Abstract
Xylan is the second most abundant polysaccharide on Earth and represents an immense quantity of stored energy for biofuel production. Despite its importance, most of the enzymes that synthesize xylan have yet to be identified. Xylans have a backbone of β-1,4–linked xylose residues with substitutions that include α-(1→2)–linked glucuronosyl, 4-O-methyl glucuronosyl, and α-1,2- and α-1,3-arabinofuranosyl residues. The substitutions are structurally diverse and vary by taxonomy, with grass xylan representing a unique composition distinct from dicots and other monocots. To date, no enzyme has yet been identified that is specific to grass xylan synthesis. We identified a xylose-deficient loss-of-function rice mutant in Os02g22380, a putative glycosyltransferase in a grass-specific subfamily of family GT61. We designate the mutant xax1 for xylosyl arabinosyl substitution of xylan 1. Enzymatic fingerprinting of xylan showed the specific absence in the mutant of a peak, which was isolated and determined by 1H-NMR to be (β-1,4-Xyl)4 with a β-Xylp-(1→2)-α-Araf-(1→3). Rice xax1 mutant plants are deficient in ferulic and coumaric acid, aromatic compounds known to be attached to arabinosyl residues in xylan substituted with xylosyl residues. The xax1 mutant plants exhibit an increased extractability of xylan and increased saccharification, probably reflecting a lower degree of diferulic cross-links. Activity assays with microsomes isolated from tobacco plants transiently expressing XAX1 demonstrated xylosyltransferase activity onto endogenous acceptors. Our results provide insight into grass xylan synthesis and how substitutions may be modified for increased saccharification for biofuel generation.
Keywords: bioenergy, plant cell wall, type II cell walls, arabinoxylan
Xylans are polysaccharides present in the cell walls of all plants. They comprise the major noncellulosic component of secondary walls of angiosperms. Hence, xylans are quantitatively second only to cellulose in biomass on earth. Xylans are valuable components of human and animal nutrition, constituting a major component of dietary fiber in cereals (1). The amount of xylan as well as its chemical composition is an important element of bread making, influencing dough yield, flavor, and shelf life (2). Xylan is of particular interest for the improvement of feedstocks for the generation of cellulosic biofuels, a currently expensive and inefficient process (3). Xylan inhibits access of cellulases to cellulose and is an additional substrate for cross-linking to lignin. Thus, genetic modification of xylan in crop species would rely on improving our knowledge of xylan biosynthesis.
Xylans play an essential structural role in plant cell walls, presumably through interactions with cellulose microfibrils and other components of the wall (4, 5). Although the organization of these various components in the cell wall is not well understood, the increased cross-linking apparently contributes to the recalcitrance of the cell wall (6, 7). Xylans are structurally diverse between taxonomic groups (4, 8). The most basal form of xylan is a main chain that lacks substitutions as seen in the green alga Caulerpa that has β-1,3-d-xylan in place of cellulose, and the red seaweeds Palmariales and Nemaliales that have a mixed linkage β-(1,3-1,4)-d-xylose backbone (8). Xylans of embryophytes have a β-1,4–linked xylose backbone. Xylans found in dicots are mostly restricted to the secondary cell walls, and hence a main component of wood. Dicot xylans are commonly substituted with α-(1→2)-linked glucuronosyl and 4-O-methyl glucuronosyl residues (1). Xylans in birch, spruce, and Arabidopsis have been found to contain the reducing end oligosaccharide β-d-Xylp-(1→4)- β-d-Xylp-(1→3)-α-l-Rhap-(1→2)-α- d-GalpA-(1→4)- d-Xylp (9–11) which, interestingly, has not been found in the xylan of grasses. Commelinid monocot xylans are unique from those of dicots and other monocots. This group, which includes the grasses, contains xylan as the main noncellulosic component in both the primary and secondary cell walls (12). These xylans have very little glucuronosyl residues, but are mostly substituted with α-1,2 and α-1,3 arabinosyl residues. A unique feature of grass xylans is the ferulate and coumarate esters linked to the O-5 of some of the α-1,3 arabinosyl residues. These ferulate esters mediate intra- and intermolecular cross-linking, possibly increasing the strength of the cell wall (7). A common feature of grass xylans is the further substitution of the feruloyl-α-l-arabinofuranosyl residues with β-d-xylose linked to O-2 of the arabinose (13–17). Some of these xylosyl residues can be further substituted with β-1,4-linked d-galactose (18). Among these ferulate-containing xylan sidechain variants, 2-O-β-d-xylopyranosyl-(5-O-feruloyl)-l-arabinose has been found to be the most abundant, and in some cases is as abundant as 5-O-feruloyl-l-arabinofuranose (13, 15, 19).
