Abstract
Abscisic acid (ABA) 8′-hydroxylase catalyzes the first step in the oxidative degradation of (+)-ABA. The development of a robust in vitro assay has now permitted detailed examination and characterization of this enzyme. Although several factors (buffer, cofactor, and source tissue) were critical in developing the assay, the most important of these was the identification of a tissue displaying high amounts of in vivo enzyme activity (A.J. Cutler, T.M. Squires, M.K. Loewen, J.J. Balsevich [1997] J Exp Bot 48: 1787–1795). (+)-ABA 8′-hydroxylase is an integral membrane protein that is localized to the microsomal fraction in suspension-cultured maize (Zea mays) cells. (+)-ABA metabolism requires both NADPH and molecular oxygen. NADH was not an effective cofactor, although there was substantial stimulation of activity (synergism) when it was included at rate-limiting NADPH concentrations. The metabolism of (+)-ABA was progressively inhibited at O2 concentrations less than 10% (v/v) and was very low (less than 5% of control) under N2. (+)-ABA 8′-hydroxylase activity was inhibited by tetcyclacis (50% inhibition at 10−6 m), cytochrome c (oxidized form), and CO. The CO inhibition was reversible by light from several regions of the visible spectrum, but most efficiently by blue and amber light. These data strongly support the contention that (+)-ABA 8′-hydroxylase is a cytochrome P450 monooxygenase.
The phytohormone ABA regulates many important physiological and developmental processes in plants. These include seed development (desiccation tolerance, storage product deposition, and dormancy), the opening and closing of stomates, and the adaptive responses of plants to imposed environmental stresses (Zeevaart and Creelman, 1988). In general, the efficacy of a hormone such as ABA in producing biochemical/biophysical changes within cells or tissues results from the magnitude of, and interaction between, hormone concentrations and cellular sensitivities. Both of these processes independently affect ABA responses, and their effects on growth and development have been documented clearly in studies of ABA-biosynthesis (aba) and ABA-insensitive (abi) mutants (for review, see Merlot and Giraudat, 1997).
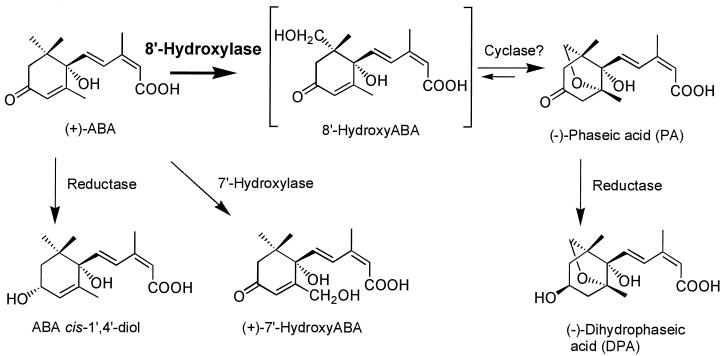
ABA concentrations in planta are controlled by the opposing forces of synthesis (and/or import) and degradation (and/or export). The predominant pathway by which ABA is metabolized in leaves, developing seeds, and seedlings is through oxidative catabolism to 8′-hydroxy-ABA, with subsequent conversion to PA and, in some tissues, reduction to DPA (Zeevaart and Creelman, 1988; Parry, 1993; Walton and Li, 1995; Fig. 1). These latter compounds are generally less biologically active than ABA and, as a consequence of these conversions, the effectiveness of ABA synthesized in or imported into a tissue is reduced (Walton and Li, 1995; Walker-Simmons et al., 1997). (+)-ABA 8′-hydroxylase catalyzes the first step in the oxidative degradation of ABA and is considered to be the pivotal enzyme controlling the rate of degradation of this plant hormone.
Figure 1.
Oxidative catabolism of (+)-ABA in plant tissues.
Experimental evidence implicating ABA turnover (and ABA 8′-hydroxylase) as a modulating factor in controlling ABA responses in planta has been indirect and circumstantial. Some of the metabolic markers used to identify potential tissues expressing this enzyme have included the disappearance of internal or applied ABA, the accumulation of PA and DPA or their Glc conjugates, and reduced sensitivity to applied ABA (Uknes and Ho, 1984; Garello and LePage-Degivry, 1995; Jia et al., 1996; Cutler et al., 1997; Qi et al., 1998). Such data imply that ABA 8′-hydroxylase is expressed at high levels in plant tissues recovering from abiotic stresses such as water stress (Creelman and Zeevaart, 1984; Walton and Li, 1995), in roots and tubers (Zhang and Davies, 1987; Vreugdenhil et al., 1994), and in leaves, developing seeds, and seedlings (Gillard and Walton, 1976; Babiano, 1995; Garello and LePage-Degivry, 1995; Jia et al., 1996; Zhang et al., 1997; Qi et al., 1998). ABA degradation can be very rapid; the half-life of radioactive ABA applied through the xylem of detached leaves of maize (Zea mays) and Commelina communis was 42 and 64 min, respectively (Jia et al., 1996), and the turnover of ABA taken up from the medium in maize root tips was greater than 60% in a 1-h period (Ribaut et al., 1996). ABA 8′-hydroxylase activity is inducible by its substrate, (+)-ABA, in some tissues (Uknes and Ho, 1984; Gergs et al., 1993; Cutler et al., 1997), and there is evidence that it is also regulated by osmotic stress and phytochrome-dependent signaling pathways (Kraepiel et al., 1994; Cutler et al., 1997).
There have been several previous reports of in vitro ABA 8′-hydroxylating activity using microsomal preparations from eastern wild cucumber endosperm (Gillard and Walton, 1976), bean leaves (Gergs et al., 1993), and chickpea seedlings (Babiano, 1995). In all of these, the reported activities were very low and the assays difficult to reproduce and thus not amenable to detailed characterization of the enzyme. However, collectively, the data suggest that ABA 8′-hydroxylase may be a Cyt P450 monooxygenase (Gillard and Walton, 1976; Gergs et al., 1993; Babiano, 1995).
Cyt P450s are a large superfamily of enzymes and to date more than 200 genes in 30 distinct families have been identified in plant species and 16 families and 29 subfamilies in animals (Nelson, 1998). In Arabidopsis alone more than 51 genes have been described (Nelson, 1998). The “classical” Cyt P450s (Schuler, 1996) are integral membrane proteins of the ER that use molecular oxygen, require NADPH and NADPH-dependent Cyt P450 reductase for activity, and are inhibited by CO (Durst, 1991; Ortiz de Montellano and Correia, 1995; Wachenfeldt and Johnson, 1995; Schuler, 1996). This inhibition is reversible by blue light (Ortiz de Montellano and Correia, 1995). More specifically, the moniker Cyt P450 (Omura, 1978) is applied to enzymes bearing a ferriprotoporphyrin IX group attached through a Cys linkage to an apoprotein (Bolwell et al., 1994). The Fe(II)-CO complex of these hemoproteins absorbs visible light maximally at 450 nm because of the electron-rich cysteinate (thiol) ligand in the trans position to CO (Ortiz de Montellano and Correia, 1995). Cyt P450 enzymes are involved in many metabolic pathways in plants (West, 1980; Bolwell et al., 1994; Schuler, 1996) and also play a significant role in detoxification of allelopathic substances and herbicides (Bolwell et al., 1994).
To identify an enzyme as a Cyt P450 using only in vivo or in vitro enzymatic data, it must be demonstrated that it satisfies several, if not all, of the following criteria (in no particular order): (a) a requirement for molecular oxygen, (b) a requirement for NADPH and a reductase (i.e. Cyt P450 reductase), (c) inhibition by CO, (d) reversal of the CO inhibition by light with a maximum in the action spectrum at 450 nm, (e) membrane association, (f) 1:1:1 stoichiometry between the amount of O2 and NADPH used and product formed, (g) the presence in the reduced enzyme preparation of a CO-binding pigment with a maximum absorption at 450 nm, (h) a substrate-dependent type I-binding spectrum, and (i) incorporation of an oxygen atom from O2 into the product (West, 1980).
