Abstract
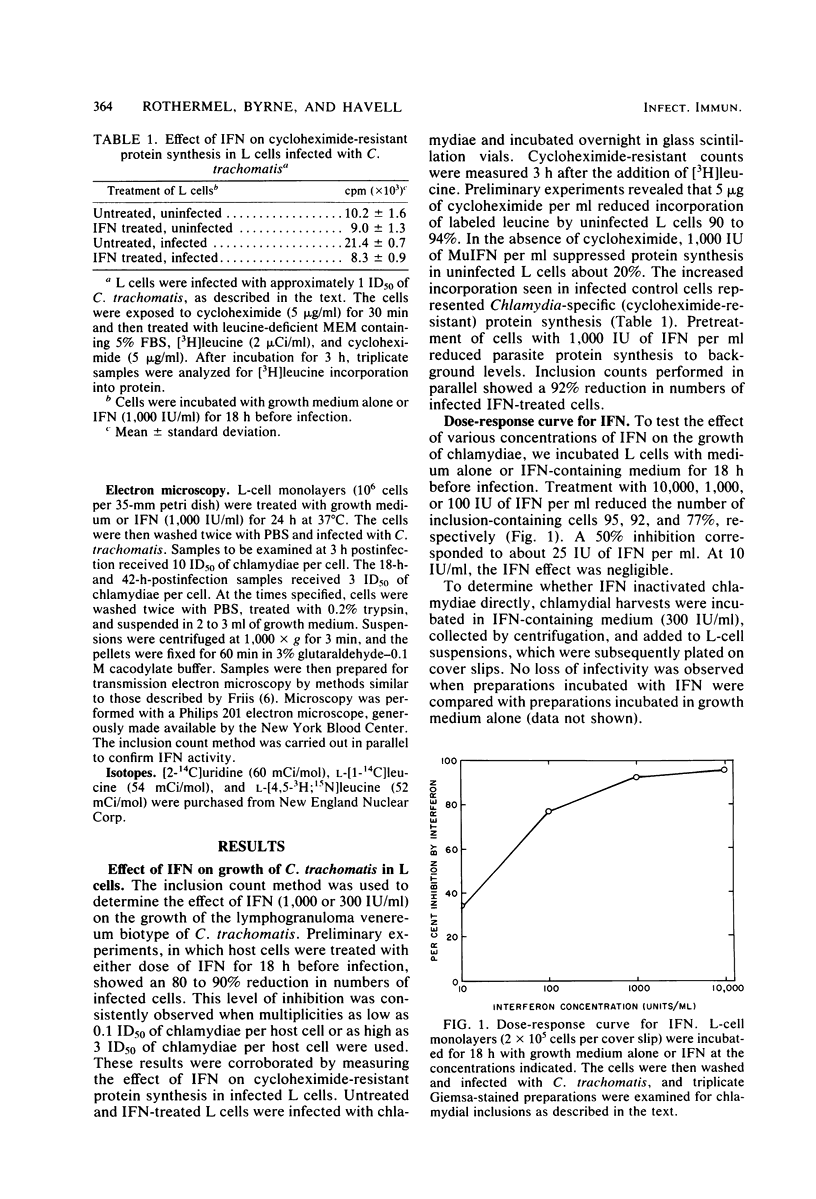
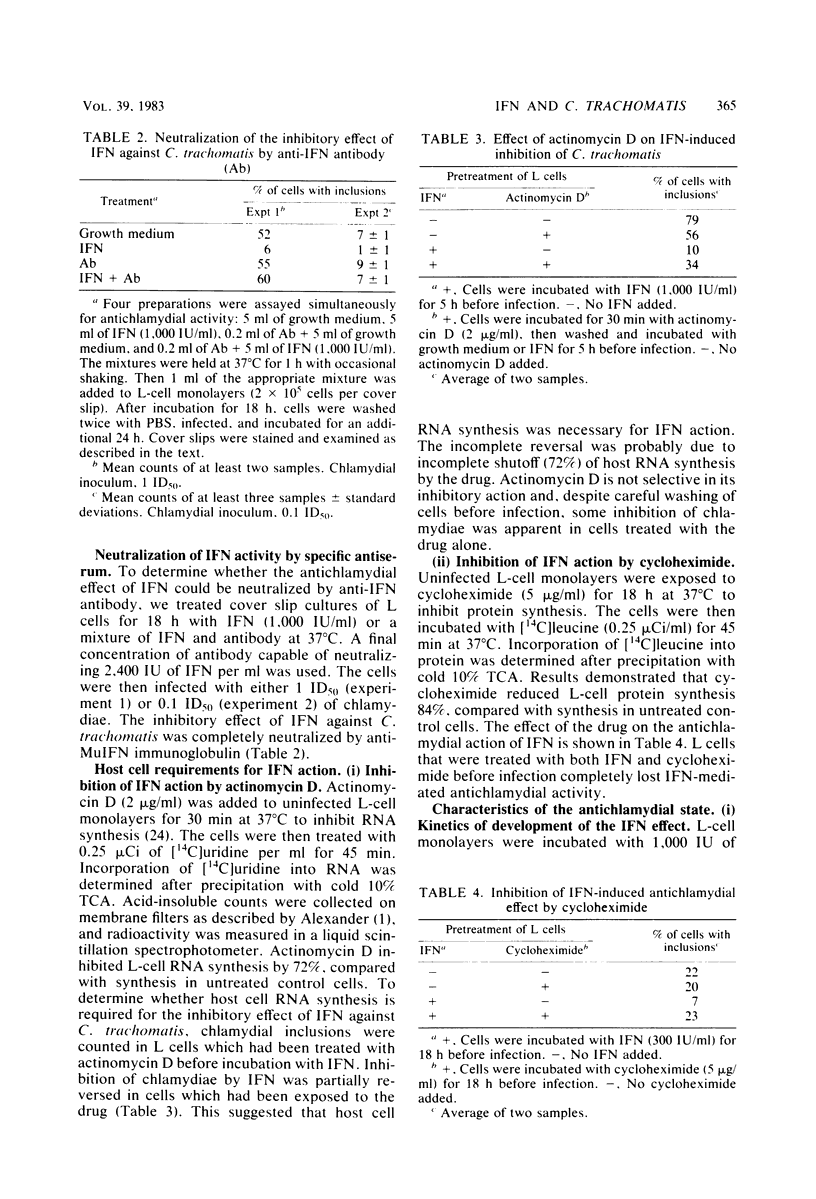
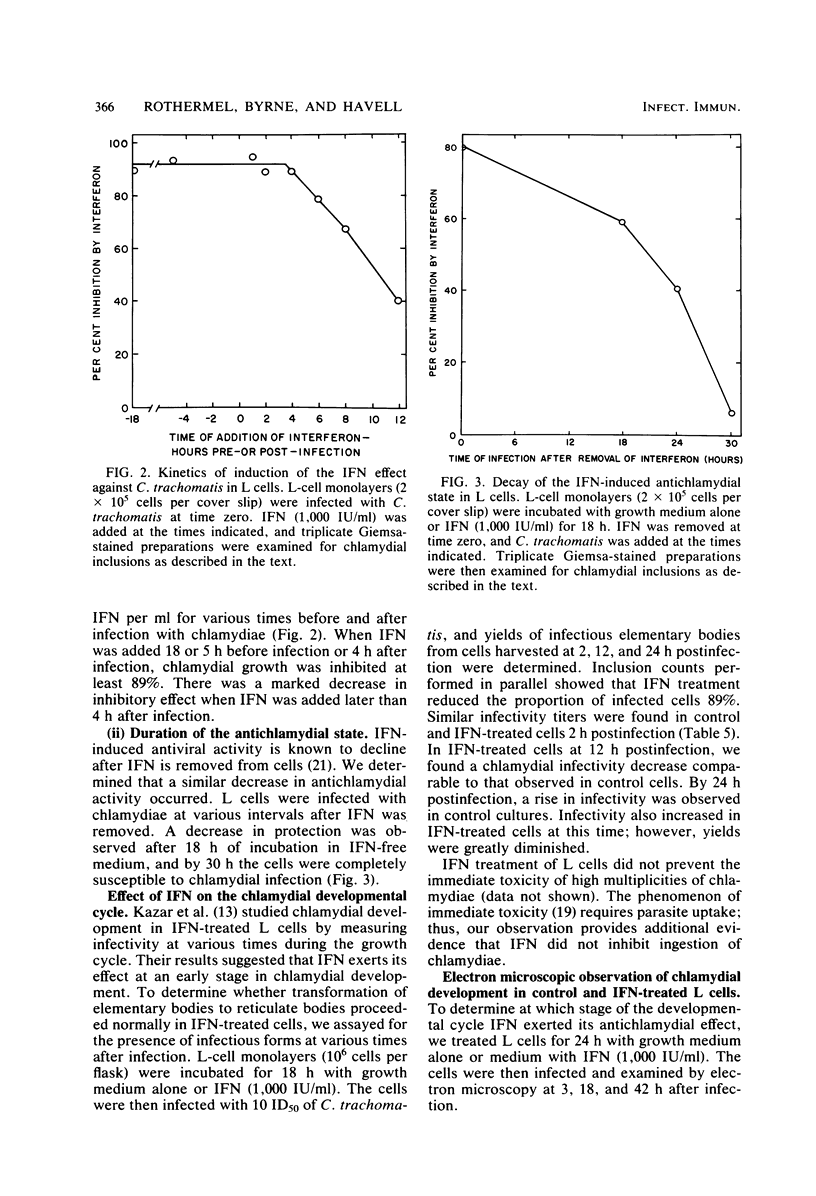
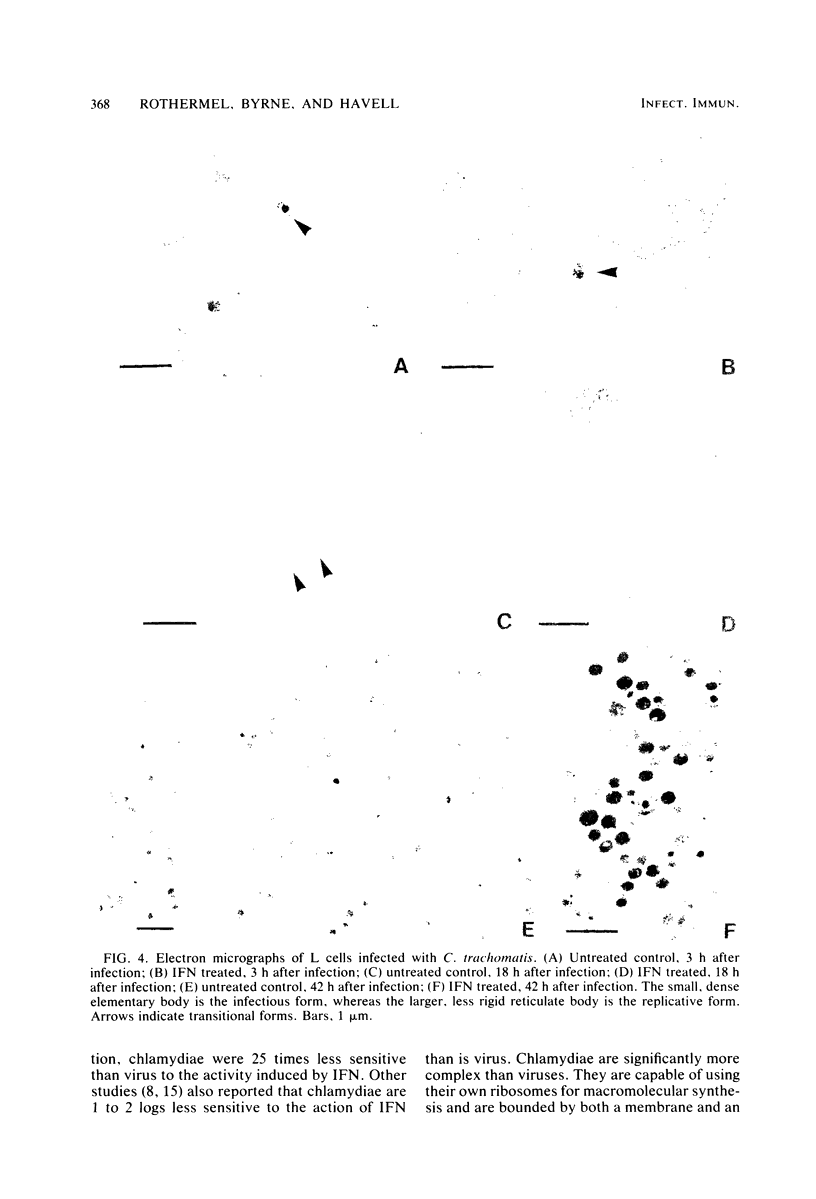
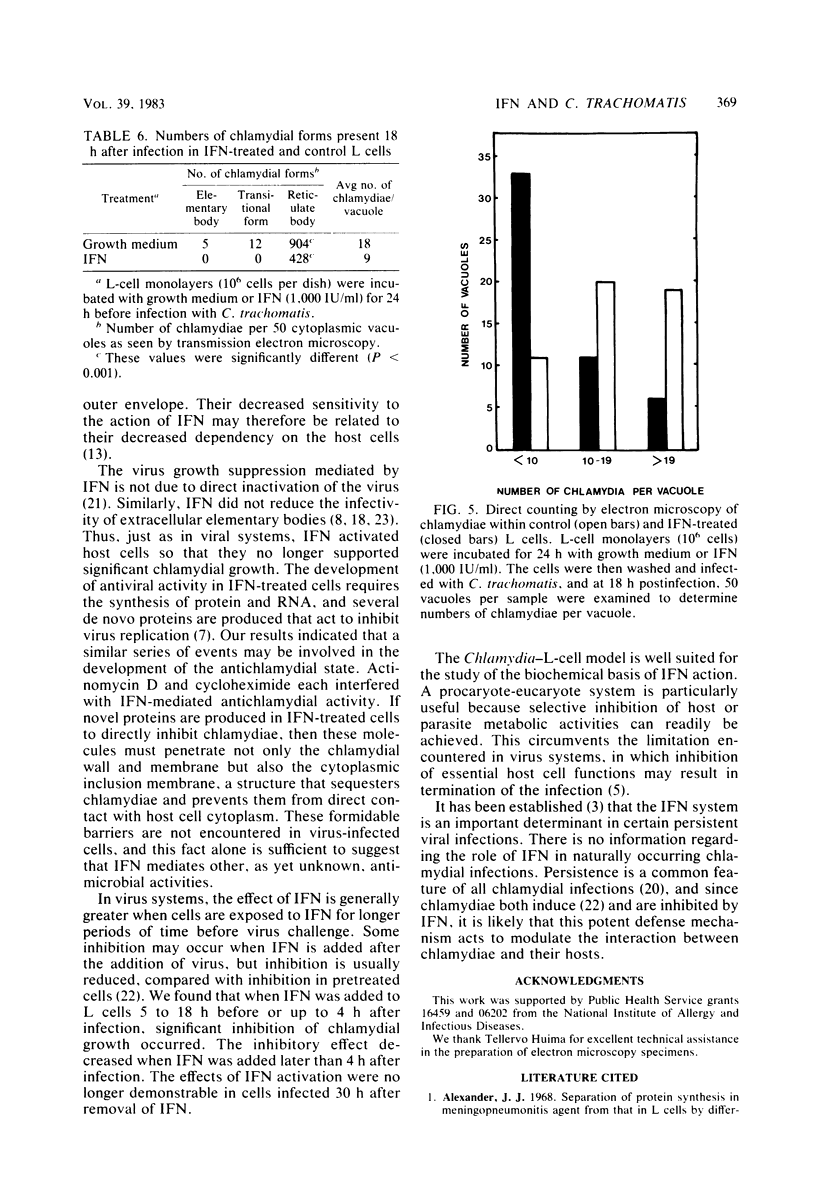
The effect of murine interferon on the growth of the lymphogranuloma venereum biotype of Chlamydia trachomatis (strain 440L) in murine fibroblasts (L cells) was examined. Treatment of infected cell cultures with interferon caused a reduction in the number of inclusion-bearing cells as seen by light and electron microscopy and a decrease in yields of chlamydiae as determined by infectivity assays. Interferon also inhibited cycloheximide-resistant (chlamydia-specific) protein synthesis in infected cells. The interferon effect was dose dependent, with 80 to 90% inhibition occurring at concentrations