During the past 6 y, there has been significant progress made toward identifying the glycosyltransferases (GTs) that synthesize xylan. The irregular xylem (irx) mutants, named for their collapsed xylem vessels due to their secondary cell wall deficiency, have been instrumental in elucidating the mechanisms of xylan biosynthesis in Arabidopsis thaliana (Arabidopsis). IRX9/IRX9L and IRX14/IRX14L from the GT43 family and IRX10/IRX10L from GT47 are thought to be responsible for elongation of the xylan backbone (20–25). IRX7 (FRA8)/IRX7L (F8H) (from GT47), IRX8 (GAUT12) (from GT8), and PARVUS (from GT8) may be responsible for synthesizing the oligosaccharide found at the reducing end of some dicot and conifer xylans (4, 21, 26, 27). GUX1 and GUX2 (from GT8) are thought to be responsible for adding both glucuronosyl and 4-O-methylglucuronosyl substitutions to the xylan backbone in Arabidopsis (28–30). Recently, rice and wheat GT61 family genes were found to be responsible for α-(1,3)-arabinosyl substitution on xylan (31). Thus, far, the enzymes that add the β-(1,2)-xylosyl residues to xylan have not been identified.
Here, we report the characterization of a mutant xax1 in a rice GT61 family gene. Our results strongly indicate that the corresponding protein possesses β-1,2-xylosyl transferase activity, transferring xylose from UDP-xylose onto xylan. Mutant xax1 rice plants have a dwarfed phenotype and the leaves are deficient in xylose, ferulic acid, and coumaric acid, and have increased saccharification efficiency. These findings add further to our understanding of xylan biosynthesis and the mechanisms of ferulic acid addition to xylan and have important implications for biofuel production.
Results
XAX1 Is a Previously Uncharacterized Xylan Biosynthesis Gene in Rice.
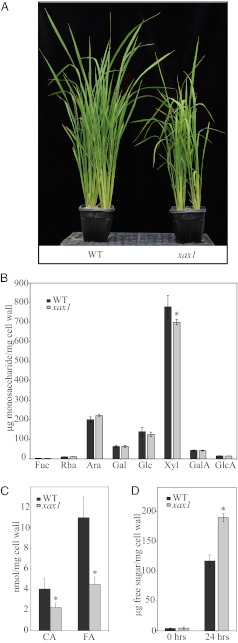
To discover genes important for grass xylan biosynthesis, we conducted a reverse genetics screen of 14 genes with insertional rice mutants that are highly expressed members of the GT61 family (Tables S1 and S2), a family previously identified through bioinformatic approaches as being expanded in grasses and containing grass-specific subgroups (29, 32, 33). Our screen prioritized lines with segregating phenotypes that had altered sugar composition, and from this screen we identified a mutant with a mutation in the third exon that knocked out Os02g22380 (Fig. S1), a gene member that is part of a grass-specific clade in the GT61 family (Fig. S2, clade C.IV). We identified this gene as a putative xylosyltransferase, based on its dwarfed phenotype and xylose deficiency in cell wall alcohol insoluble residue (AIR) in young rice leaves (Fig. 1 A and B). Whereas the majority of all xylose in rice is derived from xylan, we confirmed that the deficiency was seen in the xylan-rich fractions (1 M KOH and 4 M KOH) of sequentially extracted cell wall material (Fig. S3 F and G). Based on this biochemical phenotype and subsequent data described below, we named the mutant xylosyl arabinosyl substitution of xylan 1 (xax1).
Fig. 1.
XAX1 T-DNA insertional rice mutant. (A) Dwarfed phenotype of xax1 plants at 5 wk. (B) Monosaccharide composition of total plant cell wall after TFA hydrolysis, separated and quantified on the HPAEC. (C) Ferulic and p-coumaric acid amounts released upon base hydrolysis of total cell wall preparations and acid-catalyzed depolymerization of carbohydrate-free residues. (D) Saccharification of cell wall after 120 °C pretreatment and cellulase and hemicellulase enzymatic digestion; quantified by DNS. Error bars represent SD with at least three biological replicates. *Significantly different from WT, t test: P < 0.05.