An in vivo assay describing the inducibility of ABA 8′-hydroxylase in suspension-cultured maize cells was recently described (Cutler et al., 1997). We have now established a companion in vitro assay for ABA 8′-hydroxylase activity from this same tissue that is both robust and highly reproducible. Using this assay we have examined whether ABA 8′-hydroxylase satisfies enough of these criteria to be classified as a Cyt P450 mixed-function monooxygenase.
MATERIALS AND METHODS
Chemicals
(+)-ABA, (−)-ABA, and their metabolites were obtained as described previously: (+)-ABA, (−)-ABA, (−)-PA, 8′-hydroxy-ABA (Zou et al., 1995), DPA (according to the method of Zeevaart and Milborrow, 1976); (±)-7′-hydroxy-ABA (Nelson et al., 1991); (±)-ABA trans-1′,4′-diol and (±)-ABA cis-1′,4′-diol (Balsevich et al., 1996). Additional (+)-ABA was generously provided by Y. Kamuro (BAL Planning Co., Hanaike, Japan). Tetcyclacis was a gift from Dr. W. Rademacher (BASF, Limburgerhof, Germany) to Dr. Suzanne Abrams, who made it available for this study. Cyt c, NADPH, and NADH were obtained from Sigma.
(+)-ABA 8′-Hydroxylase Activity
Suspension cultures of maize (Zea mays L. cv Black Mexican Sweet) were maintained as described previously (Ludwig et al., 1985). (+)-ABA 8′-hydroxylase activity was induced with 200 μm (+)-ABA for 16 h, and cells were harvested by vacuum filtration and stored in aliquots at −80°C until required. Frozen tissue was ground rapidly to a powder with liquid N2 using a prechilled mortar and pestle, and extracted with 8 volumes of buffer (0.1% BSA, 0.33 m Suc, 40 mm ascorbate, and 100 mm potassium phosphate buffer, pH 7.6). In some experiments this buffer was supplemented with 20 mm EDTA and potassium phosphate was increased to 200 mm to improve enzyme stability. The tissue homogenate was filtered through two layers of Miracloth (Calbiochem) and centrifuged at 20,000g for 10 min at 4°C. The cellular debris were discarded and the supernatant was recentrifuged at 200,000g for 60 min at 4°C. The microsomal pellet was resuspended in either 100 mm NaOH-Hepes or 100 mm potassium phosphate, pH 7.6, and stored on ice until required. Protein concentration was measured using the Bio-Rad protein assay (Bradford, 1976).
The enzyme assay was initiated by the addition of 200 to 400 μm (+)-ABA and 5 mm NADPH to an aliquot of the microsomal suspension (0.3–0.6 mg of protein) in a microcentrifuge tube (total volume, 500 μL). The tube was incubated for 3 h at 30°C with gentle shaking in a temperature-controlled incubator (Thermomixer, Eppendorf), the reaction was stopped by the addition of 0.2 volume of 1 n HCl, and the assay mixture was centrifuged for 5 min to remove the precipitated protein. ABA and its metabolites were isolated from the acidified and clarified assay mixture by repeated extraction (three times) with equivalent volumes of ethyl acetate. The solvent was removed under a stream of N2 gas, and the dried residue was redissolved in methanol for analysis by TLC or HPLC. Alternatively, these compounds were purified using extraction cartridges (Oasis HLB, Waters) according to the manufacturer's instructions.
Estimation of enzyme activity was based on the conversion of (+)-ABA to (−)-PA. Initially, (+)-ABA 8′-hydroxylase activity in vitro was monitored by the appearance of radiolabeled PA on TLC plates after fluorography using (+)-[3H]ABA as the precursor (31.4 Ci mol−1). The TLC plates with fluorescence indicator (Merck, Darmstadt, Germany) were developed in toluene:ethyl acetate:acetic acid (25:15:2 [v/v]), sprayed with a fluor (Enhance, New England Nuclear), and exposed to radiographic film (Amersham) at −70°C for 24 h. The identification of substrate and products was made by comparisons with the migration of authentic standards (see above) and confirmed by the comigration of radioactive metabolites with unlabeled standards.
After initial optimization of the assay it was possible to switch from radioactive (+)-ABA and TLC separation to unlabeled (+)-ABA and HPLC separation for routine analysis and quantification of (+)-ABA 8′-hydroxylase activity. ABA and its metabolites were separated on a reverse-phase column (150 × 4.6 mm; HISEP, Supelco, Bellefonte, PA) by isocratic elution with a 75:25 (v/v) mixture of aqueous 1% acetic acid and acetonitrile and UV detection at 262 nm. Quantitative measurements of the amounts of these compounds were calculated from peak areas by comparison with known quantities of (−)-PA and (+)-ABA. We do not have a calibration curve for 8′-hydroxy-ABA, but we made the assumption that the response factor for 8′-hydroxy-ABA is the same as that for PA. For the purpose of expressing activity, the products of the degradation of ABA, 8′-hydroxy-ABA and PA, were summed and expressed as nanomoles of total PA production per milligram of protein per assay period (in hours). The standard assay period was 3 h, but this was varied in some of the experiments (it was sometimes shortened to accommodate a requirement for more samples and constraints in time and space). In all cases the data are expressed as the accumulation of product (in nanomoles per milligram of protein) over the entire period of the assay (per total number of hours), rather than per unit time (per hour), because of the increasing deviations from linearity in assays extending beyond 60 to 90 min in duration.
Gas Mixtures and Light Quality
Gas mixtures of various compositions were developed from separate sources of CO, O2, and N2. These were mixed in the proportions required by monitoring the displacement of water from an inverted flask. Care was taken to ensure the equalization of the pressure within the flask with the atmospheric pressure outside of the flask after each addition.
Culture tubes (12 × 75 mm) were sealed with a septum through which two syringe needles had been placed (one for the venting of gases and one for introducing enzyme, cofactor, or the gas mixture of choice). These culture tubes were first flushed with the required gas mixture. An enzyme and substrate mixture (deaerated under a vacuum) was added next, and finally the NADPH solution that had been deaerated and equilibrated with the required gas mixture by gentle shaking. The addition of the cofactor completed the requirements for the enzyme assay and introduced the gas mixture to the enzyme solution at the same time that the reaction was initiated. Light of defined wavelengths was generated by wrapping four high-intensity fiberoptic microscope lights (Intralux 5000, Volpi, Auburn, NY) with three layers of acetate filter (all Roscolux, Rosco, Markham, Ontario, Canada): no. 69 (Brilliant Blue) for blue light; no. 15 (Deep Straw) for amber light; and no. 19 (Fire) for red light. Total light intensities at the exterior surface of the reaction tubes in the absence of any intervening filters were in the range of 5000 to 7500 μmol photons m−2 s−1. The reaction tubes were positioned on the surface of the Thermomixer (Eppendorf) to supply continual agitation, and the temperature was maintained at 28°C through a combination of bottom heating and circulating fans. At the conclusion of the experimental period the reaction was stopped with 0.2 volume of 1 n HCl, and ABA and ABA metabolites were purified and analyzed by HPLC as described above.
RESULTS
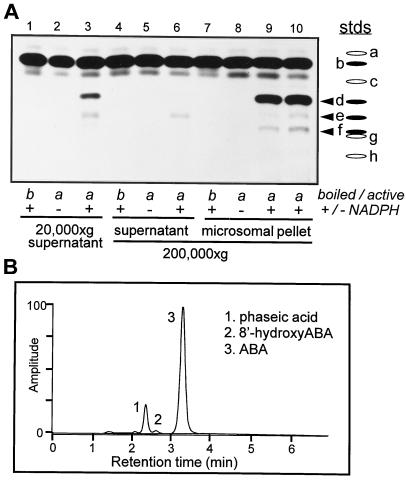
(+)-ABA metabolism in suspension-cultured maize cells occurs predominantly by oxidative catabolism to 8′-hydroxy-ABA and (−)-PA (Fig. 1; Cutler et al., 1997). The initial examinations of cell extracts for in vitro ABA 8′-hydroxylase activity were made using (+)-[3H]ABA as the assay substrate, followed by separation by TLC for visualization of the hormone and its products. Enzyme activity, identified by the appearance of a radioactive compound comigrating with unlabeled PA, was found both in the supernatant after the initial clearing spin and in a more concentrated fashion in the 20,000g to 200,000g particulate fraction after ultracentrifugation (microsomal pellet) (Fig. 2A, lanes 3, 9, and 10). This activity was dependent on the inclusion of NADPH in the assay and was abolished if the tissue extract had been heated to denature the proteins (Fig. 2A).
