Making sperm in a culture dish has been a long-term goal of reproductive biologists. In 2010, a group of investigators from Yokohama, Japan, reinvestigated an organ culture system that was originally used for testicular tissue in the 1960s (1–3). By using better molecular markers, they were able to demonstrate that some cells in this culture system underwent complete meiosis to form round spermatids (1). This work was followed by a study from the same research group that showed that the efficiency of the completion of meiosis and even the formation of flagellated cells was increased by the addition of knockout serum replacement to the organ culture medium (4). The investigators also showed that cryopreserved testicular tissue could be used in these cultures, and healthy, reproductively competent murine offspring were obtained after microinsemination using the haploid cells produced in culture. This technique was further extended when it was demonstrated that spermatogonia grown in culture in the absence of somatic cells could be transplanted into recipient testes and proceed through meiosis to form reproductively functional haploid gametes (5). Sato et al. now expand their observations again by showing that their system can be used to correct spermatogenic defects in vitro (6). The kit ligand (KITL), a cytokine type of growth factor, is produced by Sertoli cells in the testis and must interact with the kit receptor found on differentiating spermatogonia and spermatocytes for spermatogenesis to be completed (7, 8). These authors report that the addition of recombinant KITL and colony stimulating factor-1 (CSF-1) to the medium of organ cultures of testis tissue derived from mice deficient in the production of KITL induced spermatogenesis to proceed through meiosis (6). The efficiency of male gamete production in these organ cultures, and in all the earlier studies described here, is very low. Only a few tubules in the cultured testes contain spermatogenic cells, and the cells completing meiosis and becoming 1N are rare. However, haploid cells recovered from the cultures of normal testes, testes that received transplanted cultured spermatogonia, and testes from mutant mice treated with KITL and CSF-1 were then used to produce healthy offspring by microinsemination, thereby establishing proof of principle.
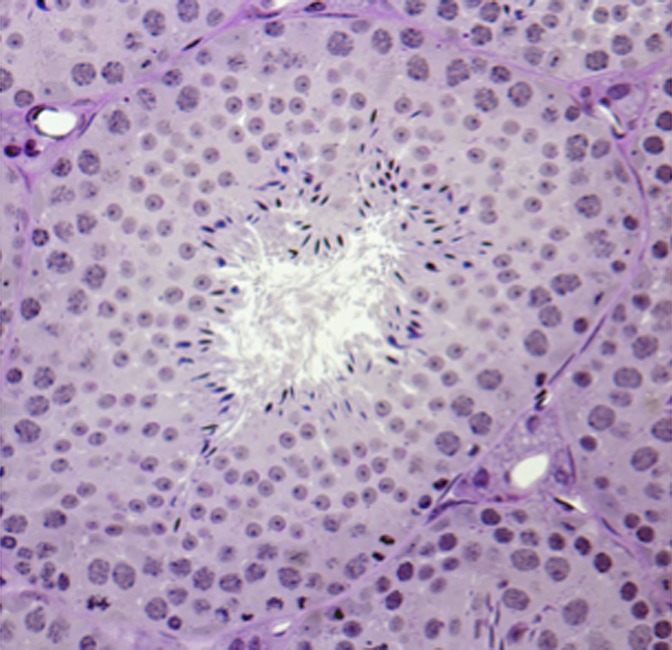
Spermatogenesis in mammals takes place in the seminiferous tubules of the testis and is under strict endocrine and exocrine control (9). The seminiferous tubules contain somatic cells (Sertoli cells and peritubular myoid cells) in addition to the germ cell component (Fig. 1). Spermatozoa originate from spermatogenic stem cells that give rise to progenitor cells that expand through mitosis, become haploid through meiosis, and differentiate into gametes with a highly specialized morphology and function. Spermatogenic stem cells may give rise to as many as 1,000 sperm per second throughout the reproductive lifetime of a human male. The success of spermatogenesis is dependent on the complex architecture of the seminiferous tubules and the appropriate and timely interactions between somatic cells and the many different stages of developing germinal cells.
Fig. 1.
Cross-section of mouse seminiferous tubule showing the complex architecture and the variety of different cell types that interact during spermatogenesis. Each tubule contains Sertoli cells, undifferentiated and differentiating spermatogonia, spermatocytes undergoing meiosis, spermatids undergoing morphological remodeling, and, finally, spermatozoa released into the tubule lumen.