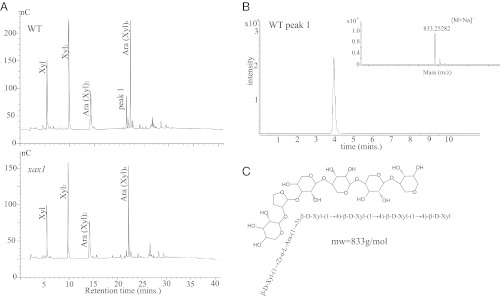
To gain further insight into the nature of the xylose deficiency, AIR was subjected to partial hydrolysis in 0.1 M trifluoroacetic acid (TFA). This treatment preferentially cleaves arabinofuranosyl residues, leaving the disaccharide relatively more susceptible to hydrolysis than the main chain xylosyl residues (13, 34). A comparison of partial versus complete hydrolysis of AIR showed a stronger decrease in xylose (7.0%) than with total hydrolysis (2.6%), which indicates that the xylose deficiency may be a substitution rather than a xylan chain deficiency (Fig. S3 A and B). Extraction of saponified AIR revealed a strong decrease in both ferulic acid (59%) and coumaric acid (44%) (Fig. 1C), aromatic compounds known to be attached to arabinosyl residues in xylans that are substituted with xylosyl residues (13). The 4 M KOH extract of depectinated AIR was further characterized by enzymatic fingerprinting with M6 endoxylanase (35). The released oligosaccharides were separated and quantified by high performance anion exchange chromatography with electrochemical detection (HPAEC-PAD) (Fig. 2A). Notably, xax1 showed an absence of one peak eluting at about 22 min, whereas the four other major peaks were found in both wild type and mutant. The peak, which was only detectable in wild type, “peak 1,” was isolated from and determined by mass spectrometry to have m/z = 833.25282 Da, which is consistent within 1.3 ppm with the sodium adduct of an oliogosaccharide composed of six pentoses (i.e., Xyl and Ara) (Fig. 2B). The secondary peaks at 834.26 and 835.26 are consistent with the naturally occurring isotopes of xylohexaose. Unlike the peaks identified as AraXyl2 and AraXyl4, peak 1 was not modified with α-arabinofuranosidase treatment (Fig. S4B), an enzyme that hydrolyzes terminal α-arabinofuranose linkages (36) indicating that the peak lacked terminal α-arabinofuranose residues, even though it contained arabinose based on total hydrolysis of the isolated peak (Fig. S4C). 1H-NMR analysis of the isolated peak strongly indicates that the oligosaccharide is (β-1,4-Xylp)4 with a β-Xylp-(1→2)-α-Araf-(1→3) sidechain most likely present on the second xylose residue from the nonreducing end (Fig. 2C and Fig. S5). Glycosidic linkage analysis of extracted xylan (4 M KOH extract of depectinated AIR) from xax1 showed a 56% decrease in arabinofuranose residues substituted at O-2 and a concomitant increase in terminal arabinose compared with wild type (Table S3), consistent with changes expected from the decrease in xax1 of arabinofuranose residues substituted at the O-2 position with xylosyl residues.
Fig. 2.
Xylan fingerprinting and characterization of oligomer lacking in mutant. (A) HPAEC profiles of oligosaccharides released after endoxylanase treatment of xylan (4 M KOH fraction following pectin removal), showing peak 1 not found in mutant plants. Profile consistent between three biological replicates. (B) LC/MS of isolated peak 1 fraction from wild-type rice. (C) Structure of peak 1 as determined by NMR spectroscopy.
XAX1 Is a Golgi-Localized Protein Specifically Expressed in Young Tissue and Has Xylosyltransferase Activity.
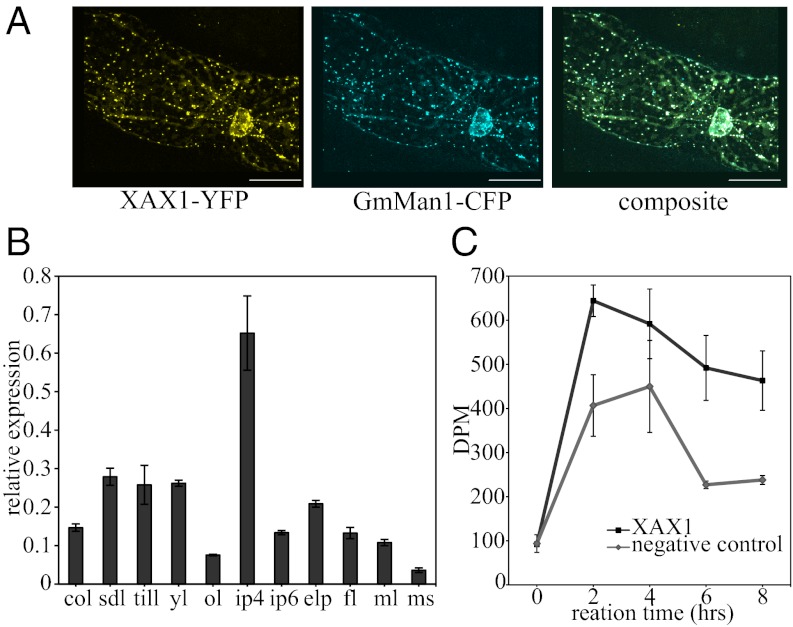
To assess the proposed xylosyltransferase activity of the XAX1 protein, we isolated microsomes from leaves of Nicotiana benthamiana transiently expressing XAX1 and measured xylose incorporation onto endogenous acceptors in the presence of UDP-14C-xylose. Peak activity was measured at 2 h for XAX1 microsomes and 4 h for endogenous XylT activity (Fig. 3C). Overall, XAX1 microsomes mediate a 62% increase in XylT activity over the endogenously measured activity. To confirm that endogenous xylose-4-epimerase activity was not a significant factor in the results, we hydrolyzed the endogenous product to monosaccharide units and separated the monosaccharides by TLC to determine radioactivity corresponding to xylose and arabinose. We found that the primary activity was from incorporation of xylose and that incorporation of arabinose was less than 22% of the total activity (Fig. S4D). GT activity studies in mung bean microsomes also found the epimerase activity to have a minimal effect on glycosyl transferase assays with UDP-arabinose (37). Whether XAX1 is acting directly on xylan substrates in the microsomes or activating endogenous xylosyl transferases in the microsomes is unknown. It is also unknown what endogenous acceptors are present in tobacco; although a detailed biochemical analysis is yet to be completed on tobacco xylan, analyses from diverse dicot species, including, sycamore (38), flax (39), psyllium (40), Litsea (41, 42), and Cinnamomum cassia (43), have been characterized to have arabinosyl residues on their xylan, indicating that such residues are common if not universal among dicots, also indicating that tobacco may also have some arabinosyl residues on xylan acting as the endogenous acceptors.
