Abstract
The proneural basic helix–loop–helix (bHLH) transcription factor neurogenin1 (Neurog1) plays a pivotal role in neuronal differentiation during mammalian development. The spatiotemporal control of the Neurog1 gene expression is mediated by several specific enhancer elements, although how these elements regulate the Neurog1 locus has remained largely unclear. Recently it has been shown that a large number of enhancer elements are transcribed, but the regulation and function of the resulting transcripts have been investigated for only several such elements. We now show that an enhancer element located 5.8–7.0 kb upstream of the mouse Neurog1 locus is transcribed. The production of this transcript, designated utNgn1, is highly correlated with that of Neurog1 mRNA during neuronal differentiation. Moreover, knockdown of utNgn1 by a corresponding short interfering RNA inhibits the production of Neurog1 mRNA in response to induction of neuronal differentiation. We also found that production of utNgn1 is suppressed by polycomb group (PcG) proteins, which inhibit the expression of Neurog1. Our results thus suggest that a noncoding RNA transcribed from an enhancer element positively regulates transcription at the Neurog1 locus.
The mammalian central nervous system (CNS) is composed of a great variety of neurons and glial cells, all of which must be generated from multipotential neural precursor cells (NPCs) in the correct number and at specific times and locations during embryogenesis (1, 2). Proneural basic helix–loop–helix (bHLH) proteins play central roles in the specification of neuronal fate and the subsequent differentiation processes (3). Among these proteins, neurogenin1 (Neurog1) is a key regulator of neuronal fate specification in various regions of the neural tube, including those that give rise to the neocortex, midbrain, hindbrain, and dorsal and ventral spinal cord of the CNS, as well as in portions of the peripheral nervous system (4–7). In the developing mouse neocortex, ablation of Neurog1 along with the related protein neurogenin2 (Neurog2) results in the loss of deep-layer neurons (8), and conversely, overexpression of Neurog1 results in premature neuronal differentiation of NPCs at the expense of glial fate (9, 10). These observations suggest that expression of the Neurog1 and Neurog2 genes must be tightly controlled in a spatiotemporal manner during development to ensure the generation of specific subsets of neurons and establishment of the fine architecture of the CNS.
Spatiotemporal control of gene expression is generally mediated by regulatory elements including enhancers. Previous studies have revealed several enhancer elements that control expression of the Neurog1 gene, including the lateral stripe element (LSE), the anterior neural plate element (ANPE), and the LATE; which were originally identified as tissue-specific enhancers in zebrafish and found to be conserved among various vertebrate genomes including the mouse genome (11–13). Deletion of the 4-kb mouse genomic region including LATE and ANPE significantly reduced overall expression of Neurog1 in a transgenic reporter assay (14). Therefore, although this 4-kb region has been implicated in tissue-specific regulation, it might also contain an essential general enhancer or locus control region. It has remained elusive, however, how these regions regulate Neurog1 expression and how their activity is regulated.
Several molecules have been implicated in the regulation of Neurog1 expression during neocortical development. For instance, Wnt signaling induces expression of Neurog1 via β-catenin and T cell specific transcription factor (TCF)/lymphoid enhancer binding factor (LEF) transcription factors in neocortical NPCs (15, 16). The homeodomain transcription factor Pax6, which contributes to neocortical regional identity, also positively regulates expression of Neurog1 (12, 17). In contrast, Polycomb group (PcG) proteins suppress the Neurog1 promoter during the gliogenic stage of neocortical development when the neurogenic potential of NPCs is restricted (18). Whether or how these molecules affect the enhancers of Neurog1 has remained unclear.
Various mechanisms have been proposed for the regulation of a gene by a corresponding enhancer (19). Classically, enhancers are viewed as clusters of DNA elements that bind transcription factors, which in turn interact with the mediator complex or transcription factor IID to facilitate the recruitment of RNA polymerase II (as well as that of chromatin modifying enzymes) to the promoter region through “DNA looping” (20). However, recent studies have indicated that enhancer sequences are not simply binding sites for transcription factors and cofactors; rather, many of them are also transcribed to generate noncoding RNAs when the transcription of corresponding genes takes place (21–26). The functions of such enhancer-associated noncoding RNAs have only just begun to be elucidated, such as in the case of those derived from the Snail1 and HoxA gene loci (23–25).
In this study, we found that an enhancer region spanning from between LATE and ANPE to LATE is transcribed in mouse neocortical NPCs and that expression of this transcript (designated utNgn1) correlates well with that of Neurog1 mRNA. Knockdown experiments revealed that utNgn1 is necessary for the effective transcription of Neurog1 in neocortical NPCs, suggesting that one of the enhancers of the Neurog1 locus functions via generation of its transcript. Furthermore, the amount of utNgn1 was found to be increased by Wnt signaling and to be down-regulated by PcG proteins. We noticed that the utNgn1 locus in neocortical NPCs harbors histone H3 lysine 27 trimethylation (H3K27me3), a histone mark catalyzed by PcG proteins, as well as histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 9 or 14 acetylation (H3K9/K14ac), marks of active transcription (27). Our results thus suggest that the enhancer of Neurog1 regulates the expression of Neurog1 via its transcript, and PcG proteins suppress a target gene not only directly by occupying promoter regions but also indirectly by occupying their enhancers.
Results
utNgn1 Is Transcribed from an Enhancer Region of the Neurog1 Locus.