Figure 2.
(+)-ABA 8′-hydroxylase activity in microsomes extracted from suspension-cultured maize cells. A, After tissue homogenization, the initial 20,000g supernatant and the subsequent 200,000g supernatant and microsomal pellet from (+)-ABA-induced suspension-cultured maize cells were assayed for in vitro hydroxylase activity with (+)-[3H]ABA as the substrate. The pellet was resuspended in 0.1 volume of the original homogenate, so the proteins were concentrated 10-fold over those in the supernatant or the initial homogenate. In some samples the enzyme was inactivated by heating (boiled/active), and in others the cofactor NADPH was omitted (+/−NADPH as controls). Identification of the substrate and products on TLC plates was based on comigration with authentic standards that were run separately and/or superimposed on the assay extracts. Standards (stds) used were: a, trans-ABA; b, ABA; c, (±)-ABA trans-1′,4′-diol; d, PA; e, (±)-ABA cis-1′,4′-diol; f, 8′-hydroxy-ABA; g, 7′-hydroxy-ABA; and h, DPA. The arrowheads indicate the ABA metabolites produced in these assays. B, Output from an HPLC separation using a reverse-phase column with UV detection at 262 nm.
In addition to PA there were minor amounts of two other radioactive products that resulted from the metabolism of (+)-ABA by these tissue extracts (Fig. 2A). Authentic metabolites of ABA were cochromatographed with the radioactive samples as standards for identification purposes; in order of increasing polarity and decreasing mobility these were: (±)-ABA trans-1′,4′-diol; PA; (±)-ABA cis-1′,4′-diol; 8′-hydroxy-ABA; 7′-hydroxy-ABA; and DPA (Fig. 2A). The less polar of the unknown compounds (upper band) co-migrated with (±)-ABA cis-1′,4′-diol, whereas the more polar compound (lower band) comigrated with 8′-hydroxy-ABA (Fig. 2A). The enzyme activity generating ABA cis-1′,4′-diol remained predominantly within the supernatant fraction after ultracentrifugation (note that the microsomal protein was 10-fold concentrated relative to the supernatant fraction; see Methods). The enzyme catalyzing this conversion was soluble and required NADPH for activity (Fig. 2A). The amount of 8′-hydroxy-ABA present in these samples was very minor relative to the quantity of PA detected (Fig. 2), but, like PA, its synthesis was associated with the microsomal fraction and required the cofactor NADPH (Fig. 2A). There was no evidence of any (+)-7′-hydroxy-ABA in these assays, although (−)-7′-hydroxy-ABA was formed in vivo and in vitro when these tissues and isolated microsomes, respectively, were incubated with (−)-ABA (in vitro data not shown; Balsevich et al., 1994).
There have been only a few reports of the isolation of the cis-1′,4′-diol of ABA from natural sources (Fig. 1; Dathe and Sembdner, 1982; Vaughan and Milborrow, 1987). It has been described in immature seeds of fava bean, in avocado fruits, and in broad bean shoots (Dathe and Sembdner, 1982; Vaughan and Milborrow, 1987), in which it is a minor metabolite resulting from ABA degradation. To our knowledge, it has never before been observed in an in vitro assay. The 1′,4′-diols of ABA, particularly the cis-diol, have been found to be unstable under strongly acidic conditions, readily interconverting and oxidizing to ABA (Vaughan and Milborrow, 1988). Acidification of the assay mixture improves the extraction of ABA and its metabolites into ethyl acetate during purification before TLC or HPLC analyses (see Methods). It is therefore perhaps not unexpected that the cis-1′,4′-diol of ABA has been reported only rarely in tissue extracts treated in this manner, and the amount measured here probably represents a minimum, not the maximum, from this tissue (Fig. 2A, lanes 3, 9, and 10). There was no evidence from the in vivo studies that this compound was present or accumulated in this tissue during incubations with (+)-ABA (Cutler et al., 1997).
Ring closure of 8′-hydroxy-ABA to form PA involves internal cyclization of the 8′-hydroxyl moiety onto the enone, resulting in an ether linkage between the 8′ and 2′ carbons (Fig. 1). Under abiotic conditions 8′-hydroxy-ABA and PA are interconvertible (Fig. 1). The forward reaction is pH dependent and occurs readily under alkaline conditions (Zou et al., 1995). The reverse reaction is more difficult to accomplish chemically; however, high yields of 8′-hydroxy-ABA have been produced recently from PA using high-temperature acid treatments and boric acid for stabilization of the opened ring structure (Zou et al., 1995). The conversion of 8′-hydroxy-ABA to PA occurred in concert with the initial hydroxylation of ABA in both the in vitro and in vivo assays (Fig. 2; Cutler et al., 1997), and it was not possible for us to separate the hydroxylation and cyclization activities or to define any conditions that would have prevented the conversion of 8′-hydroxy-ABA to PA. We cannot distinguish whether this conversion occurs enzymatically or nonenzymatically within the assay, or nonenzymatically during sample preparation. For quantitative measurements of the products resulting from (+)-ABA 8′-hydroxylase activity (PA and 8′-hydroxy-ABA), all subsequent assays were conducted with unlabeled (+)-ABA and analyzed by HPLC (Fig. 2B).
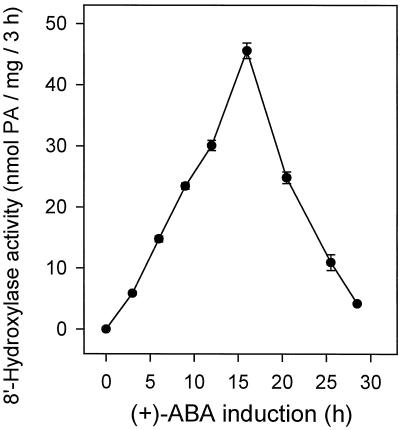
The most important factor in developing a high-activity (+)-ABA 8′-hydroxylase in vitro assay was the identification of a tissue expressing large amounts of in vivo enzyme activity. Cutler and colleagues (1997) demonstrated that the enzyme was inducible by its substrate, (+)-ABA, in suspension-cultured maize cells. In tissue that had not been incubated with (+)-ABA, there was no discernible ABA 8′-hydroxylase activity measured by the in vitro assay (Fig. 3). During the induction period some activity had developed within 3 h of the initial exposure to (+)-ABA, and this increased rapidly, reaching a peak of activity at about 16 h of exposure to (+)-ABA (Fig. 3). Thereafter, even in the continuing presence of the inducing compound, assayable enzyme activity declined rapidly and was reduced to low levels by 24 h of exposure (Fig. 3). Because induced enzyme activity was transient, tissue collections were timed carefully to correspond with this maximum activity for optimization of the in vitro assay.
Figure 3.
Time course of the induction of ABA 8′-hydroxylase activity by (+)-ABA in suspension-cultured maize cells as determined by in vitro assay. Tissue was harvested at the times indicated after the addition of (+)-ABA at 200 μm to the culture medium. PA and 8′-hydroxy-ABA were assayed by HPLC and expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per 3-h time period. Data points represent the means of triplicate samples ± se.