Fig. 3.
Characterization of XAX1 protein localization and function. (A) Subcellular localization of fluorescently tagged XAX1 proteins. Confocal imaging (40×) of onion epidermal cells expressing XAX1-YFP and GmMan1-CFP, the α-mannosidae Golgi marker. (Scale bar, 50 μm.) (B) Relative expression of XAXT in wild-type rice. col, coleoptile; sdl, 7 dpg seedling; till, 30 dpg tiller; yl, 30 dpg young leaf; ol, 30 dpg old leaf; ip4, immature panicle of 4 cm; ip6, developing panicle of 6 cm; elp, emerging panicle; fl, flag leaf; ml, mature leaf; ms, mature stem. (C) Xylosyltransferase activity reaction products. UDP-[14C]xylose incorporation to microsomes with an endogenous acceptor. Negative control is leaves infiltrated without XAX1 and represents the endogenous xylosyltransferase activity. Error bars are SEM with n = 3 biological replicates.
XAX1 is primarily expressed in younger tissue, including seedlings 7 d postgermination (dpg) and has the highest expression in the immature panicle, and the lowest expression in mature stems and older leaves (Fig. 3B and Table S4). We wanted to determine whether XAX1 localizes with other previously characterized xylan synthesis enzymes in the Golgi apparatus (44). As with other members of the GT61 family, XAX1 has an N-terminal putative transmembrane domain (Fig. S1B). To determine cellular localization, the XAX1 protein was fused to a C-terminal YFP and transiently expressed in onion cells. XAX1 colocalized with the GmMan1-CFP, the α-mannosidase I cis-Golgi marker (Fig. 3A), indicating that XAX1 is localized where xylan is known to be synthesized.
Absence of Xylose Substitution Improves Saccharification.
Cell walls from the xax1 mutant exhibited a 62% increase in the total sugars released compared with wild type after a 24-h treatment with an enzyme mixture (Ctec2; Novozymes) that contained cellulase, β-glucosidase, and hemicellulases (Fig. 1D). The cellulose content of the mutant was the same as wild type (Fig. S3H), and size exclusion chromatography found no difference in the size of the xylan molecules (Fig. S4A), which indicates that the increased extractability is not due to increased carbohydrate content in the mutant. Additionally, CDTA and Na2CO3 pectin-rich fractions, which contained ∼2.5% of the xylose content in wild type (Fig. S3C), showed that the xylose was more easily extracted in the mutant, also indicating a more easily extractable hemicellulose (Fig. S3 D and E). The improved saccharification may be due to a decrease in the diferulic cross-links (45–47) or because a more easily extracted xylan allows cellulase enzymes better access to cellulose (48).
Discussion
Evolution of a Grass-Specific Xylan Substitution.
XAX1 is a member of glycosyltransferase family GT61, and we have shown it to be essential for the grass specific β-(1,2) xylosyl substitution of α-(1,3) arabinosyl residues, a disaccharide substitution on the xylan chain that is unique to grasses. The GT61 family was recently identified through bioinformatic analysis to be highly grass diverged (32, 33, 49). Enzymes are grouped into the GT61 family on the basis of a transmembrane domain and a DUF563 protein sequence [carbohydrate-active enzyme (CAZy) database] (50) (Fig. S1B). We have found a single DUF563 sequence in the brown algae Ectocarpus siliculosus, which suggests that this family originated around the time brown algae evolved or before; we have used this brown algae sequence to root our GT61 tree. There are at least three distinct clades identifiable in the GT61 phylogenetic tree (Fig. S2, clades A, B, and C) (49). Clades A and B, which both include moss genes, are represented relatively evenly between grasses and dicots. Clade B genes have diverged far less than other genes in the family, and are generally represented by only a single sequence per genome (At5G55500 and Os08g39380 in Arabidopsis and rice, respectively). One member of this clade, At5g55500, has been previously characterized to have β-(1,2)-xylosyltransferase activity, transferring xylose to N-linked glycans (51). There is nothing yet known about clade A, but it does contain five Selaginella representatives. We found Selaginella leaves to have no detectable arabinose on their xylan (Fig. S4E) and propose that this clade does not contain arabinosyl transferases involved in xylan biosynthesis. Most likely clade A has a function similar to that of clade B or an as yet uncharacterized function. However, clade C, which contains Os02g22380 (XAX1), is dramatically expanded in the grasses, which is particularly true for groups III and IV, which have no known dicot orthologs. The recently characterized rice and wheat α-(1,3)-arabinosyltransferases are part of clade C.I and clade C.II (XAT1, XAT2, TaXAT1, and TaXAT2) (31). Clade C.II contains the only dicot orthologs of Clade C (Fig. S2) (31). Arabinose substitutions on xylan are abundant in grasses, but not unique to them. Whereas O-3 substituted arabinose is characteristic of the grasses, many dicot species have O-2 substituted arabinose (38, 41, 42). In addition, flax and psyllium seed mucilage have been shown to contain α-(1,2) α-(1,3) disubstituted or α-(1,3) monosubstituted arabinose on xylan, respectively (39, 40). Thus, having dicot orthologs in Clade C.II is not surprising. That XAX1 is located in the grass-unique clade C.IV, appears correct, because the β-Xyl-α-Ara disaccharide substitution is a grass-specific trait in xylan and associated with ferulate esters, which are also unique features of grass xylans.
