Abstract
We previously reported the discovery of P7C3, an aminopropyl carbazole having proneurogenic and neuroprotective properties in newborn neural precursor cells of the dentate gyrus. Here, we provide evidence that P7C3 also protects mature neurons in brain regions outside of the hippocampus. P7C3 blocks 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated cell death of dopaminergic neurons in the substantia nigra of adult mice, a model of Parkinson disease (PD). Dose–response studies show that the P7C3 analog P7C3A20 blocks cell death with even greater potency and efficacy, which parallels the relative potency and efficacy of these agents in blocking apoptosis of newborn neural precursor cells of the dentate gyrus. P7C3 and P7C3A20 display similar relative effects in blocking 1-methyl-4-phenylpyridinium (MPP+)-mediated death of dopaminergic neurons in Caenorhabditis elegans, as well as in preserving C. elegans mobility following MPP+ exposure. Dimebon, an antihistaminergic drug that is weakly proneurogenic and neuroprotective in the dentate gyrus, confers no protection in either the mouse or the worm models of PD. We further demonstrate that the hippocampal proneurogenic efficacy of eight additional analogs of P7C3 correlates with their protective efficacy in MPTP-mediated neurotoxicity. In vivo screening of P7C3 analogs for proneurogenic efficacy in the hippocampus may thus provide a reliable means of predicting neuroprotective efficacy. We propose that the chemical scaffold represented by P7C3 and P7C3A20 provides a basis for optimizing and advancing pharmacologic agents for the treatment of patients with PD.
Parkinson disease (PD) is an incurable and progressive neurodegenerative disorder of predominantly idiopathic origin that is characterized by the death of dopaminergic neurons in the substantia nigra pars compacta (SNc), a region of the brain that controls motor activity by projecting dopaminergic axons to the striatum (1). Early symptoms in PD are primarily movement related, including shaking, rigidity, brady- and hypokinesia, tremor, and difficulty walking. More advanced stages of PD are associated with cognitive and behavioral problems, including dementia. Current treatment strategies for PD consist primarily of partial management of early motor symptoms with drugs that enhance dopaminergic signaling, such as l-DOPA or dopamine receptor agonists. Unfortunately, as greater numbers of dopaminergic neurons in the SNc die, these drugs fail to alleviate symptoms and additionally produce dyskinesia. There is thus a significant unmet need for new pharmacologic strategies to slow the progression of PD, such as drugs capable of blocking the death of SNc dopaminergic neurons.
We have previously reported the identification of an aminopropyl carbazole (P7C3) discovered via an unbiased, in vivo screen for small molecules capable of enhancing postnatal hippocampal neurogenesis. P7C3 displays enantiomeric-selective stabilization of mitochondrial membrane potential and enhances neurogenesis by blocking apoptosis of newborn neurons in the dentate gyrus (2). Prolonged oral or i.p. administration of P7C3 to rodents safely improves hippocampal functioning. For example, administration of P7C3 to mice suffering from pathologically high levels of neuronal apoptosis in the dentate gyrus, neuronal PAS domain protein 3 (NPAS3)-deficient mice (3), restored hippocampal structure and function with no obvious physiologic side effects (2). In addition, extended administration of P7C3 to aged rats safely impeded hippocampal cell death and preserved cognitive ability as a function of terminal aging (2).
Through an in vivo structure–activity relationship (SAR) study, we have identified analogs of P7C3 displaying either increased or decreased activity. In particular, a chemical variant known as P7C3A20 was observed to have greater potency and efficacy than P7C3. P7C3A20 differs from P7C3 by virtue of replacing the hydroxyl group at the chiral center of the linker with a fluorine and the addition of a methoxy group to the aniline ring (Fig. 1). This analog displays a more favorable toxicity profile than P7C3, with no hERG channel binding, histamine receptor binding, or toxicity to HeLa cells (2, 4, 5). We have also found that Dimebon, an antihistaminergic drug long deployed in Russia that is claimed to have anti-apoptotic and mitochondrial protective properties (6, 7), displays modest efficacy in the same biologic assays used to discover and characterize P7C3 and P7C3A20 (2). Here, we report that the neuroprotective activity of these agents extends beyond promoting long-term survival of newborn cells in the adult hippocampus. Specifically, we show that the most active variants of P7C3 exhibit robust protection of mature dopaminergic neurons in both mouse and worm models of neurodegeneration and propose that substituted carbazoles may represent attractive chemical scaffolds for the optimization of therapeutic agents for the treatment of Parkinson disease.