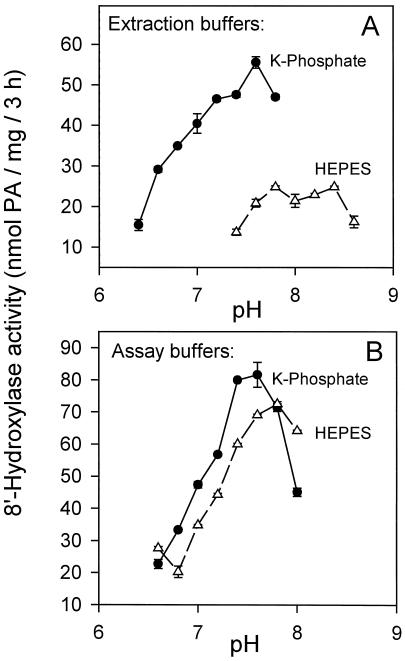
The extraction buffer we have used has very few components (buffer, Suc, ascorbate, and BSA; see Methods) and is much simpler then many other buffers that have been used to develop similar in vitro assays for membrane-bound enzymes (Gergs et al., 1993). We have found that the choice of buffering agent within the extraction buffer was critical in determining the final enzyme activity. Phosphate buffer was always the buffer of choice (Fig. 4A). Compared with Hepes-NaOH, potassium phosphate buffer gave 2- to 3-fold more activity over the same range of pH values (Fig. 4A). The pH optimum was between 7.4 and 7.8, and we used pH 7.6 routinely (Fig. 4A). By contrast, after extraction and separation of the microsomal pellet using potassium phosphate as the initial buffering agent, either of these buffers (Hepes or potassium phosphate) could be used to resuspend the pellet and support the assay with equal efficiency (Fig. 4B). We investigated this apparent protective effect by the phosphate buffer by quantifying the efficiencies of extraction buffers containing combinations of Hepes and/or phosphate at various ratios and concentrations with and without the addition of EDTA and/or EGTA. The results suggest that Hepes buffer is not itself inhibitory to the activity, but that phosphate exerts a concentration-dependent positive effect on activity that can be supplemented by, but not replaced by, EDTA (data not presented). An osmotic agent (e.g. Suc) was not required within the assay to maintain activity (data not presented).
Figure 4.
Effect of buffers and pH on in vitro assayable ABA 8′-hydroxylase activity. A, Comparison of the efficacy of 100 mm Hepes-NaOH and 100 mm potassium phosphate as the initial extraction buffers over a range of pH values. The microsomal pellets and the cofactor were resuspended in 100 mm Hepes-NaOH buffer, pH 7.4, for each of the assays. B, Comparison of 100 mm Hepes-NaOH and 100 mm potassium phosphate as assay buffers over a range of pH values. The initial extraction was performed using 100 mm potassium phosphate buffer, pH 7.6. After ultracentrifugation the microsomal pellets and the cofactor were resuspended for the assay in the buffers indicated at each data point on the graph. PA and 8′-hydroxy-ABA were assayed by HPLC and expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per 3-h time period. Data points represent the means of triplicate samples ± se.
All of the initial assays were incubated for a period of 3 h at 30°C to permit substantial accumulation of PA for analysis. The time course of the accumulation of PA during an extended assay indicated that PA accumulation was linear (and enzyme activity relatively constant) for at least the 1st h (data not shown). There was a gradual reduction in activity during longer periods, but PA production was measurable even at 6 h. Losses during short-term storage on ice were slight: no more than 20% over 6 h (data not shown). It was apparent that after tissue extraction the enzyme did not deteriorate readily in the assay, during storage on ice, or with repeated centrifugation and resuspension (data not shown). This was in marked contrast to its limited persistence within the tissue during long incubations with (+)-ABA measured by in vivo (Cutler et al., 1997) or in vitro activity (Fig. 3).
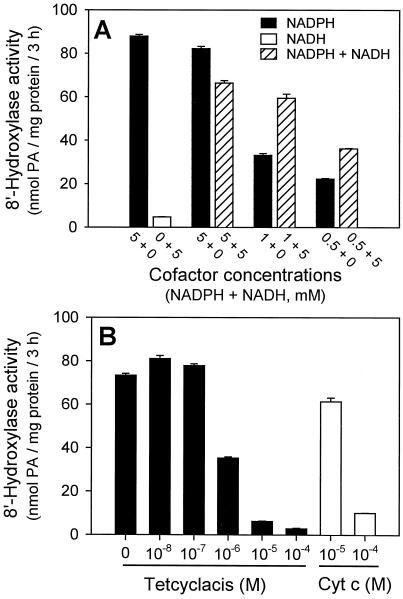
ABA 8′-hydroxylase requires NADPH as a cofactor (Fig. 5A) and high concentrations (5 mm) were necessary for maximum activity (data not shown). NADH did not substitute for NADPH in supporting this assay and the rate at equivalent concentrations of NADH was only 3% to 5% of the rate with NADPH (Fig. 5A). This was no higher than the background activities that occur in the absence of added cofactor (data not shown). When 5 mm NADH was added to assay mixtures containing suboptimal concentrations of NADPH (1 or 0.5 mm), there was substantial improvement in hydroxylase activity over that obtained with NADPH alone (Fig. 5A). Such synergism between NADPH and added NADH has been reported previously for other Cyt P450 enzymes (Donaldson and Luster, 1991; Durst, 1991). Oxidized Cyt c (10 or 100 μm) added to the standard reaction also inhibited (+)-ABA 8′-hydroxylase activity (Fig. 5B), presumably by siphoning off reducing power from NADPH through the involved reductase (West, 1980).
Figure 5.
A, The relative efficacies of NADPH, NADH, and various combinations of these cofactors in supporting (+)-ABA 8′-hydroxylase activity. Concentrations: NADPH, 5, 1, and 0.5 mm; NADH, 5 mm; and NADPH, 5, 1, or 0.5 mm in combination with NADH at 5 mm. The other components were standard (3 h of incubation at 30°C; see Methods). Activities were determined by HPLC and expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per 3-h time period. Data points represent the means of triplicate samples ± se. B, Effect of tetcyclacis and Cyt c (oxidized form) on (+)-ABA 8′-hydroxylase activity. Tetcyclacis (dissolved in ethanol) or Cyt c was added to the assay mixture (enzyme, substrate, and buffer) immediately before the addition of the cofactor. The other components and procedures were standard (3 h of incubation at 30°C; see Methods). Activities were determined by HPLC and are expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per 3-h time period. Data points represent the means of triplicate samples ± se.
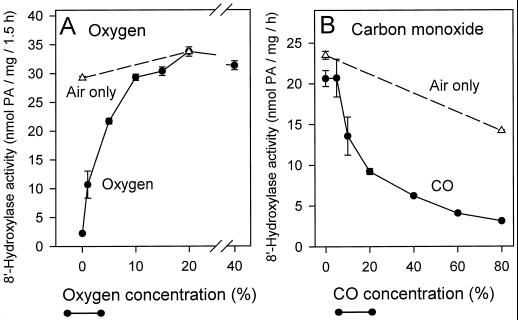
Tetcyclacis is a norbornanodiazetine derivative that has been found to be detrimental to the activity of many Cyt P450 enzymes (Rademacher et al., 1987). Cyt P450s differ markedly in their sensitivities to this compound (10−4 − 10−7 m for 50% inhibition) (Rademacher et al., 1987). Tetcyclacis is not a specific inhibitor of Cyt P450-type enzymes; inhibition is thought to result from the interaction between the lone pair of electrons on the sp2-hybridized N of the heterocyclic ring of tetcyclacis and the exposed heme in the active site of the enzyme, which then results in the displacement of O2 (Rademacher et al., 1987). (+)-ABA 8′-hydroxylase was very sensitive to this inhibitor, having a 50% inhibitory concentration of 10−6 m (Fig. 5B). Enzyme assays were incubated with gas mixtures other than air (20% [v/v] O2) to determine the effects of various O2 concentrations, with and without added CO, on (+)-ABA 8′-hydroxylase activity. Enzyme activity under N2 gas was very low, about 5% of the control rate in air (Fig. 6A); however, at 1% (v/v) O2 hydroxylase activity already had increased to about 33% of the control rate (Fig. 6A). At these low O2 tensions (+)-ABA 8′-hydroxylase very efficiently scavenged for O2 from the buffered medium. With increasing O2 tensions (+)-ABA metabolism increased, reaching control values when O2 concentrations were 10% (v/v) (Fig. 6A). At elevated O2 tensions (40% [v/v]) there was no inhibition of enzyme activity over the control rate at 20% (v/v) O2 (Fig. 6A). Clearly, there was an obligate requirement for molecular oxygen for (+)-ABA metabolism in this assay, but a substantial amount of enzyme activity also occurred under strictly limiting O2 tensions.