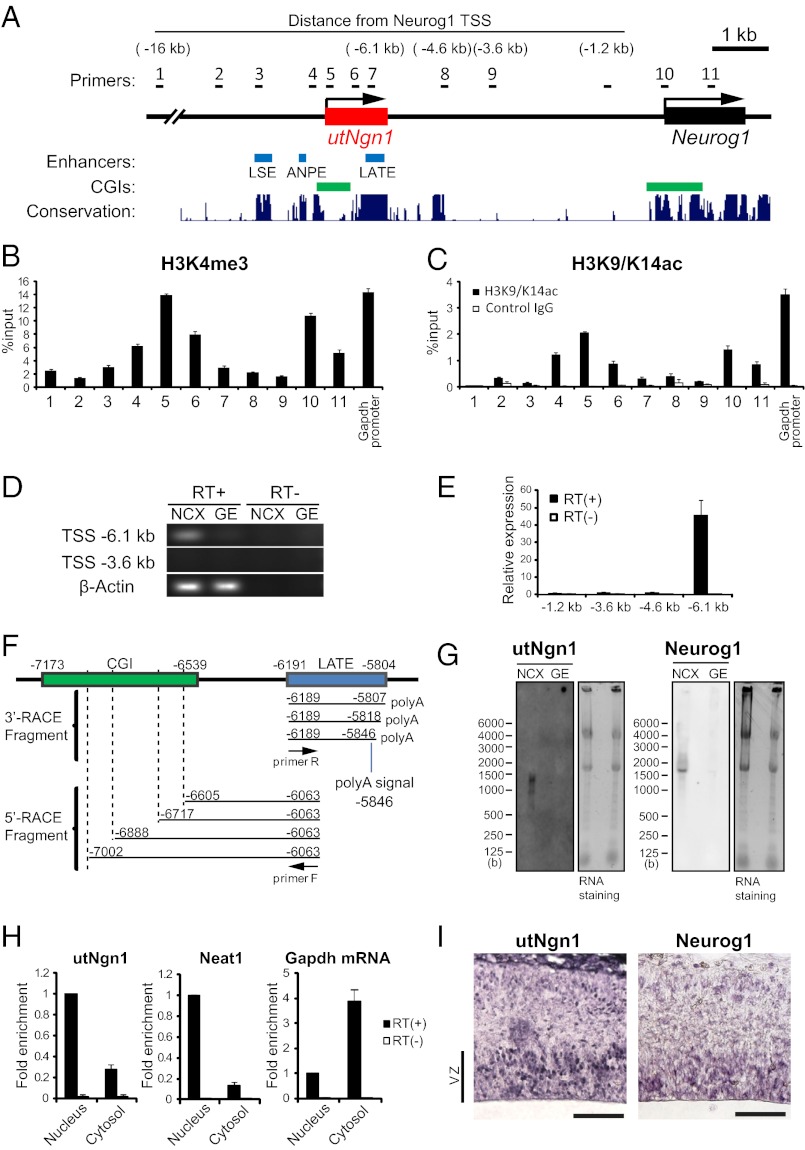
A 4-kb region located 3.8–7.8 kb upstream of the transcription start site (TSS) of mouse Neurog1 has been implicated in regulation of Neurog1 expression in most expression domains of the mouse embryo (14), suggesting the existence of a general enhancer within this region. Given that this region contains a CpG island (CGI) between ANPE and LATE (Fig. 1A), we hypothesized that it might be transcriptionally active (28). We therefore first examined whether the region surrounding this CGI contains the H3K4me3 mark, which is associated with the promoters of actively transcribed genes (or genes in a state permissive for transcription). We cultured NPCs prepared from the mouse neocortex at embryonic day (E)11.5 for 3 d in vitro (DIV) as neurospheres (E11.5 + 3DIV culture). Chromatin immunoprecipitation (ChIP) assay revealed the presence of this modification in the region ∼7 kb upstream of the TSS of Neurog1 (Fig. 1A, primer 5), with a peak in H3K4me3 at the CGI (Fig. 1B). The amount of H3K4me3 at the CGI was similar to that at the promoter of Neurog1 as well as at the promoter of the actively transcribed gene Gapdh, suggesting that the region surrounding the CGI might be transcribed. This notion was further supported by the observation that H3K9/K14ac, another histone modification associated with actively transcribed genes, was also present in the region surrounding the CGI (Fig. 1C).
Fig. 1.
A noncoding RNA, utNgn1, is transcribed from an enhancer region of Neurog1. (A) Schematic representation of enhancers and CpG islands (CGIs) of the mouse Neurog1 locus. Blue and green boxes indicate enhancers (LATE, ANPE, and LSE) identified in zebrafish and CGIs, respectively. Black arrows indicate the direction of transcription of utNgn1 (red box) and Neurog1 (black box). Conservation plot and CGIs are adapted from the University of California Santa Cruz Genome Browser. Black bars indicate the positions of PCR primers used for RT-PCR or ChIP analysis. (B and C) Primary NPCs isolated from the E11.5 mouse neocortex (NCX) were cultured in suspension for 3DIV in the presence of fibroblast growth factor 2 (FGF2) and epidermal growth factor (EGF). Cells were then subjected to ChIP analysis with antibodies to H3K4me3 (B) or H3K9/K14ac (or with control IgG) (C) and with the PCR primers indicated in A or those specific for the promoter of Gapdh. Data are expressed as percentage of the input and are means ± SEM for three samples. (D) Total RNA extracted from the NCX or ganglionic eminences (GE) of E13.5 mouse embryos was subjected to RT (or not) with an oligo (dT) primer followed by PCR with primers targeted to regions located 6.1 kb or 3.6 kb upstream of the transcription start site (TSS) of Neurog1 as well as with those specific for β-actin (internal control). (E) Relative expression levels of utNgn1 locus (Neurog1 TSS −6.1 kb) in the neocortex compared with other intergenic regions (Neurog1 TSS −1.2, −3.6, and −4.6 kb) are determined by qRT-PCR. Data represent means ± SEM from three embryos at E13.5. (F) 5′ and 3′ RACE analysis of utNgn1 with cDNA prepared from poly(A)+ or total RNA of the mouse neocortex at E12.5. Black arrows indicate PCR primers F and R. Four 5′ ends and three 3′ ends were determined by DNA sequencing in 5′ RACE and 3′ RACE, respectively. Amplified fragments are mapped on the Neurog1 upstream region, and a polyadenylation signal (AAATAA) is shown. (G) Northern blot analysis of total RNA prepared from the E13.5 NCX or GE were performed with DIG-labeled RNA probes specific for utNgn1 or Neurog1 mRNA. Blots were also stained with SYBR Gold (Right). (H) Relative concentration of utNgn1 in the nuclear and cytosolic fractions. Cells were prepared from the E12.5 mouse NCX and cultured in suspension for 3DIV. The same amount of total RNA extracted from nuclear or cytosolic fractions of NPCs were reverse transcribed. Relative amounts of the cDNA in each fraction were determined by qRT-PCR. Data are expressed relative to the corresponding value for concentration in the nuclear fraction and are means ± SEM from three independent experiments. (I) In situ hybridization analysis of utNgn1 and Neurog1 mRNA in coronal sections of the E13.5 mouse NCX. VZ, ventricular zone. (Scale bar, 50 μm.)
