Abstract
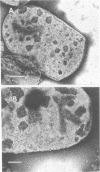
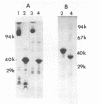
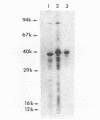
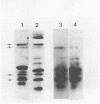
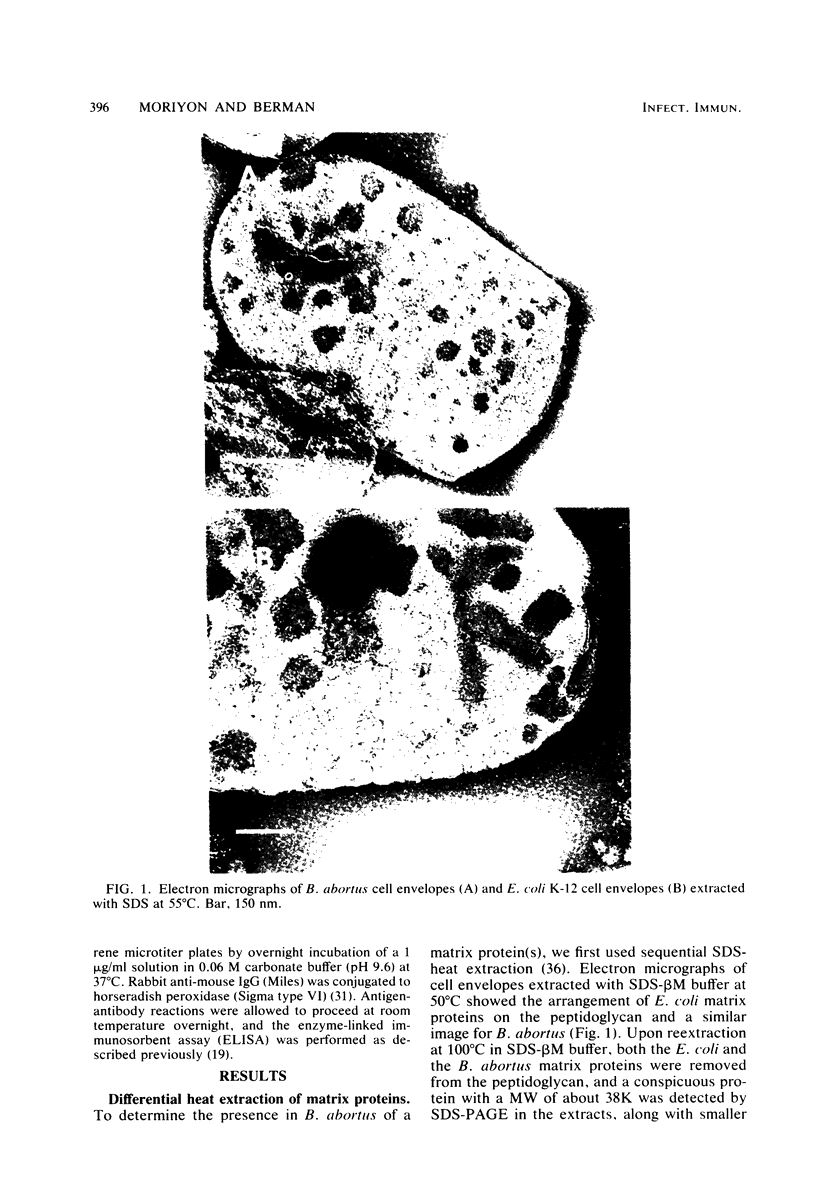
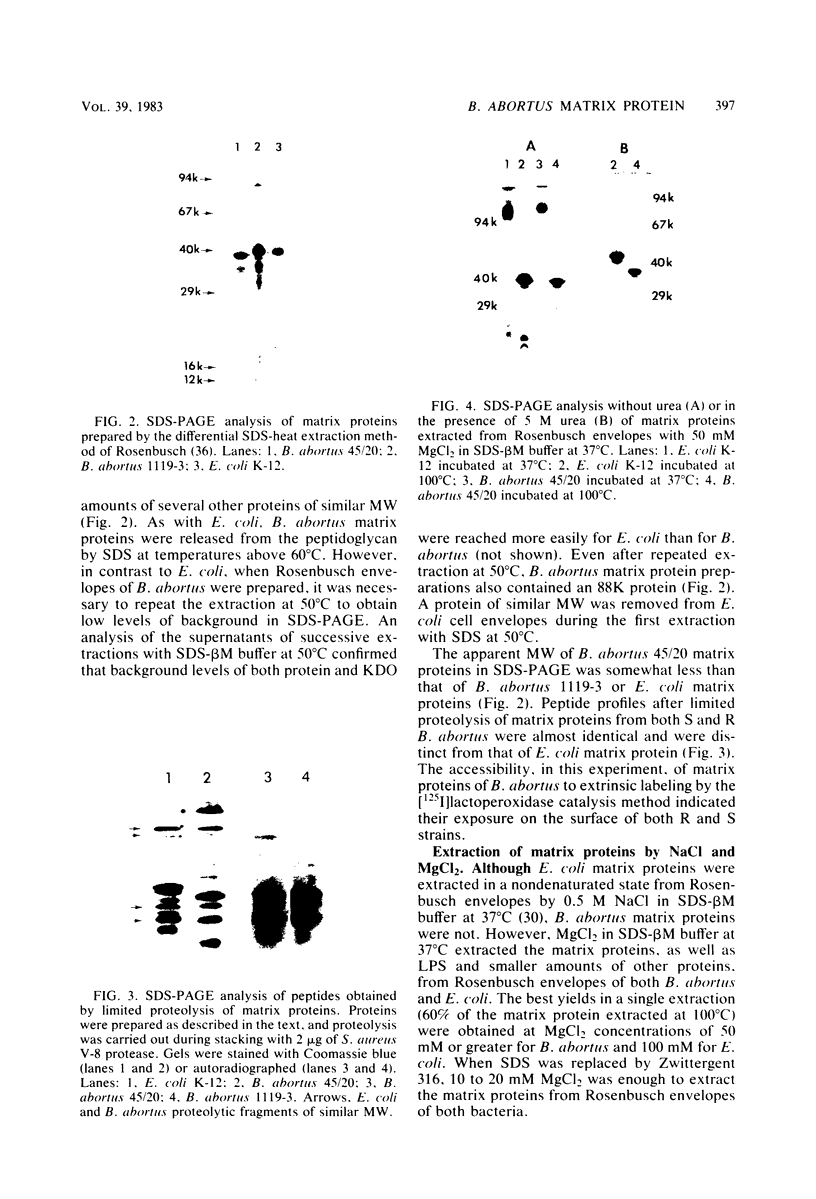
Peptidoglycan sacculi with peptidoglycan-associated proteins were prepared from cell envelopes of Brucella abortus by extraction with sodium dodecyl sulfate (SDS) at 50 degrees C. On extraction of these preparations with SDS at 100 degrees C, a protein was obtained whose removal from peptidoglycan was confirmed by electron microscopy. Incubation of the 50 degrees C SDS-extracted cell envelopes with 50 mM MgCl2 in SDS-2-beta-mercaptoethanol at 37 degrees C also extracted the protein, along with lipopolysaccharide. At temperatures below 60 degrees C, the protein did not bind SDS strongly and had an apparent molecular weight greater than 92,000 in SDS-polyacrylamide gel electrophoresis. At higher temperatures, SDS bound strongly, and the apparent molecular weight was 38,000. Urea at 5 M did not alter the electrophoretic mobility of this 38,000-molecular-weight form. Immunoelectrophoresis in detergents with antisera to cell envelopes, carbohydrate staining of SDS-polyacrylamide gels, and production of anti-lipopolysaccharide antibodies by mice immunized with the purified protein indicated that lipopolysaccharide was present in free and protein-bound forms. Sequential gel filtration in SDS-EDTA and SDS-NaCl removed most lipopolysaccharide. After further purification by preparative SDS-polyacrylamide gel electrophoresis, a gas-liquid chromatographic analysis showed residual lipid tightly associated with the protein. The results suggested that the interactions between matrix proteins and other outer membrane components are stronger in B. abortus than in Escherichia coli, which was used as a control throughout.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobo R. A., Eagon R. G. Lipids of cell walls of Pseudomonas aeruginosa and Brucella abortus. Can J Microbiol. 1968 May;14(5):503–513. doi: 10.1139/m68-086. [DOI] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Purification of protein A, an outer membrane component missing in Escherichia coli K-12 ompA mutants. Biochim Biophys Acta. 1977 Jul 22;493(1):210–215. doi: 10.1016/0005-2795(77)90274-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Diedrich D. L., Summers A. O., Schnaitman C. A. Outer membrane proteins of Escherichia coli. V. Evidence that protein 1 and bacteriophage-directed protein 2 are different polypeptides. J Bacteriol. 