Figure 6.
A, The effect of O2 concentrations on (+)-ABA 8′-hydroxylase activity. Seven gas mixtures of varying O2 content were developed by combining N2 and O2 gases within a stoppered flask. These contained 0%, 1%, 5%, 10%, 15%, 20%, and 40% O2 (v/v) (•). The reaction was started with the introduction of NADPH solutions preequilibrated with the gas mixture required. There was some concern that activity of the enzyme stock (stored at 4°C) might deteriorate during the course of the experiment. The air-only control (▵) provided an indication of enzyme activity in the stock assayed under standard conditions during the period of this experiment. Activities were expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per 1.5-h time period. Data points represent the means of triplicate samples ± se. B, The effect of CO concentration on (+)-ABA 8′-hydroxylase activity. Seven gas mixtures were developed in stoppered flasks using various combinations of N2, O2, and CO gases. All mixtures contained 20% (v/v) O2 and varying amounts of CO: 0%, 5%, 10%, 20%, 40%, 60%, and 80% (v/v) (•). As described above, the assays were initiated by the introduction of NADPH solutions preequilibrated with the gas mixtures. The air-only control assayed under standard conditions provided an indication of enzyme activity in the stock during the period of this experiment (▵). Activities are expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per hour. Data points represent the means of triplicate samples ± se.
The inhibitory influence of CO on (+)-ABA 8′-hydroxylase was examined at O2 tensions that simulated atmospheric concentrations (20% [v/v] O2) and had previously been shown to support the maximal rates of PA accumulation under standard assay conditions (Fig. 6A). When 5% (v/v) CO was added there was little inhibition of (+)-ABA metabolism (Fig. 6B). At higher CO concentrations there was considerable reduction in activity (Fig. 6B): 10% (v/v) CO caused about 30% inhibition and 20% (v/v) CO about 55% inhibition of enzyme activity compared with the control (Fig. 6B). There were continued and progressive reductions in enzyme activity at the higher CO concentrations (Fig. 6B). The greatest inhibition, 90% compared with control, was achieved at the maximum CO concentration (80% CO:20% O2 [v/v] ) (Fig. 6B).
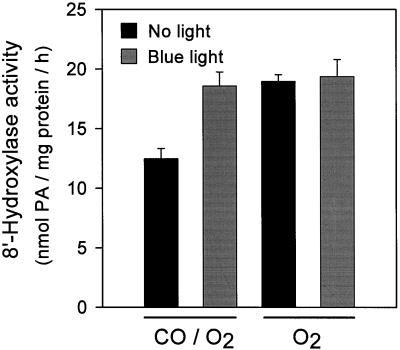
The photoreversibility by blue light of the CO inhibition of enzyme activity is one of the standard diagnostic tests for a Cyt P450 (West, 1980; Donaldson and Luster, 1991; Mihaliak et al., 1993; Schuler, 1996). (+)-ABA 8′-hydroxylase activity was determined in assays at 20% (v/v) O2 with and without CO (20% [v/v]) and with and without blue light (Fig. 7); this concentration of CO consistently produced a 40% to 50% inhibition of enzyme activity (Figs. 6B and 7). This inhibition was light sensitive (Fig. 7); there was a full recovery to control levels of activity with blue light (Fig. 7). In other samples it was demonstrated that blue light had no effect on enzyme activity in the absence of inhibition by CO (Fig. 7).
Figure 7.
The effect of blue light on the CO inhibition of (+)-ABA 8′-hydroxylase activity. Assay mixtures equilibrated with O2 (20% O2:80% N2 [v/v]) and O2 and CO mixtures (20% O2:20% CO:60% N2 [v/v]) were positioned under blue light or under dim laboratory light immediately after the addition of NADPH. The assays were incubated at 28°C for 1 h. The amounts of PA and 8′-hydroxy-ABA were determined by HPLC and expressed as nanomoles of total PA produced (the sum of PA plus 8′-hydroxy-ABA) per milligram of protein per hour. Data points represent the means of at least three samples per treatment ± se.
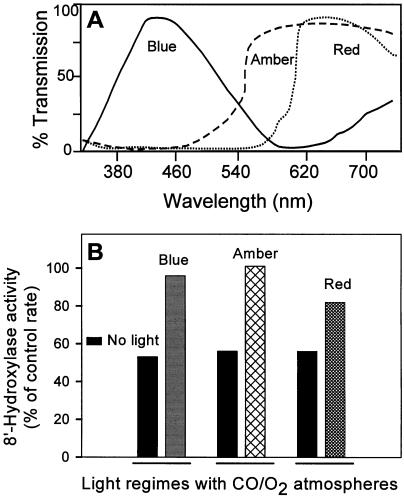
For comparison, filter sets that defined other specific wavelength ranges were tested to determine whether they could overcome the inhibitory effects of CO (20% [v/v]) on this enzyme. In addition to the blue-light reversibility, amber and red light relieved the CO-induced inhibition of (+)-ABA 8′-hydroxylase (Fig. 8). For the CO to photodissociate, the excitation wavelengths must correspond to a heme absorption band. Blue light targets the intense heme absorption band at 450 nm; however, there are also weaker absorption bands at higher wavelengths, in particular, a broad band centered at 570 nm (Markowitz et al., 1992). Reversal by blue light of the CO-induced inhibition was expected given that this enzyme was possibly a Cyt P450 (Gillard and Walton, 1976; Gergs et al., 1993; Babiano, 1995). Reversal by amber light was a surprise, but not unique among Cyt P450s if the incident light intensities are strong enough (Markowitz et al., 1992). The partial reversal of the CO-induced inhibition by red light was totally unexpected; these data indicate that there is a heme absorption band in this Cyt P450 enzyme that extends into the red region of the visible spectrum and overlaps with the wavelengths of the red light transmitted. This red-light reversal of the CO-induced inhibition of (+)-ABA 8′-hydroxylase was less efficient than that of either blue or amber light (Fig. 8).
Figure 8.
The effectiveness of various portions of the visible spectrum in the photoreversibility of the CO inhibition of (+)-ABA 8′-hydroxylase. A, Transmission spectrum for a single layer of acetate for each of the filters (blue, amber, and red; see text). B, Samples were set up as in Figure 7 (20% O2:20% CO:60% N2 [v/v]) except that three different filter sets were used to isolate different wavelength ranges of incident light. The assays were incubated at 28°C for 1 h, and the amounts of PA and 8′-hydroxy-ABA were determined by HPLC analysis. The data for illuminated and nonilluminated samples are expressed as a percentage of the activity of control samples that had been incubated in atmospheres containing 20% (v/v) O2 but without CO and intense illumination. Replicate samples differed by less than 10%.
DISCUSSION
The activities for ABA 8′-hydroxylase that we have established in this in vitro assay are much greater than those described in previous reports (Gillard and Walton, 1976; Gergs et al., 1993; Babiano, 1995). The maximum activities (30 nmol PA mg−1 protein h−1; Fig. 4B) were 30,000-fold greater than those obtained by Gergs et al. (1993) using bean leaves as the source tissue (1 pmol PA mg−1 protein h−1), and 750-fold greater than those obtained by Babiano (1995) using embryonic axes from chickpea (calculated as 0.04 nmol PA mg−1 protein h−1, assuming 1 mg of microsomal protein g−1 tissue).
There has been some difficulty in developing a robust and reproducible in vitro assay for ABA 8′-hydroxylase. A comparison of activities during optimization of this assay demonstrates why this might have been so. The most important factor in obtaining a robust in vitro assay was identification of a tissue source already capable of metabolizing ABA to PA (or DPA) in large quantities. In suspension-cultured maize cells the enzyme was inducible with (+)-ABA, providing high potential activities (Fig. 3). Cutler et al. (1997) have shown that the development of enzyme activity in this tissue is dependent on both RNA and protein synthesis, and we surmise that this involves specifically the transcription of the gene(s) for ABA 8′-hydroxylase and translation of that mRNA into protein. There is no experimental evidence of any preexisting enzyme activity in this tissue before induction (Fig. 3), or that there was latent activity that was then activated during the induction period. Induction of (+)-ABA 8′-hydroxylase by its substrate, (+)-ABA, also has been reported in barley and wheat aleurones (Uknes and Ho, 1984), in potato and Arabidopsis suspension cultures (Windsor and Zeevaart, 1997), in bean leaves (Gergs et al., 1993), and in embryonic axes of chickpea (Babiano, 1995). Only in the latter study (Babiano, 1995) was this activity confirmed by an in vitro assay.