Role of XAX1 in the Context of Ferulic Acid Esters.
The decreased content in feruloyl and coumaroyl esters in the xax1 mutant is interesting and somewhat unexpected. It is not clear how hydroxycinnamate esters are added to xylan, but it is known to take place in the Golgi, and there is evidence that acyltransferases belonging to the BAHD family are involved (17, 52). The BAHD enzymes are cytoplasmic, suggesting that they do not directly ferulate xylan, but add a feruloyl residue to an intermediate. A likely intermediate that has been proposed is UDP-arabinofuranose (53), which is also synthesized in the cytoplasm—again somewhat surprisingly because the precursor, UDP-arabinopyranose, is largely synthesized inside the Golgi lumen (54). However, if UDP-feruloyl-arabinose were the substrate for feruloylation of xylan, we would not expect to see a decrease in ferulate ester content in xax1. The decreased hydroxycinnamate in the mutant suggests that ferulic acid is added subsequently to the addition of xylose to the unferuloylated arabinose residues. In that case, the intermediate for feruloylation would be another as yet unidentified compound. Alternatively, hydroxycinnamates attached to arabinose in xax1 are more susceptible to being removed by ferulic acid esterases. If the latter is the case, it would suggest a role of β-1,2-xylosyl substitutions on grass xylans in stabilizing the ferulic acid esters.
Rice Xylan Lacking β-(1,2) Xyl Substitution Have Improved Release of Sugars.
Mutant xax1 plants exhibit an increased saccharification efficiency (Fig. 1D) and an increased extractability of xylan (Fig. S3 D and E), most likely due to the lower ferulic cross-links (Fig. 1C), but it may also be due to increased solubility of the hemicelluloses and hence possible increased access of cellulases to cellulose. There does appear to be a slight increase in arabinose substitutions (Fig. 1B) possibly as a compensatory mechanism due to the xax1 mutation. Based on the linkage analysis (Table S3), there is an increase in disubstituted xylose (2,3,4-Xyl) presumably with α-(1,2) and α-(1,3) arabinose. This increase in arabinose would account for a degree of the increased extractability of xylan, but we propose that, whereas this ∼10% increase in arabinose may account for a fraction of the 62% increase in saccharification efficiency, it most likely does not account for the majority of the increased saccharification observed in xax1. We find it more likely that the increased saccharification is largely a consequence of the 59% decrease in ferulic acid (Fig. 1C).
In conclusion, mutant xax1 plants show a dwarfed phenotype and increased extractability that demonstrates the importance of the β-1,2-xylosyl substitutions on arabinoxylan. These findings also provide us with new insights into xylan synthesis and a unique capability to modify ferulic cross-links by targeting a glycosyltransferase. As ferulic cross-links inhibit sugar release as well as microbial fermentation, this is a potentially important biotechnological tool for grass biofuel feedstocks.
Materials and Methods
Details of plant material, reagents, quantitative RT-PCR, microscopy, construction of GT61 phylogeny, liquid chromatography (LC)/TOF mass spectrometry, NMR, and primers are described in SI Materials and Methods.
AIR Preparation and Analysis.
AIR was prepared and analyzed with a HPAEC-PAD as described (21) and in SI Materials and Methods. The extraction of aromatic compounds from AIR was performed as described (55) and in SI Materials and Methods. For the saccharification assay, 5 mg destarched AIR was autoclaved at 120 °C for 1 h. A 500-μL enzyme mixture containing 50 mM citrate buffer pH 6.2, 1.6% (wt/vol) tetracycline, 2 μL Ctec2 enzyme mixture (Novozymes), which contains cellulases, β-glucosidases, and hemicellulases, was added and incubated at 50 °C for 24 h with shaking. After enzyme treatment, samples were pelleted, and released sugars in the supernatant were measured using DNS reagent [1% (wt/vol) 3,5-dinitrosalicylic acid, 30% (wt/vol) potassium sodium tartrate, 400 mM NaOH] reading absorbance at 540 nm.
Enzymatic Fingerprinting of Xylan.