1977 Aug;131(2):598–607. doi: 10.1128/jb.131.2.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli 0111. J Immunol. 1981 Oct;127(4):1290–1294. [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Outer membrane proteins and cell surface structure of Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):899–907. doi: 10.1128/jb.141.2.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxén H., Mäkelä P. H. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun. 1981 Nov;34(2):328–332. doi: 10.1128/iai.34.2.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Wiltberger H. A., Winter J. Major outer membrane protein of Campylobacter fetus: physical and immunological characterization. Infect Immun. 1976 Apr;13(4):1258–1265. doi: 10.1128/iai.13.4.1258-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. In vitro reassembly of the membranous vesicle from Escherichia coli outer membrane components. Role of individual components and magnesium ions in reassembly. Biochim Biophys Acta. 1975 Dec 16;413(3):371–393. doi: 10.1016/0005-2736(75)90122-4. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schurig G. G., Jones L. M., Speth S. L., Berman D. T. Antibody response to antigens distinct from smooth lipopolysaccharide complex in Brucella infection. Infect Immun. 1978 Sep;21(3):994–1002. doi: 10.1128/iai.21.3.994-1002.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Heggeler B., Müller R., Kistler J., Rosenbusch J. P. Ultrastructure of a periodic protein layer in the outer membrane of Escherichia coli. J Cell Biol. 1977 Feb;72(2):292–301. doi: 10.1083/jcb.72.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Reconstitution of an ordered structure from major outer membrane constituents and the lipoprotein-bearing peptidoglycan sacculus of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1024–1031. doi: 10.1128/jb.135.3.1024-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Ichihara S., Mizushima S. A major outer membrane protein (O-8) of Escherichia coli K-12 exists as a trimer in sodium dodecyl sulfate solution. FEBS Lett. 1979 Apr 1;100(1):71–74. doi: 10.1016/0014-5793(79)81133-3. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]