The most surprising thing about the induction response in suspension-cultured maize cells was the sharp spike of induced ABA 8′-hydroxylase activity (Fig. 3). ABA 8′-hydroxylase activity was more labile in vivo than it was after extraction; therefore, the timing for the harvest of induced tissue was critical in obtaining maximum in vitro activity. Such rapid turnover and/or inactivation of this enzyme was unexpected given that sufficient (+)-ABA was still present in the medium to support both continued enzyme induction and metabolism of the substrate. This observation underscores the difficulties in developing a robust in vitro assay for (+)-ABA 8′-hydroxylase from any tissue, and suggests how precisely these activities must be regulated in vivo during the course of normal plant growth and how developmentally sensitive such regulation might be.
The main point of this investigation was to determine whether (+)-ABA 8′-hydroxylase from suspension-cultured maize cells was a member of the Cyt P450 superfamily of enzymes. (+)-ABA 8′-hydroxylase was shown to be an integral membrane protein (Fig. 2). Activity was always recovered in the pellet after ultracentrifugation, even when the primary extract had been incubated with 0.5 m NaCl to remove extrinsic proteins loosely bound to the lipid bilayer (data not shown; Ward, 1984; Rodgers et al., 1993). ABA hydroxylation required both molecular oxygen and NADPH (Figs. 5A and 6A) and was inhibited by tetcyclacis (Fig. 5B) and CO (Fig. 6B). The inhibition by CO was reversible by visible light (Figs. 7 and 8). Attempts to establish spectrophotometrically the CO difference spectra using dithionite-reduced microsomes and specific ABA-dependent type I-binding spectra were unsuccessful, and no absorption peaks attributable to this enzyme were found (J.E. Krochko, unpublished results). This difficulty probably reflects the low amounts of enzyme protein within this tissue. Lacking such spectral data, we cannot say with certainty that the CO-complexed enzyme absorbs light specifically and maximally at 450 nm; however, all of the other data are consistent with the enzyme being a classical Cyt P450 monooxygenase.
Inhibition by tetcyclacis has sometimes been used as a diagnostic tool for Cyt P450-type enzymes in plants in in vitro assays and in vivo studies (Rademacher et al., 1987; Vreugdenhil et al., 1994). Tetcyclacis is not a specific inhibitor of Cyt P450-type enzymes; rather, any enzyme having an exposed Fe group that could interact with the unpaired electrons of the N on the heterocyclic ring of tetcyclacis may be a target (Rademacher et al., 1987). Therefore, inhibition by tetcyclacis does not confirm that (+)-ABA 8′-hydroxylase is a Cyt P450, but is consistent with that classification (Rademacher et al., 1987; Hauser et al., 1990). The sensitivity that we have established for this inhibitor in these assays is several orders of magnitude greater than that reported in some in vivo studies involving ABA hydroxylase (Daeter and Hartung, 1990; Wasternack et al., 1995).
All of the Cyt P450 and Cyt P450-type enzymes identified in plants so far have been localized to the microsomal fraction (Durst, 1991; Schuler, 1996). The majority of these membrane-bound proteins are ER bound, although there are some cases in which Cyt P450s have been found in the plasma membrane (Kjellbom et al., 1985), in the vacuolar fraction (Madyastha et al., 1977), and perhaps in the glyoxysomes and mitochondria (Donaldson and Luster, 1991). There is some controversy regarding the precise cellular location of the (+)-ABA-degrading enzyme(s) in plant tissues. The question has centered on whether (+)-ABA must enter the cell (symplast) to be metabolized, or if it can be degraded to PA while still remaining outside of the cell proper (apoplast). Based on the rapid disappearance of extracellular ABA from the medium of germinating barley embryos, it has been suggested that ABA is degraded by an enzyme that is both soluble and secreted into the medium (Visser et al., 1996). However, this isolated report is inconsistent with the evidence provided here and elsewhere (Gillard and Walton, 1976; Gergs et al., 1993; Babiano, 1995) that ABA 8′-hydroxylase is a membrane-bound Cyt P450.
Additionally, in suspension-cultured maize cells there is evidence for a time-dependent redistribution of (−)-PA from the interior of the cells to the medium, which supports the contention that (+)-ABA is metabolized within the cell (Balsevich et al., 1994). Nonetheless, other authors have provided indirect and circumstantial experimental data in addition to some theoretical considerations to argue that ABA metabolism occurs primarily at the junction of the symplast and the apoplast (Hartung and Slovik, 1991; Betz et al., 1993). The identity of the cellular membrane fraction with which ABA 8′-hydroxylase is associated has not yet been determined. Most Cyt P450s are inserted into the ER; however, the plasma membrane is another possibility and perhaps not an inappropriate one considering the importance of this enzyme in modulating rapidly fluctuating ABA concentrations in leaves (Trejo et al., 1993; Jia et al., 1996).
NADH does not support the hydroxylation of (+)-ABA, although when it was added to reactions already containing limiting NADPH there was a substantial stimulation of hydroxylating activity over that normally found with NADPH alone (Fig. 5A). The synergism of NADPH by NADH in promoting Cyt P450 activity was first described by Cohen and Estabrook in the 1970s using liver microsomal membranes (for review, see Peterson and Prough, 1986). Similar enhancements of NADPH-dependent P450 activity by NADH have been reported for many other Cyt P450 enzymes extracted from plant tissues (e.g. p-coumaroylshikimate 3′-hydroxylase, ferulic acid 5-hydroxylase, and isoflavone synthase; for review, see Durst, 1991).
For each molecule of substrate that is acted upon by a Cyt P450 enzyme, two electrons are transferred sequentially from the cofactor to the Cyt P450 (Groves and Han, 1995). Cyt P450s do not interact directly with reduced pyridine nucleotide cofactors; rather, electron flow is mediated through other accessory proteins such as NADPH-dependent Cyt c P450 reductase (Peterson and Prough, 1986). It has been suggested that whereas the first electron likely is provided by NADPH-dependent Cyt P450 reductase, the second electron might be donated by another component, presumably NADH-dependent Cyt b5 reductase and Cyt b5 (Peterson and Prough, 1986; Donaldson and Luster, 1991). Studies using antibodies specific for each of these reductases (NADH-Cyt b5 reductase and NADPH-dependent Cyt P450 reductase) have confirmed this hypothesis (Benveniste et al., 1989; Ortiz de Montellano, 1995). Our in vitro data for ABA 8′-hydroxylase are consistent with the proposal that Cyt b5 and Cyt b5 reductase, in addition to NADPH-dependent Cyt P450 reductase, are involved in the transfer of electrons to ABA 8′-hydroxylase.
(+)-ABA 8′-hydroxylating activity was inhibited by CO in a concentration-dependent manner, and blue light effectively reversed this inhibition (Figs. 6B and 7). The results of this experiment satisfy the major criterion for the classification of this enzyme as a Cyt P450. However, both amber and red light were also very effective in reversing the CO inhibition (Fig. 8). Absorption of amber light (570 nm) by CO-inhibited Cyt P450s has been exploited in the studies of laser-induced CO photolysis, and it has been established that these proteins have a broad heme-absorption band around this wavelength (Markowitz et al., 1992). There is little published information about light absorption at longer wavelengths, although a Cyt P450 that was purified from tulip bulb microsomes showed maxima at 392, 525, and 645 nm in the oxidized form (Durst, 1991). Red-light reversal of the CO inhibition of a Cyt P450 enzyme has not been demonstrated to our knowledge, which may indicate some unusual properties of the apoprotein-heme-CO interaction in ABA 8′-hydroxylase.