Xylan was extracted from AIR essentially as previously described (56). Following 1,2-Cyclohexylenedi nitrilo-tetraacetic Acid (CDTA) and Na2CO3 extraction, 5 mg AIR was extracted with 4 M KOH, neutralized with glacial acetic acid, dialyzed extensively against water, and incubated with 1 unit endoxylanase (Megazyme) in 0.05 M ammonium acetate buffer (pH 6) at 42 °C for 24 h. The reaction mixture was heated at 100 °C for 10 min to stop the reaction and centrifuged before HPAEC analysis. In some experiments, the endoxylanase-treated extract was further incubated with 0.25 units α-L arabinofuranosidase from Aspergillus niger (Megazyme) in 0.05 M sodium acetate buffer (pH 4) at 42 °C for 1 h. The reaction was stopped by boiling at 100 °C for 10 min and centrifuged. The oligosaccharides released after enzymatic digestion were separated by HPAEC on a Dionex DX 600 system equipped with a pulsed amperometric detector (PAD). A CarboPac PA200 column (3× 250 mm) eluted with a gradient of 0–250 mM sodium acetate in 100 mM NaOH over 40 min at 0.4 mL/min was used. Xylose, arabinose, and xylooligosaccharides (xylobiose, xylotriose, xylotetraose, xylopentaose, and xylohexaose) (Megazyme) were run as standards.
XylT Activity Assay.
Infiltration of 4-wk-old N. benthamiana leaves was done using Agrobacterium tumefasciens strain C58 (OD = 1), following the method described in ref. 57. For details on the plasmid constructs, see SI Materials and Methods. Leaves were collected 5 d after infiltration, flash frozen, ground in a mortar and pestle in 15 mL of buffer [50 mM Hepes pH 7.0, 400 mM sucrose, 1 mM PMSF, 1% (wt/vol) PVPP, protease inhibitor mixture]. The buffer with plant material was filtered through Miracloth mesh and centrifuged at 3,000 × g for 10 min. The supernatant was centrifuged at 50,000 × g for 1 h. (Beckman Ultracentrifuge). The pellets containing the microsomes were resuspended in a 50-mM Hepes pH 7.0, 400-mM sucrose buffer and stored at −80 °C. Protein concentration was determined using the Bradford method. Microsomes corresponding to 100 μg protein were incubated with 50 mM Hepes (pH 7.0), 400 mM sucrose, 5 mM MnCl2, 20 nCi UDP-[14C]xylose (American Radiolabeled Chemicals) in a total reaction volume of 50 μL. After incubation at 24 °C for various times, the reaction was stopped by adding 70% ice-cold ethanol and 100 µg dextran as a carrier. The products were resuspended and washed in 70% ethanol in a sonicator bath (VWR) at 30 °C for 30 min, vortexed, precipitated at 20 °C for 30 min, and repeated the wash until no counts were found in the wash solution. The pellet was resuspended in water, an equal volume of scintillation fluid was added (National Diagnostics), and the amount of activity was determined using a scintillation counter set to measure 14C counts for 2 min (Beckman; LS 6500).
Supplementary Material
Acknowledgments
We thank Dr. Ahn of Postech for providing rice mutant seeds; Dr. Peijian Cao for assistance with global gene expression analyses; and Huong Nguyen, Sherry Chan, and Dr. A. Michelle Smith-Moritz for technical assistance. This work, conducted by the Joint BioEnergy Institute, was supported by the Office of Science, Office of Biological and Environmental Research, of the Department of Energy under Contract DE-AC02-05CH11231. Funding for this work was provided in part by National Science Foundation Collaborative Research and Education in Agricultural Technologies and Engineering- Integrative Graduate Education and Research Traineeship (CREATE-IGERT) (NSF DGE-0653984). Peter Ulvskov was supported by a Villum-Kann Rasmussen grant to the Pro-Active Plant Centre (www.proactiveplants.life.ku.dk). J.H. was supported by the Danish Research Council Grant FTP-09-066624 (to P.U.) and by the Villum Foundation’s Young Investigator Programme.
Footnotes
Conflict of interest statement: The authors declare that a related patent application has been filed. Chiniquy DM, Ronald PC, Scheller HV. Rice Os02g22380 encodes a glycosyltransferase critical for xylose biosynthesis in the cell wall. US Patent application serial number: 61/676,536.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202079109/-/DCSupplemental.