We next performed RT-PCR analysis to search for transcripts derived from the region surrounding the CGI and detected PCR amplicons originating from a position ∼6.1 kb upstream of the TSS of Neurog1 in the mouse neocortex at E13.5 (Fig. 1 A and D). Furthermore, quantitative RT-PCR (qRT-PCR) analysis revealed that a significant level of transcription occurs in this position compared with other intergenic regions such as ∼1.2, 3.6, and 4.6 kb upstream of the TSS of Neurog1 (Fig. 1 A and E). Next, to determine the 5′ and 3′ ends of the transcripts, we performed rapid amplification of cDNA ends (RACE) analysis (Fig. 1F). 5′ RACE revealed multiple 5′ ends within the CGI, consistent with previous observations showing that CGI promoters are accompanied by multiple initiation points within the CGI (29). 3′ RACE revealed a polyadenylation signal at position −5.8 kb relative to the TSS of Neurog1. Consistent with these observations, Northern blot analysis with an antisense probe corresponding to positions −6.0 to −6.4 kb revealed a transcript of ∼1.4 kb in a total RNA fraction isolated from the mouse neocortex at E13.5 (Fig. 1G). We now refer to this transcript derived from an enhancer region of Neurog1 as utNgn1 (upstream transcript of the Neurog1 locus). The longest ORF of utNgn1 potentially encodes a 49-amino-acid protein with no sequence similarity to any expressed sequences currently deposited in protein databases. Moreover, it may not be translated because fractionation of neocortical NPCs (E12.5 + 3DIV culture) revealed that the concentration of utNgn1 is higher in the nucleus compared with the cytosol, which is characteristic of noncoding RNA (for instance, Neat1, Malat1, or Xist) (30) in contrast to the cytosol-enriched localization of coding RNA (mRNA) (Fig. 1H). We cannot, however, exclude the possibility that utNgn1 is translated. Although utNgn1 and Neurog1 mRNA are encoded on the same strand, they do not appear to be components of the same transcript, given that neither the probe for utNgn1 nor one for Neurog1 mRNA detected a transcript spanning both target RNAs (>3 kb) in Northern blot analysis (Fig. 1G). In addition, we could not detect a transcript, by qRT-PCR, or association with histone H3 lysine 36 trimethylation (H3K36me3) (27), by ChIP analysis, for the region between the coding sequences for utNgn1 and Neurog1 mRNA (Fig. 1E and Fig. S1).
Correlation Between utNgn1 and Neurog1 mRNA Expression Patterns.
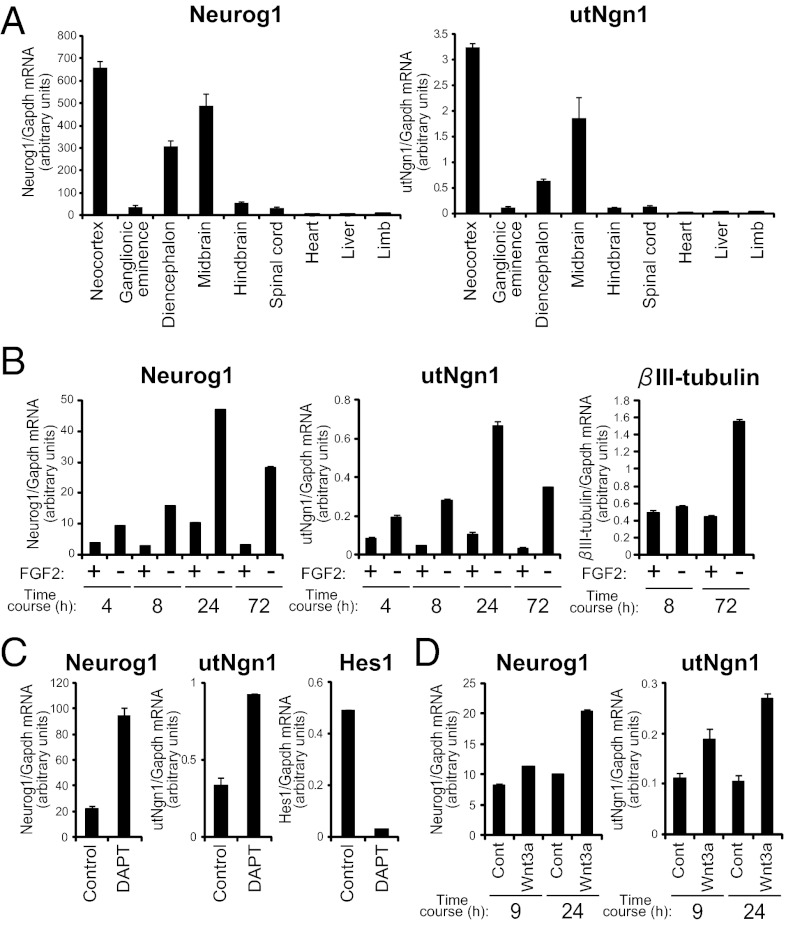
Long noncoding RNAs have previously been found to affect gene expression both positively and negatively (31). We therefore compared the expression pattern of utNgn1 with that of Neurog1 mRNA. We isolated various regions of the developing CNS, including the neocortex, ganglionic eminences, diencephalon, midbrain, hindbrain, and spinal cord, as well as nonneural tissues, including the heart, liver, and limb, from mouse embryos at E13.5. qRT-PCR analysis revealed that utNgn1 was expressed in all regions of the CNS that expressed Neurog1 mRNA, but neither RNA was detected in the nonneural tissues (Fig. 2A) (14). Moreover, the amount of utNgn1 correlated well with that of Neurog1 mRNA. The expression level of utNgn1 is very low (1/30–1/300 of that of Neurog1) (Fig. 2 A–D). Northern blot analysis also showed that both utNgn1 and Neurog1 mRNA were more abundant in the neocortex than in the ganglionic eminences (Fig. 1G). These results thus suggested that the expression of utNgn1 is positively correlated with that of Neurog1 mRNA.