It is likely that the enzyme (+)-ABA 8′-hydroxylase plays a more important role in regulating ABA responses in plant tissues than is appreciated at present. Low sensitivity to ABA (i.e. high concentrations for a predicted effect) has often been assumed to be attributable to reduced receptor or signal transduction effectiveness. An equally likely (and sometimes testable) interpretation is that the applied ABA was rapidly degraded, thereby reducing its effective concentration and biological activity (J.E. Krochko, G.D. Abrams, M.K. Loewen, S.R. Abrams, and A.J. Cutler, unpublished data). Circumstantial evidence suggests that this enzyme is particularly active in developing seeds and germinating seedlings, in young and expanding leaves, in roots and tubers, and in tissues recovering from abiotic stresses (Creelman and Zeevaart, 1984; Zhang and Davies, 1987; Vreugdenhil et al., 1994; Garello and LePage-Degivry, 1995; Walton and Li, 1995; Jia et al., 1996; Zhang et al., 1997; Qi et al., 1998). This supposition was examined in germinating cress seed using a deuterated analog of (+)-ABA, (+)-d9-ABA (Lamb et al., 1996). This compound had been shown to be metabolized to PA more slowly than (+)-ABA in suspension-cultured maize cells, with an observed isotope effect of approximately 2 (Lamb et al., 1996), reflecting the higher strength of the C–D bond relative to the C–H bond. When applied in equivalent concentrations to cress seeds, the deuterated analog retarded germination more than (+)-ABA (Lamb et al., 1996), clearly demonstrating the importance of ABA metabolism and ABA 8′-hydroxylase in the modulation of germination rates in vivo.
ABA 8′-hydroxylase scavenges efficiently for O2 at low O2 tensions (Fig. 6A) and maintains substantial activities both at high and low temperatures (data not shown). In the absence of matching rates of ABA synthesis/import, this may result in drastically reduced ABA concentrations in plant tissues exposed to deteriorating environmental conditions. For example, it has been observed that high temperatures prevent stomatal closure in leaves of intact cotton plants, even during water stress, and this was associated with reduced ABA accumulation and evidence of continued high rates of ABA metabolism (Cornish and Radin, 1990). In this manner, ABA concentrations and physiological responses may be affected by the balance between the rates of synthesis and degradation under changing environmental conditions.
In summary, our data developed from an in vitro assay confirm and extend the characterization by previous authors and strongly suggest that ABA 8′-hydroxylase is a Cyt P450 and a homolog of the enzymes described previously from liquid endosperm of eastern wild cucumber (Gillard and Walton, 1976), bean leaves (Gergs et al., 1993), and chickpea seedlings (Babiano, 1995). (+)-ABA 8′-hydroxylase from suspension-cultured maize cells is membrane associated, requires O2 and NADPH, is inhibited by tetcyclasis and CO, and shows light reversibility of the CO inhibition. Like many other Cyt P450s, this enzyme is inducible and is probably transcriptionally regulated (Cutler et al., 1997). Although it has not been possible to confirm spectrally the presence of a heme-thiolate protein (Cyt P450) in these enzyme extracts, all of the data are consistent with the identification of (+)-ABA 8′-hydroxylase as a classical Cyt P450 monooxygenase.
ACKNOWLEDGMENTS
We are grateful to R. Estabrook (University of Texas Southwestern Medical Center), F.K. Friedman (Laboratory of Molecular Carcinogenesis, National Institutes of Health), and K. Degtyarenko (University of Leeds) for their timely comments and discussions regarding Cyt P450s. John Balsevich kindly provided the (+)-[3H]ABA. We extend our appreciation to Tim Squires for assisting with the HPLC analysis, and David Taylor and Pat Covello for their thoughtful comments and critiques.
Abbreviations:
- DPA
dihydrophaseic acid
- PA
phaseic acid
Footnotes
This is National Research Council of Canada paper no. 40,731.
LITERATURE CITED
- Babiano MJ. Metabolism of [2-14C]abscisic acid by a cell-free system from embryonic axes of Cicer arietinum L. seeds. J Plant Physiol. 1995;145:374–376. [Google Scholar]
- Balsevich JJ, Bishop G, Jacques SL, Hogge LR, Olson DJH, Laganiere N. Preparation and analysis of some acetosugar esters of abscisic acid and derivatives. Can J Chem. 1996;74:238–245. [Google Scholar]
- Balsevich JJ, Cutler AJ, Lamb N, Friesen LJ, Kurz EU, Perras MR, Abrams SR. Response of cultured maize cells to (+)-abscisic acid, (−)-abscisic acid, and their metabolites. Plant Physiol. 1994;106:135–142. doi: 10.1104/pp.106.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Lesot A, Hasenfratz MP, Durst F. Immunochemical characterization of NADPH-cytochrome P-450 reductase from Jerusalem artichoke and other plants. Biochem J. 1989;259:847–853. doi: 10.1042/bj2590847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C, Ullrich CI, Hartung W. Tetcyclacis and abscisic acid-sensitive reduction of extracellular ferricyanide by mesophyll cells of Valerianella locusta and Lemna gibba. J Exp Bot. 1993;44:35–39. [Google Scholar]
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cornish K, Radin JW. From metabolism to organism: an integrated view of water stress emphasizing abscisic acid. In: Katterman F, editor. Environmental Injury to Plants. San Diego, CA: Academic Press; 1990. pp. 89–112. [Google Scholar]
- Creelman RA, Zeevaart JAD. Incorporation of oxygen into abscisic acid and phaseic acid from molecular oxygen. Plant Physiol. 1984;75:166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler AJ, Squires TM, Loewen MK, Balsevich JJ. Induction of (+)-abscisic acid 8′ hydroxylase by (+)-abscisic acid in cultured maize cells. J Exp Bot. 1997;48:1787–1795. [Google Scholar]
- Daeter W, Hartung W. Compartmentation and transport of abscisic acid in mesophyll cells of intact leaves of Valerianella locusta. J Plant Physiol. 1990;136:306–312. [Google Scholar]
- Dathe W, Sembdner G. Isolation of 4′-dihydroabscisic acid from immature seeds of Vicia faba. Phytochemistry. 1982;21:1798–1799. [Google Scholar]
- Donaldson RP, Luster DG. Multiple forms of plant cytochromes P-450. Plant Physiol. 1991;96:669–674. doi: 10.1104/pp.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F. Biochemistry and physiology of plant cytochrome P-450. In: Ruckpaul K, Rein H, editors. Microbial and Plant Cytochromes P-450: Biochemical Characteristics, Genetic Engineering and Practical Implications. Akademie- Berlin: Verlag; 1991. pp. 191–232. [Google Scholar]
- Garello G, LePage-Degivry MT. Desiccation-sensitive Hopea odorata seeds: sensitivity to abscisic acid, water potential and inhibitors of gibberellin biosynthesis. Physiol Plant. 1995;95:45–50. [Google Scholar]
- Gergs U, Hagemann K, Zeevaart JAD, Weiler EW. The determination of phaseic acid by monoclonal antibody-based enzyme immunoassay. Bot Acta. 1993;106:404–410. [Google Scholar]
- Gillard DF, Walton DC. Abscisic acid metabolism by a cell-free preparation from Echinocystis lobata liquid endosperm. Plant Physiol. 1976;58:790–795. doi: 10.1104/pp.58.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JT, Han Y-Z (1995) Models and mechanisms of cytochrome P450 action. In PR Ortiz de Montellano, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp 3–48
- Hartung W, Slovik S. Physico-chemical properties of plant growth regulators and plant tissues determine their distribution and redistribution: stomatal regulation by abscisic acid in leaves. New Phytol. 1991;119:361–382. [Google Scholar]