References
- 1.Ebringerova A, Heinze T. Xylan and xylan derivatives–biopolymers with valuable properties, 1. Naturally occurring xylans structures, isolation procedures and properties. Macromol Rapid Commun. 2000;21:542–556. [Google Scholar]
- 2.Vinkx C, Delcour J. Rye (Secale cereale L.) arabinoxylans: A critical review. J Cereal Sci. 1996;24:1–14. [Google Scholar]
- 3.Klein-Marcuschamer D, Holmes B, Simmons B, Blanch H. Biofuel economics. In: Hood E, Powell R, Nelson P, editors. Plant Biomass Conversion. 1 Ed. Wiley, New York: 2011. pp. 329–348. [Google Scholar]
- 4.Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 5.Grabber JH, Ralph J, Hatfield RD. Cross-linking of maize walls by ferulate dimerization and incorporation into lignin. J Agric Food Chem. 2000;48:6106–6113. doi: 10.1021/jf0006978. [DOI] [PubMed] [Google Scholar]
- 6.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 7.Grabber J. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 2005;45:820–831. [Google Scholar]
- 8.Ebringerová A, Heinze T. Hemicellulose. Adv Polym Sci. 2005;186:1–67. [Google Scholar]
- 9.Johansson M, Samuelson O. Reducing end groups in birch xylan and their alkaline degradation. Wood Sci Technol. 1977;11:251–263. [Google Scholar]
- 10.Andersson S, Samuelson O, Ishihara M, Shimizu K. Structure of the reducing end-groups in spruce xylan. Carbohydr Res. 1983;111:283–288. [Google Scholar]
- 11.Peña MJ, et al. Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel J. Unique aspects of the grass cell wall. Curr Opin Plant Biol. 2008;11:301–307. doi: 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Wende G, Fry S. 2-O-β-D-xylopyranosyl-(5-O-feruloyl)-L-arabinose, a widespread component of grass cell walls. Phytochemistry. 1997;44:1019–1030. [Google Scholar]
- 14.Kusakabe I, Ohgushi S, Yasui T, Kobayashi T. Structures of the arabinoxylo-oligosaccharides from the hydrolytic products of corncob arabinoxylan by a xylanase from Streptomyces. Agric Biol Chem. 1983;47:2713–2723. [Google Scholar]
- 15.Höije A, et al. Evidence of the presence of 2-O-β-D-xylopyranosyl-α-l-arabinofuranose side chains in barley husk arabinoxylan. Carbohydr Res. 2006;341:2959–2966. doi: 10.1016/j.carres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Yui T, Imada K, Shibuya N, Ogawa K. Conformation of an arabinoxylan isolated from the rice endosperm cell wall by X-ray diffraction and a conformational analysis. Biosci Biotechnol Biochem. 1995;59:965–968. doi: 10.1271/bbb.59.965. [DOI] [PubMed] [Google Scholar]
- 17.Obel N, Porchia AC, Scheller HV. Intracellular feruloylation of arabinoxylan in wheat: Evidence for feruloyl-glucose as precursor. Planta. 2003;216:620–629. doi: 10.1007/s00425-002-0863-9. [DOI] [PubMed] [Google Scholar]
- 18.Saulnier L, Vigouroux J, Thibault JF. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr Res. 1995;272:241–253. doi: 10.1016/0008-6215(95)00053-v. [DOI] [PubMed] [Google Scholar]
- 19.Pastell H, Virkki L, Harju E, Tuomainen P, Tenkanen M. Presence of 1→3-linked 2-O-β-d-xylopyranosyl-α-l-arabinofuranosyl side chains in cereal arabinoxylans. Carbohydr Res. 2009;344:2480–2488. doi: 10.1016/j.carres.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Persson S, et al. The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell. 2007;19:237–255. doi: 10.1105/tpc.106.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DM, et al. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J. 2007;52:1154–1168. doi: 10.1111/j.1365-313X.2007.03307.x. [DOI] [PubMed] [Google Scholar]
- 22.Geisler-Lee J, et al. A predicted interactome for Arabidopsis. Plant Physiol. 2007;145:317–329. doi: 10.1104/pp.107.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown DM, Zhang Z, Stephens E, Dupree P, Turner SR. Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J. 2009;57:732–746. doi: 10.1111/j.1365-313X.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu AM, et al. The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J. 2009;57:718–731. doi: 10.1111/j.1365-313X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- 25.Faik A. Xylan biosynthesis: News from the grass. Plant Physiol. 2010;153:396–402. doi: 10.1104/pp.110.154237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, et al. The PARVUS gene is expressed in cells undergoing secondary wall thickening and is essential for glucuronoxylan biosynthesis. Plant Cell Physiol. 2007;48:1659–1672. doi: 10.1093/pcp/pcm155. [DOI] [PubMed] [Google Scholar]
- 27.Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. Arabidopsis: A powerful model system for plant cell wall research. Plant J. 2010;61:1107–1121. doi: 10.1111/j.1365-313X.2010.04161.x. [DOI] [PubMed] [Google Scholar]
- 28.Mortimer JC, et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA. 2010;107:17409–17414. doi: 10.1073/pnas.1005456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oikawa A, et al. An integrative approach to the identification of Arabidopsis and rice genes involved in xylan and secondary wall development. PLoS ONE. 2010;5:e15481. doi: 10.1371/journal.pone.0015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennie E, et al. Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant Physiol. 2012;159:1408–1417. doi: 10.1104/pp.112.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders N, et al. Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci USA. 2012;109:989–993. doi: 10.1073/pnas.1115858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao PJ, Bartley LE, Jung KH, Ronald PC. Construction of a rice glycosyltransferase phylogenomic database and identification of rice-diverged glycosyltransferases. Mol Plant. 2008;1:858–877. doi: 10.1093/mp/ssn052. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell RA, Dupree P, Shewry PR. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol. 2007;144:43–53. doi: 10.1104/pp.106.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry S. The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Caldwell, NJ: Blackburn Press; 1988. [Google Scholar]