Fig. 2.
Expression pattern of utNgn1 is highly correlated with that of Neurog1 mRNA. (A) Tissues were isolated from the neocortex, ganglionic eminences, diencephalon, midbrain, hindbrain, spinal cord, heart, liver, and limb of E13.5 mouse embryos. (B) Primary NPCs prepared from the E11.5 neocortex were cultured for 1 d and then incubated for the indicated times in medium with or without FGF2. (C) Primary NPCs prepared from the E11.5 neocortex were cultured for 2 d and then exposed to DAPT (5 μM) or dimethyl sulfoxide vehicle (control) for 7 h. (D) Primary NPCs prepared from the E11.5 neocortex were cultured without (control) or with Wnt3a for 9 or 24 h. (A–D) Total RNA was isolated from the cells or tissues and subjected to qRT-PCR analysis of each gene. Relative amounts of Neurog1 and utNgn1 were determined using a standard curve derived from a BAC clone, which contained both Neurog1 and utNgn1 loci, making the values of Neurog1 and utNgn1 transcripts comparable. Data are normalized by the amount of Gapdh mRNA and are means ± SEM (n = 3).
We also examined the expression of utNgn1 by in situ hybridization. An antisense probe for utNgn1 yielded robust signals in a subpopulation of cells located within the ventricular zone (which contains NPCs) of the E13.5 mouse neocortex (Fig. 1I), the midbrain, and a dorsal part of the diencephalon (Fig. S2 A and B). Importantly, the expression pattern of utNgn1 largely overlapped with that of Neurog1 mRNA, providing further support for a positive correlation between utNgn1 and Neurog1 mRNA expression in the developing CNS.
We next asked whether the expression level of utNgn1 changes during neuronal differentiation of neocortical NPCs. NPCs freshly prepared from the neocortex at E11.5 were maintained in an undifferentiated state in the presence of fibroblast growth factor (FGF)2. Removal of FGF2 in such primary cultures results in neuronal differentiation and promotes the production of Neurog1 mRNA and subsequently that of βIII-tubulin mRNA (32) (Fig. 2B). Here we found that, under the same conditions, growth factor deprivation also induced expression of utNgn1 with a time course similar to that of Neurog1 mRNA (Fig. 2B), indicating that the expression of utNgn1 positively correlates with that of Neurog1 mRNA and is induced during an early phase of neuronal differentiation of neocortical NPCs.
We next investigated how the production of utNgn1 is regulated by extracellular signals, which regulate neuronal differentiation of NPCs. Notch signaling is essential for the maintenance of undifferentiated state of NPCs and suppress neuronal differentiation. We then induced neuronal differentiation of NPCs by treatment with N-[N-(3,5-difluoro-phenacetyl)-l-alanyl]-(S)-phenylglycine t-butyl ester (DAPT), a presenilin inhibitor that suppresses Notch signaling and thereby reduces the amount of Hes1 and Hes5 mRNA, a downstream effector of Notch signaling (33) (Fig. 2C). Exposure of the NPC cultures to DAPT induced the expression of utNgn1 and that of Neurog1 mRNA (Fig. 2C). Wnt signaling is another extracellular signaling that regulates neuronal differentiation and Neurog1 expression in the mouse neocortex. We therefore examined whether Wnt signaling might also regulate utNgn1 expression. Treatment of E11.5 neocortical NPCs with recombinant Wnt3a for 9 or 24 h increased the amounts of utNgn1 and of Neurog1 mRNA (Fig. 2D). These results further indicate that the expression of utNgn1 positively correlates with that of Neurog1 mRNA and is induced during neuronal differentiation of neocortical NPCs.
We also examined the expression of utNgn1 in embryonic stem (ES) cells and ES-derived NPCs (Fig. S3) (34). Neither utNgn1 nor Neurog1 mRNA was detected in ES cells in the undifferentiated state (day 0), but both transcripts were found to be expressed after culturing the cells for 7 d under conditions that induce differentiation into NPCs (day 7). Again, these results supported a positive correlation between transcription of utNgn1 and that of Neurog1 mRNA during development.
utNgn1 Positively Regulates Neurog1 Expression.