- Hauser C, Kwiatkowski J, Rademacher W, Grossman K. Regulation of endogenous abscisic acid levels and transpiration in oilseed rape by plant growth retardants. J Plant Physiol. 1990;137:201–207. [Google Scholar]
- Jia W, Zhang J, Zhang DP. Metabolism of xylem-delivered ABA in relation to ABA flux and concentration in leaves of maize and Commelina communis. J Exp Bot. 1996;47:1085–1091. [Google Scholar]
- Kjellbom P, Larsson C, Askerlund P, Schelin C, Widell S. Cytochrome P-450/420 in plant plasma membranes: a possible component of the blue-light-reducible flavoprotein-cytochrome complex. Photochem Photobiol. 1985;42:779–783. [Google Scholar]
- Kraepiel Y, Rousselin P, Sotta B, Kerhoas L, Einhorn J, Caboche M, Miginiac E. Analysis of phytochrome- and ABA-deficient mutants suggests that ABA degradation is controlled by light in Nicotiana plumbaginifolia. Plant J. 1994;6:665–672. [Google Scholar]
- Lamb N, Wahab N, Rose PA, Shaw AC, Abrams SR, Cutler AJ, Smith PJ, Gusta LV, Ewan B. Synthesis, metabolism and biological activity of a deuterated analogue of the plant hormone S-(+)-abscisic acid. Phytochemistry. 1996;41:23–28. [Google Scholar]
- Ludwig SR, Somers DA, Peterson WL, Pohlmann BF, Zarovitz MA, Gengenbach BG, Messing J. High frequency callus formation from maize protoplasts. Theor Appl Genet. 1985;71:344–350. doi: 10.1007/BF00252078. [DOI] [PubMed] [Google Scholar]
- Madyastha KM, Ridway JE, Dwyer JG, Coscia CJ. Subcellular localization of a cytochrome P-450-dependent monooxygenase in vesicles of the higher plant Catharanthus roseus. J Cell Biol. 1977;72:302–313. doi: 10.1083/jcb.72.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz A, Robinson RC, Omata Y, Friedman FK. A flash photolysis instrument with digital smoothing of data using a fast Fourier transform. Anal Instrum. 1992;20:313–321. [Google Scholar]
- Merlot S, Giraudat J. Genetic analysis of abscisic acid signal transduction. Plant Physiol. 1997;114:751–757. doi: 10.1104/pp.114.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaliak CA, Karp F, Croteau R (1993) Cytochrome P-450 terpene hydroxylases. In PJ Lea, ed, Methods in Plant Biochemistry, Vol 9: Enzymes of Secondary Metabolism. Academic Press, New York, pp 261–279
- Nelson DR (1998) Cytochrome P450 database. http://www.drnelson.utmem.edu/nelsonhomepage
- Nelson LAK, Shaw AC, Abrams SR. Synthesis of (+)-, (−)- and (±)-7′ hydroxyabscisic acid. Tetrahedron. 1991;47:3259–3270. [Google Scholar]
- Omura T (1978) Introduction: short history of cytochrome P450. In R Sato, T Omura, eds, Cytochrome P-450. Academic Press, New York, pp 1–21
- Ortiz de Montellano PR (1995) Oxygen activation and reactivity. In PR Ortiz de Montellano, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp 245–303
- Ortiz de Montellano PR, Correia MA (1995) Inhibition of cytochrome P450 enzymes. In PR Ortiz de Montellano, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp 305–364
- Parry AD (1993) Abscisic acid metabolism. In PJ Lea, ed, Methods in Plant Biochemistry, Vol 9: Enzymes of Secondary Metabolism. Academic Press, New York, pp 381–402
- Peterson JA, Prough RA (1986) Cytochrome P-450 reductase and cytochrome b5 in cytochrome P-450 catalysis. In PR Ortiz de Montellano, ed, Cytochrome P-450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp 89–117
- Qi Q, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998;117:979–987. doi: 10.1104/pp.117.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher W, Fritsch H, Graebe JE, Sauter H, Jung J. Tetcyclacis and triazole-type plant growth retardants: their influence on the biosynthesis of gibberellins and other metabolic processes. Pestic Sci. 1987;21:241–252. [Google Scholar]
- Ribaut JM, Martin HV, Pilet PE. Abscisic acid turnover in intact maize roots: a new approach. J Plant Physiol. 1996;148:761–764. [Google Scholar]
- Rodgers MW, Zimmerlin A, Werck-Reichhert D, Bolwell GP. Microsomally associated heme proteins from French bean: characterization of the cytochrome P450 cinnamate-4-hydroxylase and two peroxidases. Arch Biochem Biophys. 1993;304:74–80. doi: 10.1006/abbi.1993.1323. [DOI] [PubMed] [Google Scholar]
- Schuler MA. Plant cytochrome P450 monooxygenases. Crit Rev Plant Sci. 1996;15:235–284. [Google Scholar]
- Trejo CL, Davies WJ, Ruiz LMP. Sensitivity of stomata to abscisic acid. An effect of the mesophyll. Plant Physiol. 1993;102:497–502. doi: 10.1104/pp.102.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes SJ, Ho THD. Mode of action of abscisic acid in barley aleurone layers. Abscisic acid induces its own conversion to phaseic acid. Plant Physiol. 1984;75:1126–1132. doi: 10.1104/pp.75.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan GT, Milborrow BV. The occurrence and metabolism of the 1′,4′-diols of abscisic acid. Aust J Plant Physiol. 1987;14:593–604. [Google Scholar]
- Vaughan GT, Milborrow BV. The stability of the 1′,4′-diols of abscisic acid. Phytochemistry. 1988;27:339–343. [Google Scholar]
- Visser K, Vissers APA, Cagirgan MI, Kijne JW, Wang M. Rapid germination of a barley mutant is correlated with a rapid turnover of abscisic acid outside the embryo. Plant Physiol. 1996;111:1127–1133. doi: 10.1104/pp.111.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil D, Bindels P, Reinhoud P, Klocek J, Hendriks T. Use of the growth retardant tetcyclacis for potato tuber formation in vitro. Plant Growth Regul. 1994;14:257–265. [Google Scholar]
- Wachenfeldt C, Johnson EF (1995) Structures of eukaryotic cytochrome P450 enzymes. In PR Ortiz de Montellano, ed, Cytochrome P450: Structure, Mechanism, and Biochemistry. Plenum Press, New York, pp 183–223
- Walker-Simmons MK, Holappa LD, Abrams GD, Abrams SR. ABA metabolites induce group 3 LEA mRNA and inhibit germination in wheat. Physiol Plant. 1997;100:474–480. [Google Scholar]
- Walton DC, Li Y. Abscisic acid biosynthesis and metabolism. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 140–157. [Google Scholar]
- Ward GM (1984) Labelling of cell membrane proteins. In JC Venter, LC Harrison, eds, Receptor Biochemistry and Methodology: Membranes, Detergents, and Receptor Solubilization. Alan R Liss, New York, pp 109–118
- Wasternack C, Atzorn R, Leopold J, Feussner I, Rademacher W, Parthier B. Synthesis of jasmonate-induced proteins in barley (Hordeum vulgare) is inhibited by the growth retardant tetcyclacis. Physiol Plant. 1995;94:335–341. [Google Scholar]
- West CA (1980) Hydroxylases, monooxygenases, and cytochrome P450. In DD Davies, ed, The Biochemistry of Plants, Vol 2. Academic Press, New York, pp 317–364
- Windsor ML, Zeevaart JAD. Induction of ABA 8′-hydroxylase by (+)-S-, (−)-R- and 8′,8′,8′-trifluoro-S-abscisic acid in suspension cultures of potato and Arabidopsis. Phytochemistry. 1997;45:931–934. doi: 10.1016/s0031-9422(97)00022-8. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]
- Zeevaart JAD, Milborrow BV. Metabolism of abscisic acid and the occurrence of epi-dihydrophaseic acid in Phaseolus vulgaris. Phytochemistry. 1976;15:493–500. [Google Scholar]
- Zhang J, Davies WJ. ABA in root and leaves of flooded pea plants. J Exp Bot. 1987;38:649–659. [Google Scholar]
- Zhang J, Jia W, Zhang D. Re-export and metabolism of xylem-delivered ABA in attached maize leaves under different transpirational fluxes and xylem ABA concentrations. J Exp Bot. 1997;48:1557–1564. [Google Scholar]
- Zou J, Abrams GD, Barton DL, Taylor DC, Pomeroy MK, Abrams SR. Induction of lipid and oleosin biosynthesis by (+)-abscisic acid and its metabolites in microspore-derived embryos of Brassica napus L cv. Reston. Plant Physiol. 1995;108:563–571. doi: 10.1104/pp.108.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]