- 35.Ingelbrecht JA, Verwimp T, Delcour JA. Endoxylanases in durum wheat semolina processing: Solubilization of arabinoxylans, action of endogenous inhibitors, and effects on rheological properties. J Agric Food Chem. 2000;48:2017–2022. doi: 10.1021/jf991299b. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko S, Ishii T, Kobayashi H, Kusakabe I. Substrate specificities of α-L-arabinofuranosidases produced by two species of Aspergillus niger. Biosci Biotechnol Biochem. 1998;62:695–699. doi: 10.1271/bbb.62.695. [DOI] [PubMed] [Google Scholar]
- 37.Nunan KJ, Scheller HV. Solubilization of an arabinan arabinosyltransferase activity from mung bean hypocotyls. Plant Physiol. 2003;132:331–342. doi: 10.1104/pp.102.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darvill JE, McNeil M, Darvill AG, Albersheim P. Structure of plant cell walls. XI. Glucuronoarabinoxylan, a second hemicellulose in the primary cell walls of suspension-cultured sycamore cells. Plant Physiol. 1980;66:1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naran R, Chen G, Carpita NC. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 2008;148:132–141. doi: 10.1104/pp.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer MH, et al. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk) Carbohydr Res. 2004;339:2009–2017. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Herath HM, Kumar NS, Wimalasiri KM. Structural studies of an arabinoxylan isolated from Litsea glutinosa (Lauraceae) Carbohydr Res. 1990;198:343–351. doi: 10.1016/0008-6215(90)84304-d. [DOI] [PubMed] [Google Scholar]
- 42.Wimalasiri K, Kumar N. A water-soluble polysaccharide from the leaves of Litsea gardneri (Lauraceae) Carbohydrate Polymers. 1995;26(1):19–23. [Google Scholar]
- 43.Kanari M, et al. A reticuloendothelial system-activating arabinoxylan from the bark of Cinnamomum cassia. Chem Pharm Bull (Tokyo) 1989;37:3191–3194. doi: 10.1248/cpb.37.3191. [DOI] [PubMed] [Google Scholar]
- 44.Porchia AC, Sørensen SO, Scheller HV. Arabinoxylan biosynthesis in wheat. Characterization of arabinosyltransferase activity in Golgi membranes. Plant Physiol. 2002;130:432–441. doi: 10.1104/pp.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buanafina MM, Langdon T, Hauck B, Dalton SJ, Morris P. Manipulating the phenolic acid content and digestibility of italian ryegrass (Lolium multiflorum) by vacuolar-targeted expression of a fungal ferulic acid esterase. Appl Biochem Biotechnol. 2006;129-132:416–426. doi: 10.1007/978-1-59745-268-7_34. [DOI] [PubMed] [Google Scholar]
- 46.Buanafina MM, Langdon T, Hauck B, Dalton S, Morris P. Expression of a fungal ferulic acid esterase increases cell wall digestibility of tall fescue (Festuca arundinacea) Plant Biotechnol J. 2008;6:264–280. doi: 10.1111/j.1467-7652.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 47.Lam TB, Iiyama K, Stone BA. Hot alkali-labile linkages in the walls of the forage grass Phalaris aquatica and Lolium perenne and their relation to in vitro wall digestibility. Phytochemistry. 2003;64:603–607. doi: 10.1016/s0031-9422(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Wyman CE. Effect of xylanase supplementation of cellulase on digestion of corn stover solids prepared by leading pretreatment technologies. Bioresour Technol. 2009;100:4203–4213. doi: 10.1016/j.biortech.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 49.Vogel J, et al. International Brachypodium Initiative Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature. 2010;463:763–768. doi: 10.1038/nature08747. [DOI] [PubMed] [Google Scholar]
- 50.Cantarel BL, et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strasser R, et al. Molecular cloning and functional expression of beta1, 2-xylosyltransferase cDNA from Arabidopsis thaliana. FEBS Lett. 2000;472:105–108. doi: 10.1016/s0014-5793(00)01443-5. [DOI] [PubMed] [Google Scholar]
- 52.Piston F, et al. Down-regulation of four putative arabinoxylan feruloyl transferase genes from family PF02458 reduces ester-linked ferulate content in rice cell walls. Planta. 2010;231:677–691. doi: 10.1007/s00425-009-1077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buanafina M. Feruloylation in grasses: Current and future perspectives. Molecular Plant. 2009;2:5861–5872. doi: 10.1093/mp/ssp067. [DOI] [PubMed] [Google Scholar]
- 54.Rautengarten C, et al. The interconversion of UDP-arabinopyranose and UDP-arabinofuranose is indispensable for plant development in Arabidopsis. Plant Cell. 2011;23:1373–1390. doi: 10.1105/tpc.111.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rautengarten C, et al. Arabidopsis deficient in cutin ferulate encodes a transferase required for feruloylation of ω-hydroxy fatty acids in cutin polyester. Plant Physiol. 2012;158:654–665. doi: 10.1104/pp.111.187187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harholt J, et al. ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol. 2006;140:49–58. doi: 10.1104/pp.105.072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin L, et al. The cooperative activities of CSLD2, CSLD3, and CSLD5 are required for normal Arabidopsis development. Mol Plant. 2011;4:1024–1037. doi: 10.1093/mp/ssr026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.