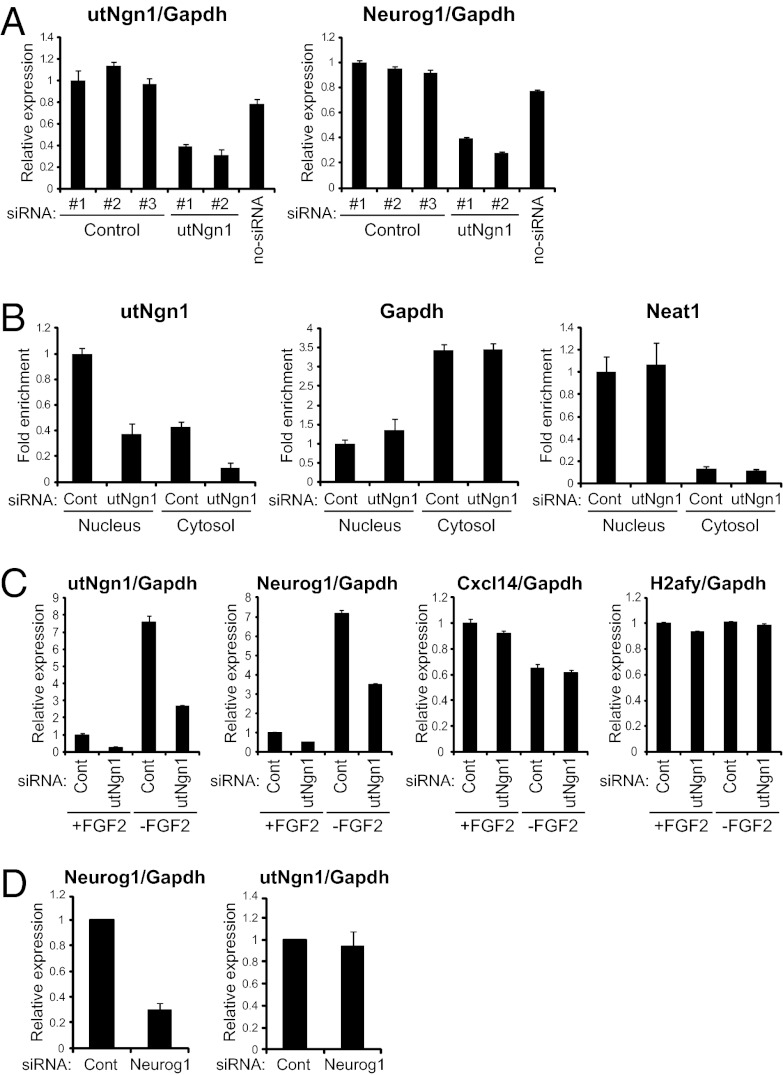
Transcription of neighboring loci can be positively correlated as a result of the phenomenon known as transcriptional noise or “ripples” (35), which might be dependent on mechanisms including chromatin remodeling. We therefore investigated whether utNgn1 functions in gene regulation or is simply a byproduct of Neurog1 expression. To assess its possible role in Neurog1 expression, we knocked down utNgn1 in E11.5 NPCs using small interfering RNAs (siRNAs) targeted to three different sequences within utNgn1 and three control siRNAs. Growth factor deprivation for 10 h induced Neurog1 expression in cells without siRNA or with control siRNA, but this effect was markedly inhibited in cells harboring any of the siRNAs specific for utNgn1 (Fig. 3A and Fig. S4). We confirmed that the nuclear level of utNgn1 was effectively decreased by siRNAs targeting utNgn1 (Fig. 3B). We examined the expression of genes within 1 Mb of the utNgn1 locus and found that knockdown of utNgn1 did not reduce the expression of any of these genes except for Neurog1 (Fig. 3C and Fig. S5 A and B). We also found that depletion of Neurog1 mRNA with a corresponding siRNA did not affect the amount of utNgn1 (Fig. 3D). These results thus suggest that utNgn1 is not simply a byproduct of transcription of a neighboring gene, but rather is a positive regulator of Neurog1 expression, providing an example of gene regulation through the production of an enhancer-derived transcript.
Fig. 3.
utNgn1 is required for the expression of Neurog1 during neuronal differentiation. (A, C, and D) Primary NPCs prepared from the E11.5 neocortex were transfected with three independent control (siRNAs 1, 2, and 3), two independent siRNAs against utNgn1 (utNgn1 siRNA 1 and 2), or without siRNAs (no-siRNA) (A). Similarly, cells were transfected with control (siRNA 1), utNgn1 (siRNA 1), or siRNA against Neurog1 (C and D). Cells were cultured for 2 d with FGF2 and then for 10 h with or without FGF2. Total RNA was then extracted from the cells and subjected to qRT-PCR analysis of each gene. Data are normalized with the amount of Gapdh mRNA, are expressed relative to the corresponding value for cells transfected with the control siRNA 1, and are means ± SEM (n = 3). (B) Primary NPCs prepared from the E11.5 neocortex were transfected with a control (siRNA 1) or with a combination of two independent siRNAs against utNgn1 (utNgn1 siRNAs 1 and 2). Cells were cultured for 3 d in suspension with FGF2 and EGF, harvested and fractionated, as described in Fig. 1H, and the amount of RNA in each fraction was determined.
Next we examined the biological function of utNgn1. Consistent with the proposed role of Neurog1 in neuronal fate commitment in neocortical NPCs (8), we found that utNgn1 knockdown partially inhibited the induction of Tbr2 and NeuroD1, early markers of neocortical neuronal fate commitment, under a differentiation-inducing condition (Fig. S5C). Therefore, utNgn1 might play a role in neuronal fate commitment of these cells.
utNgn1, but Not Its Truncated Form, Positively Regulates Expression of Its Downstream Gene.
