecol. 2005;40:25–35. [Google Scholar]
- 27.Cherrier J, Bauer JE, Druffel ERM, Coffin RB, Chanton JP. Radiocarbon in marine bacteria: Evidence for the ages of assimilated carbon. Limnol Oceanogr. 1999;44:730–736. [Google Scholar]
- 28.Mopper K, et al. Photochemical degradation of dissolved organic-carbon and its impact on the oceanic carbon-cycle. Nature. 1991;353:60–62. [Google Scholar]
- 29.Kieber DJ, McDaniel J, Mopper K. Photochemical source of biological substrates in seawater: Implications for carbon cycling. Nature. 1989;341:637–639. [Google Scholar]
- 30.Schiff SL, et al. Export of DOC from forested catchments on the Precambrian Shield of Central Ontario: Clues from δ13C and Δ14C. Biogeochemistry. 1997;36:43–65. [Google Scholar]
- 31.Tipping E, Billett MF, Bryant CL, Buckingham S, Thacker SA. Sources and ages of dissolved organic matter in peatland streams: Evidence from chemistry mixture modelling and radiocarbon data. Biogeochemistry. 2010;100:121–137. [Google Scholar]
- 32.Sanderman J, Lohse KA, Baldock JA, Amundson R. Linking soils and streams: Sources and chemistry of dissolved organic matter in a small coastal watershed. Water Resour Res. 2009;45:W03418. doi: 10.1029/2008WR006977. [DOI] [Google Scholar]
- 33.Schindler DW, et al. Climate-induced changes in the dissolved organic carbon budgets of boreal lakes. Biogeochemistry. 1997;36:9–28. [Google Scholar]
- 34.Liu J, Chen M, Cihlar J, Chen W. Net primary productivity mapped for Canada at 1-km resolution. Glob Ecol Biogeogr. 2002;11:115–129. [Google Scholar]
- 35.Tremblay S, Ouimet R, Houle D. Prediction of organic carbon content in upland forest soils of Quebec, Canada. Can J For Res. 2002;32:903–914. [Google Scholar]
- 36.Rasmussen JB, Godbout L, Schallenberg M. The humic content of lake water and its relationship to watershed and lake morphometry. Limnol Oceanogr. 1989;34:1336–1343. [Google Scholar]
- 37.Moore TR. Dissolved organic carbon in a northern boreal landscape. Global Biogeochem Cycles. 2003 doi: 10.1029/2003GB002050. [DOI] [Google Scholar]
- 38.Eckhardt BW, Moore TR. Controls on dissolved organic carbon concentrations in streams, southern Québec. Can J Fish Aquat Sci. 1990;47:1537–1544. [Google Scholar]
- 39.Gaudinski JB, Trumbore S, Davidson EA, Zheng SH. Soil carbon cycling in temperate forest: Radiocarbon-based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry. 2000;51:33–69. [Google Scholar]
- 40.Luyssaert S, et al. CO2 balance of boreal, temperate and tropical forests derived from a global database. Glob Change Biol. 2007;13:2509–2537. [Google Scholar]
- 41.Dickens AF, Gélinas Y, Masiello CA, Wakeham SG, Hedges JI. Reburial of fossil organic carbon in marine sediments. Nature. 2004;427:336–339. doi: 10.1038/nature02299. [DOI] [PubMed] [Google Scholar]
- 42.Schuur EAG, et al. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 2009;459:556–559. doi: 10.1038/nature08031. [DOI] [PubMed] [Google Scholar]
- 43.Chapman PJ, et al. Changes in water colour between 1986-2006 in the headwaters of the River Nidd, Yorkshire, UK. Biogeochemistry. 2010;101:281–294. [Google Scholar]
- 44.Zhang J, et al. Long-term patterns of dissolved organic carbon in lakes across eastern Canada: Evidence of a pronounced climate effect. Limnol Oceanogr. 2010;55:30–42. [Google Scholar]
- 45.Schindler DW, et al. The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Ontario. Limnol Oceanogr. 1996;41:1004–1017. [Google Scholar]
- 46.Couture S, Houle D, Gagnon C. Increases of dissolved organic carbon in temperate and boreal lakes in Quebec, Canada. Environ Sci Pollut Res Int. 2012;19:361–371. doi: 10.1007/s11356-011-0565-6. [DOI] [PubMed] [Google Scholar]
- 47.del Giorgio PA, Cole JJ, Caraco NF, Peters RH. Linking planktonic biomass and metabolism to net gas fluxes in northern temperate lakes. Ecology. 1999;80:1422–1431. [Google Scholar]
- 48.del Giorgio PA, Pace ML, Fisher D. Relationship of bacterial growth efficiency to spatial variation in bacterial activity in the Hudson River. Aquat Microb Ecol. 2006;45:55–67. [Google Scholar]
- 49.Demarty M, Prairie YT. In situ dissolved organic carbon (DOC) release by submerged macrophyte-epiphyte communities in southern Quebec lakes. Can J Fish Aquat Sci. 2009;66:1522–1531. [Google Scholar]
- 50.Peng CH, Liu JX, Dang QL, Apps MJ, Jiang H. TRIPLEX: A generic hybrid model for predicting forest growth and carbon and nitrogen dynamics. Ecol Modell. 2002;153:109–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.