Producing male gametes from spermatogenic stem cells in culture has proven to be a difficult undertaking. Generally, cells destined to become sperm in vivo fail to proceed through meiosis when placed in culture (10). These difficulties have made it necessary to use in vitro techniques such as organ culture that allow the original testicular architecture and complex cellular relationships to, in part, be maintained. However, even under these more in vivo-like conditions, germ cells generally fail to undergo meiosis. The observation that the addition of knockout serum replacement to these cultures allowed some cells to undergo meiosis and a few to proceed to form haploid round spermatids was an important step toward being able to generate healthy gametes in culture.
Spermatogonial stem cells can be maintained in culture, but they will not proceed through meiosis. When cultured spermatogonial stem cells are transplanted into recipient testes, they colonize the seminiferous tubules and efficiently form spermatozoa as long as they are maintained in the animal (11). The inability to coax germ cells cultured alone through meiosis and the organ culture-based successes in the production of gametes ex vivo demonstrate that the testicular architecture or the interplay between germ and somatic cells in the form of growth or endocrine factors and structural support are essential elements in this process. Because of its limitations, this organ culture approach, in its current version, is unlikely to be clinically useful. Live murine offspring can be produced by microinsemination by using the haploid cells produced in vitro, but the efficiency of gamete production is very low, and the biochemical and genetic integrity of the average haploid cells produced in suboptimal conditions are unknown. The major usefulness of this approach lies in the ability to dissect out which components of the system are necessary for gamete production and to determine what external factors can improve the efficiency of completion of meiosis. For example, although genetics has previously shown the requirement for KITL during spermatogenesis, Sato et al. (6) are able to effectively ameliorate the lesion by using soluble KITL. In addition, the results with the use of CSF-1 provide new information on the potential synergistic actions of these two cytokines.
Successful spermatogenesis requires the orchestrated expression of hundreds of unique testicular gene products. The function of some of these gene products is known but many remain on array spreadsheets as “unknown” or “unannotated.” Hidden among these genes are likely required products for male fertility or potential targets for contraception. At some point in the future, it is likely that the intricacies and mysteries for efficiently making functional male gametes from human or animal stem cells in a culture dish will all be revealed. Ideally, the efficient production of spermatozoa in culture from spermatogenic stem cells—or, more generally, from pluripotent stem cells—has important applications. Human spermatogenic stem cells in culture could be a source of gametes from individuals with a number of infertility issues. As described by Sato et al. (6), defects in endocrine or paracrine factors could be corrected, and the potential exists for transgenesis to correct any known genetic defect. Spermatogenic stem cell cultures could be used to replace stem cells destroyed by toxic agents or anticancer drugs. This may be a technique useful in the expansion and the maintenance of germplasm from endangered species. The ability to maintain cultures of spermatogenic stem cells from a variety of animal species that can be induced to efficiently undergo meiosis and can be genetically modified by transgenesis opens a world of possibilities to many groups, including research scientists, clinicians, and livestock producers.
Footnotes
The author declares no conflict of interest.
See companion article on page 16934.
References
- 1.Gohbara A, et al. In vitro murine spermatogenesis in an organ culture system. Biol Reprod. 2010;83(2):261–267. doi: 10.1095/biolreprod.110.083899. [DOI] [PubMed] [Google Scholar]
- 2.Steinberger A, Steinberger E, Perloff WH. Mammalian testes in organ culture. Exp Cell Res. 1964;36:19–27. doi: 10.1016/0014-4827(64)90156-9. [DOI] [PubMed] [Google Scholar]
- 3.Steinberger E, Steinberger A, Perloff WH. Studies on growth in organ culture of testicular tissue from rats of various ages. Anat Rec. 1964;148:581–589. doi: 10.1002/ar.1091480409. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472. doi: 10.1038/ncomms1478. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, et al. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci USA. 2012;109:16934–16938. doi: 10.1073/pnas.1211845109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: The role of c-kit and its ligand SCF. Development. 2000;127(10):2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 8.Vincent S, et al. Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: A Kit-KL interaction critical for meiosis. Development. 1998;125(22):4585–4593. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 9.Jan SZ, et al. Molecular control of rodent spermatogenesis. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbadis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Hunter D, Anand-Ivell R, Danner S, Ivell R. Models of in vitro spermatogenesis. Spermatogenesis. 2012;2(1):32–43. doi: 10.4161/spmg.19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–282. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]