To investigate whether utNgn1 is capable of cis-regulating the transcription of its downstream gene, we performed a reporter gene assay. We designed Firefly luciferase (luc2) reporter constructs as schematized in Fig. 4A. In these constructs, the luc2 gene is under the control of the Neurog1 promoter either alone (a), with an intact utNgn1 (b), or with a truncated utNgn1 harboring early termination sequences (c). In vector c, we inserted early termination sequences in utNgn1 between the CGI and the LATE enhancer to inhibit the expression of full-length utNgn1 without affecting the LATE enhancer sequence itself. A synthetic poly(A) signal transcriptional pause site was inserted between utNgn1 and the Neurog1 promoter to prevent the direct effect of upstream transcription on reporter transcription. We found that the insertion of utNgn1 into an upstream region of the Neurog1 promoter significantly enhanced reporter gene transcription (Fig. 4B). Importantly, insertion of early termination sequences in utNgn1 resulted in a loss of the increased reporter gene expression, suggesting the importance of an intact utNgn1 transcript for this action (Fig. 4B). This result provides additional evidence indicating the importance of utNgn1 for Neurog1 gene transcription.
Fig. 4.
Full-length utNgn1, but not truncated utNgn1, promotes expression of reporter gene. (A) Schematic representation of the reporter constructs. A 2.7-kb region of the Neurog1 promoter was cloned into the promoter multicloning site of the pGL4.20 vector and used as a control (a). Full-length utNgn1 was cloned and inserted into the upstream region of vector a (b). Two SV40 poly(A) sequences were tandemly inserted into the utNgn1 region between the CGI and the LATE enhancer (c). (B) Luciferase reporter assay. Firefly vectors shown in A and the control Renilla vector were transfected into NPCs prepared from E11.5 neocortices. All cells were cultured with FGF2 for 12 h and then half were cultured with and half without FGF2 for 20 h. Data are normalized by the Renilla luciferase activity and are means ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student t test.
PcG Proteins Suppress utNgn1 Expression.
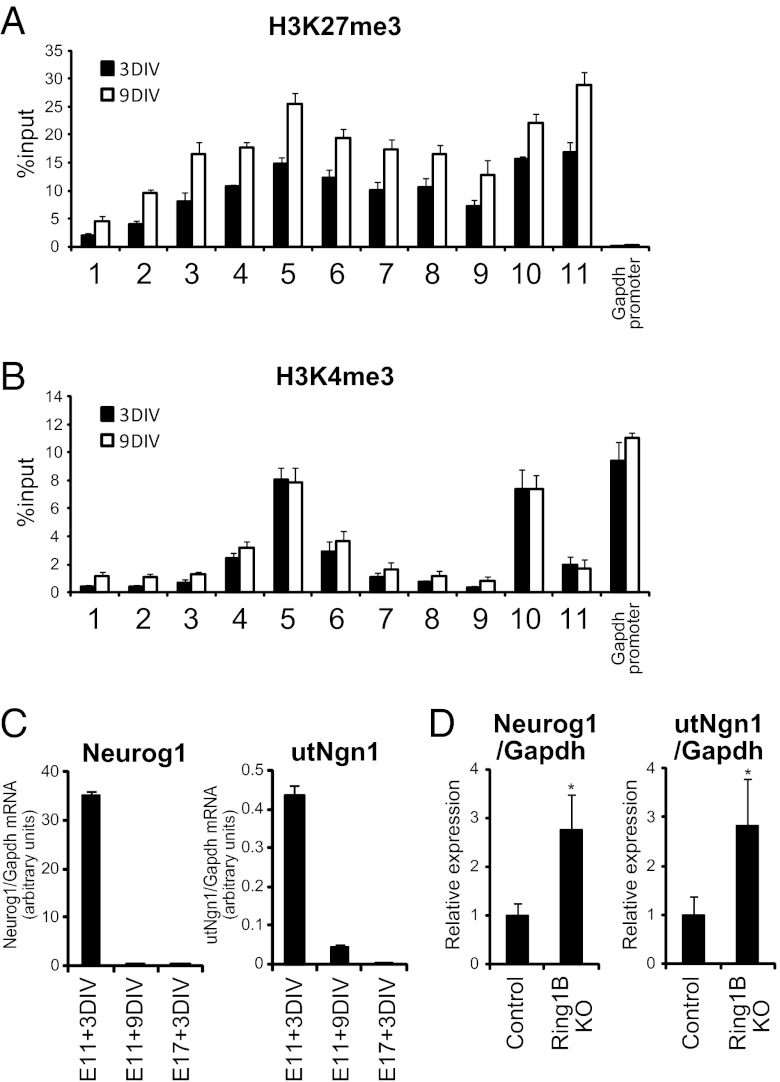
During the late stage of neocortical development, which is associated with the restriction of neuronal fate and activation of the gliogenic phase of NPCs, PcG proteins suppress the Neurog1 promoter (18). We therefore asked whether PcG proteins also regulate utNgn1 expression. ChIP analysis with neocortical NPCs (E11.5 + 3DIV or 9DIV) revealed that the utNgn1 locus was broadly occupied by H3K27me3, a histone mark catalyzed by PcG proteins, at levels comparable to those at the promoter of Neurog1 (Fig. 5A). Therefore, like the Neurog1 locus, the utNgn1 locus is also “bivalent,” harboring both H3K4me3 (active) and H3K27me3 (repressive) histone marks (36). Neocortical E11.5 + 3DIV and E11.5 + 9DIV NPC cultures are characterized by preferential differentiation into neurons and astrocytes, respectively, under the conditions adopted in the present study (18). The level of H3K27me3 at the utNgn1 locus was substantially higher in E11.5 + 9DIV cultures than in E11.5 + 3DIV cultures (Fig. 5A), suggesting that this change is associated with the late (gliogenic) stage of neocortical NPC development. In contrast, the level of H3K4me3 at both utNgn1 and Neurog1 loci remained largely unchanged between E11.5 + 3DIV and E11.5 + 9DIV (Fig. 5B).