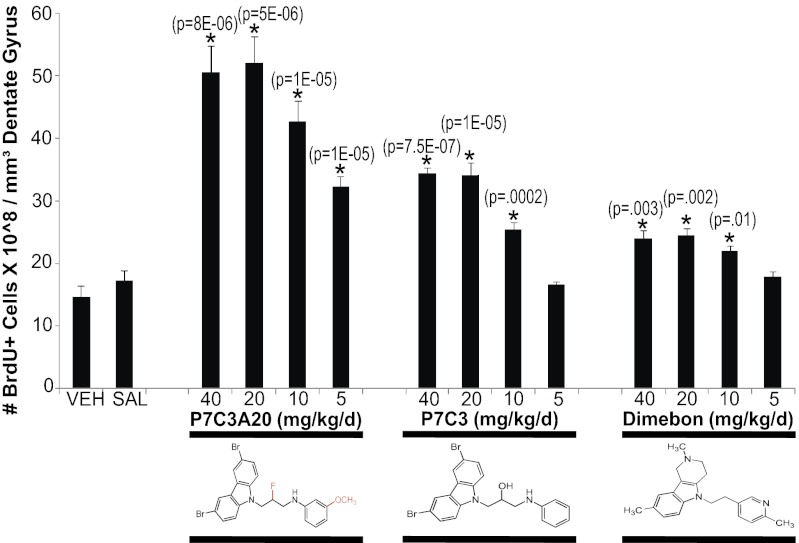
Fig. 1.
Neuroprotective efficacy of P7C3, P7C3A20, and Dimebon for newborn neurons in the adult hippocampus. Test compounds were evaluated by dose–response assay for their ability to block normal apoptotic cell death of newborn neural precursor cells in the adult dentate gyrus. P7C3A20 exhibits the greatest potency and ceiling of efficacy, and Dimebon the least. P7C3 is intermediate in both measures. Six animals were tested per group. Dosing is expressed as total milligrams per day, and compounds were administered intraperitoneally in divided doses twice daily. Data are expressed as mean ± SEM. Values for P7C3 and P7C3A20 were compared with those of vehicle (VEH), and values for Dimebon were compared with those of saline (SAL).
Results
Neuroprotective Efficacy of P7C3, P7C3A20, and Dimebon for Newborn Hippocampal Neurons.
Adult hippocampal neurogenesis in mice is an approximately 1-mo-long process, during which time the majority of newborn cells die as they transition through a “differentiation gauntlet” lasting about 1 mo before surviving cells become functionally wired into the central nervous system. We have previously found that ∼40% of these newborn cells die within the first week following their birth in the subgranular zone of the dentate gyrus (2). P7C3 was originally discovered through a 1-wk-long in vivo screen designed to identify small molecules that might enhance either proliferation or survival of newborn hippocampal neural precursor cells. Subsequent bromodeoxyuridine (BrdU) pulse-chase labeling studies revealed that P7C3 does not affect neural precursor proliferation, but instead augments survival of newborn cells by blocking apoptosis. P7C3A20 was found to be active at lower doses and to have a higher ceiling of efficacy (CoE) than P7C3 in this 7-d in vivo assay, whereas Dimebon was shown to be substantially less potent and efficacious than P7C3 (2).