of greater than 200 IU/ml. The inhibitory effect was neutralized by anti-murine interferon globulin. Interferon did not inactivate extracellular chlamydiae, and both host cell RNA and protein synthesis were required for the development of the interferon-induced antichlamydial state. Inhibition of chlamydial growth by interferon was demonstrable in cells treated 18 h before infection or up to 4 h after infection. Cells infected after interferon was removed exhibited an antichlamydial activity decline which was complete by 30 h after interferon removal. We show that interferon treatment did not affect either entry of chlamydiae into host cells or chlamydial conversion to reticulate bodies but rather caused a reduction in the rate of reticulate body replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Minks M. A. The interferon renaissance: molecular aspects of induction and action. Microbiol Rev. 1981 Jun;45(2):244–266. doi: 10.1128/mr.45.2.244-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Effect of interferon on TRIC agents and induction of interferon by TRIC agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1115–1119. doi: 10.1016/0002-9394(67)94092-5. [DOI] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M., Lu Y. K. Induction of interferon by the Bour strain of trachoma in HeLa 229 cells. Am J Ophthalmol. 1967 May;63(5 Suppl):1110–1115. doi: 10.1016/0002-9394(67)94091-3. [DOI] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg K. R., Horoschak K. D., Moulder J. W. Toxicity of low and moderate multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1977 Nov;18(2):531–541. doi: 10.1128/iai.18.2.531-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozikowski E. H., Hahon N. Quantitative assay of interferon by the immunofluorescent cell-counting technique. J Gen Virol. 1969 Apr;4(3):441–443. doi: 10.1099/0022-1317-4-3-441. [DOI] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Mordhorst C. H., Reinicke V., Schonne E. In ovo inhibition by concentrated chick interferon of the growth of TRIC agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1107–1109. doi: 10.1016/0002-9394(67)94090-1. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueltenfuss E. A., Pollard M. Cytochemical Assay of Interferon Produced by Duck Hepatitis Virus. Science. 1963 Feb 15;139(3555):595–596. doi: 10.1126/science.139.3555.595. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]