Fig. 5.
PcG proteins repress the expression of utNgn1 in the late stage of NPC development. (A and B) Primary NPCs isolated from the E11.5 neocortex were cultured for 3 or 9 DIV in suspension in the presence of FGF2 and EGF. Cells were then subjected to ChIP assays with antibodies to H3K27me3 (A) or to H3K4me3 (B) and with the PCR primers indicated in Fig. 1A. Data are means ± SEM from three independent experiments. (C) Primary NPCs isolated from the E11.5 or E17.5 neocortex were cultured for 3 or 9 DIV as in A, after which total RNA was isolated and subjected to qRT-PCR analysis of utNgn1 and Neurog1 mRNA. Data are normalized by the amount of Gapdh mRNA and are means ± SEM (n = 3). (D) Total RNA prepared from the neocortex of E18.5 Ring1bflox/flox; NestinERT2-Cre (Ring1B KO) and Ring1bflox/flox (control) embryos that were exposed to tamoxifen in utero at E12.5 were subjected to qRT-PCR analysis of Neurog1 mRNA and utNgn1. Data are normalized by the amount of Gapdh mRNA, are expressed relative to the corresponding value for control embryos, and are means ± SD from three embryos of each genotype. *P < 0.05 (Student t test).
The high levels of H3K27me3 at the utNgn1 locus suggest that PcG proteins may suppress the production of the enhancer-encoded transcript during the late developmental stage of neocortical NPCs. To test this hypothesis, we first examined whether the expression of utNgn1 is indeed repressed in the late stage of neocortical NPC development. The level of utNgn1, as well as that of Neurog1 mRNA, was greatly reduced in E11.5 + 9DIV cultures compared with that in E11.5 + 3DIV cultures (Fig. 5C). We observed similar reductions of both utNgn1 and Neurog1 in E17.5 + 3DIV cultures relative to E11.5 + 3DIV cultures (Fig. 5C), suggesting that the suppression of utNgn1 expression is the result of developmental progression rather than culture duration. Consistent with this, the expression levels of both Neurog1 and utNgn1 in the neocortex were dramatically reduced during development (Figs. S2C and S6). We then examined whether the suppression of utNgn1 production in the late stage of development is mediated by PcG proteins. To this end, we induced the conditional ablation of Ring1B, an essential component of Polycomb repressive complex 1 (37), by injecting tamoxifen intraperitoneally into Ring1bflox/flox; NestinERT2-Cre mice (18, 38) at E12.5. The amount of utNgn1 was markedly greater in the neocortex isolated from the Ring1B-deficient embryos at E18.5 compared with control embryos (Fig. 5D). Together, these results indicate that PcG proteins suppress utNgn1 expression during the late stage of neocortical NPC development. PcG proteins thus appear to regulate Neurog1 expression not simply by direct suppression of the gene itself but also by that of its enhancer.
Discussion
A significant portion of the mammalian genome generates noncoding RNAs (ncRNAs) including long intervening noncoding RNAs (lincRNAs) (39–41), some of which have been implicated in gene silencing associated with processes such as imprinting and X chromosome inactivation (42). However, recent studies have also begun to reveal roles of lincRNAs in gene activation (23–25). For instance, a set of ncRNAs (ncRNA-a) was shown to positively regulate their neighboring protein-coding genes, including those for SCL, Snail1, and Snail2 (24). Similarly, HOTTIP, a lincRNA transcribed from the 5′ end of the HoxA locus, was found to positively regulate the HoxA gene cluster (23). Our results now indicate that utNgn1 is another lincRNA that positively regulates gene expression.
Recent genome-wide studies have revealed that gene promoters are marked by H3K4me3, whereas enhancers are often associated with H3K4me1 but are devoid of H3K4me3 (43). The enhancer region now shown to encode utNgn1 therefore appears to be atypical in that it is associated with H3K4me3. Because this is also the case for the ncRNA-a and HOTTIP loci, the presence of H3K4me3 in the enhancer regions might be a specific characteristic of regions encoding this “gene activator” class of lincRNAs. H3K4me1-enriched enhancers have also been shown to express RNA (eRNA), although the functions of these RNA molecules are unknown (21, 26). The transcripts from H3K4me1-enriched enhancers are devoid of polyadenylation, whereas long noncoding RNAs transcribed from H3K4me3-enriched enhancers including utNgn1 harbor polyadenylation, possibly reflecting a difference in mode of regulation and/or function (21, 23–26). utNgn1 is more similar to lincRNAs in this context, although the utNgn1 locus does not harbor the H3K36me3 mark, which is a characteristic of lincRNAs.
Development is associated with strict regulation of the enhancers of developmental genes. It has been proposed that H3K4me1-enriched enhancers can be classified as either “poised” and “active” enhancers by the additional presence of the histone marks H3K27me3 and H3K27ac, respectively (44, 45). It remains unclear, however, whether these histone marks are functionally important for enhancer activity. Our results now suggest that the utNgn1 locus is maintained in a poised (or inactive) state in the late stage of neocortical NPC development by a PcG protein-mediated mechanism, given that the locus is enriched with both H3K27me3 and H3K4me3 and that utNgn1 expression was derepressed by ablation of Ring1B. These findings thus suggest that PcG proteins may directly suppress enhancers in addition to promoters for a class of developmental genes in a developmental context-dependent manner.
The mechanism of enhancers’ regulation of gene expression and that of the regulation of enhancers themselves are fundamental to an understanding of gene control mechanisms. Given our finding that utNgn1 is necessary for the efficient activation of Neurog1 expression, a next key question will be how this transcript promotes gene activation, which is likely by recruitment of effector proteins, including histone modifiers or transcription factors, to the Neurog1 promoter. Although recruitment or maintenance of mixed lineage leukemia (MLL) complex is implicated in the action of lincRNAs such as HOTTIP and Mistral (23, 25), other mechanisms likely account for the action of utNgn1, because the level of H3K4me3 (mediated by MLL complex) of the Neurog1 promoter was not significantly reduced, despite a marked reduction of utNgn1 expression, in the late developmental stages (Fig. 5). It will also be important to elucidate how PcG protein-mediated regulation of the utNgn1 locus relates to that of the Neurog1 promoter. It is possible that PcG proteins associated with the enhancer might physically and functionally interact with those associated with the promoter. Further elucidation of utNgn1’s regulation and function should bring new insights into the role of enhancers and the regulation of transcription.
Materials and Methods
Full details of procedures are provided in SI Materials and Methods. Briefly, primary NPCs were isolated and cultured as described previously (18). The siRNA were transfected using Neon transfection system (Invitrogen). Antibodies used in ChIP assay were anti-H3K9K14Ac (Upstate; 06–599), anti-H3K27me3 (Upstate; 07–449), anti-H3K4me3 (Active Motif; 39916), anti-H3K36me3 (Abcam; 9050), and control rabbit IgG (Santa Cruz; sc-2027).
Supplementary Material
Acknowledgments
We thank Drs. Yoshiki Sasai, Mototsugu Eiraku, and Tokushige Nakano for instruction, materials, and cell lines on ES cell culture and NPC induction; and Dr. Ryoichiro Kageyama for Nes-CreERT2 mice; and Dr. Yukihide Tomari, Kei Akaiwa, and Taruho Endoh for technical assistance. This work was supported by Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency, Grant-in-Aid for Scientific Research on Innovative Areas “Neural Diversity and Neocortical Organization” and “Cell Fate” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, by Grant-in-Aid for Scientific Research (A) from MEXT of Japan, by Grant-in-Aid for JSPS fellows from JSPS, and by the Global COE Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms), MEXT, Japan. M.O. is a research fellow of the Japan Society for the Promotion of Science (JSPS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202956109/-/DCSupplemental.
References
- 1.Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci. 2010;11:377–388. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- 2.Miller FD, Gauthier AS. Timing is everything: Making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Ma QF, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 5.Fode C, et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Gowan K, et al. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 7.Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- 8.Schuurmans C, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Morrow EM, Cepko CL. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- 11.Blader P, Plessy C, Strähle U. Multiple regulatory elements with spatially and temporally distinct activities control neurogenin1 expression in primary neurons of the zebrafish embryo. Mech Dev. 2003;120:211–218. doi: 10.1016/s0925-4773(02)00413-6. [DOI] [PubMed] [Google Scholar]
- 12.Blader P, et al. Conserved and acquired features of neurogenin1 regulation. Development. 2004;131:5627–5637. doi: 10.1242/dev.01455. [DOI] [PubMed] [Google Scholar]
- 13.Nakada Y, Parab P, Simmons A, Omer-Abdalla A, Johnson JE. Separable enhancer sequences regulate the expression of the neural bHLH transcription factor neurogenin 1. Dev Biol. 2004;271:479–487. doi: 10.1016/j.ydbio.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Quiñones HI, Savage TK, Battiste J, Johnson JE. Neurogenin 1 (Neurog1) expression in the ventral neural tube is mediated by a distinct enhancer and preferentially marks ventral interneuron lineages. Dev Biol. 2010;340:283–292. doi: 10.1016/j.ydbio.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirabayashi Y, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 16.Israsena N, Hu M, Fu WM, Kan LX, Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Holm PC, et al. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Mol Cell Neurosci. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi Y, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63:600–613. doi: 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Ong CT, Corces VG. Enhancer function: New insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackwood EM, Kadonaga JT. Going the distance: A current view of enhancer action. Science. 1998;281:60–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 21.Kim TK, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Santa F, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ørom UA, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertani S, Sauer S, Bolotin E, Sauer F. The noncoding RNA Mistral activates Hoxa6 and Hoxa7 expression and stem cell differentiation by recruiting MLL1 to chromatin. Mol Cell. 2011;43:1040–1046. doi: 10.1016/j.molcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang D, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- 28.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandelin A, et al. Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet. 2007;8:424–436. doi: 10.1038/nrg2026. [DOI] [PubMed] [Google Scholar]
- 30.Chen LL, Carmichael GG. Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol. 2010;22:357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 32.Maric D, Fiorio Pla A, Chang YH, Barker JL. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci. 2007;27:1836–1852. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 34.Eiraku M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Ebisuya M, Yamamoto T, Nakajima M, Nishida E. Ripples from neighbouring transcription. Nat Cell Biol. 2008;10:1106–1113. doi: 10.1038/ncb1771. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: Knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- 38.Calés C, et al. Inactivation of the polycomb group protein Ring1B unveils an antiproliferative role in hematopoietic cell expansion and cooperation with tumorigenesis associated with Ink4a deletion. Mol Cell Biol. 2008;28:1018–1028. doi: 10.1128/MCB.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carninci P, et al. FANTOM Consortium RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group) The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 40.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalil AM, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 44.Rada-Iglesias A, et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creyghton MP, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.