To more carefully compare the neuroprotective efficacy of these compounds in abetting hippocampal neurogenesis, we conducted dose–response studies in a 30-d BrdU pulse-chase survival assay (Fig. 1). Briefly, newborn cells were labeled with a single i.p. injection of BrdU (150 mg/kg), followed by daily treatment with the three test compounds beginning the following day. After 30 d of compound administration, mice were transcardially perfused with 4% paraformaldehyde, brains were dissected, and immunohistochemical detection of BrdU followed by standard microscopic imaging and normalization for dentate gyrus volume were used to quantify the number of surviving cells. As shown in Fig. 1, P7C3A20 increased neuron survival by almost 100% at the lowest dose tested (5 mg⋅kg−1⋅d−1). By contrast, neither P7C3 nor Dimebon exhibited any effect at this dose. At the next higher dose (10 mg⋅kg−1⋅d−1), P7C3 and Dimebon showed 65% and 15% neuroprotective efficacy, respectively, whereas P7C3A20 showed neuroprotective CoE of about 175%. All three compounds exhibited their maximal individual CoEs at the two highest doses tested (20 or 40 mg⋅kg−1⋅d−1), with P7C3A20’s CoE being highest (∼230% increase in survival), P7C3’s CoE being intermediate (∼130% increase in survival), and Dimebon’s CoE being the lowest (∼30% increase in survival).
Efficacy Assays for P7C3, P7C3A20, and Dimebon for Protection from MPTP Toxicity to Dopaminergic Neurons in Mice.
The protective efficacy of the three test compounds for newborn hippocampal neurons in the 30 d survival assay prompted us to investigate whether they might also have neuroprotective efficacy in mature neurons outside of the hippocampus. To investigate this hypothesis, we used the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of neuronal cell death. MPTP is a potent toxin that selectively kills neurons in the substantia nigra of both rodents and primates, causing clinical manifestations resembling PD (8). MPTP is lipophilic and readily crosses into the brain, where it is metabolized by monoamine oxidase B in glial cells into the highly toxic cation 1-methyl-4-phenylpyridinium (MPP+). MPP+ is selectively concentrated in SNc dopaminergic neurons by virtue of its high affinity for the plasma membrane dopamine transporter (9), and toxicity is further potentiated by binding of MPP+ to melanin in these cells, creating a depot mechanism that maintains prolonged high intracellular concentrations of MPP+ (10). MPTP toxicity is routinely used to study the death of dopaminergic neurons as a possible means of discovering new treatments for PD on the basis of neuroprotective strategies found to be effective in this model.
We compared the protective efficacy of our agents in the Tatton and Kish model of MPTP administration, which induces prolonged apoptotic death of SNc dopaminergic neurons lasting about 3 wk after a short course of daily MPTP administration (11, 12). Mice were treated daily for 5 d with 30 mg⋅kg−1⋅d−1 free base MPTP. On the sixth day, 24 h after receiving the fifth and final dose of MPTP, daily treatment with P7C3, P7C3A20, Dimebon, or vehicle was initiated. This testing paradigm ensured that any observed activity of P7C3 or its analogs could be attributed to neuroprotective effects and not to disruption of MPTP uptake or metabolism. Dose–response studies were conducted in which mice received twice daily doses of each compound (or vehicle) for the ensuing 21 d (Fig. 2A). Treatment groups consisted of 15 animals each. At the end of the 21-d treatment period, mice were killed by transcardial perfusion with 4% paraformaldehyde, and fixed brains were sectioned through the striatum and SNc at 30-μm intervals. Every fourth section (spaced 120 μm apart) was stained with antibodies specific to tyrosine hydroxylase (TH) (Abcam; rabbit anti-TH, 1:2,500). The TH enzyme catalyzes the conversion of the amino acid l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA), which serves as the precursor for dopamine. TH staining thus provides a means to immunohistochemically identify dopaminergic neurons. By counting the number of TH+ cells in the SNc, we were able to assess the neuroprotective efficacy of the three chemicals following MPTP exposure. All microscopic analysis was performed by two investigators, blind to treatment group.
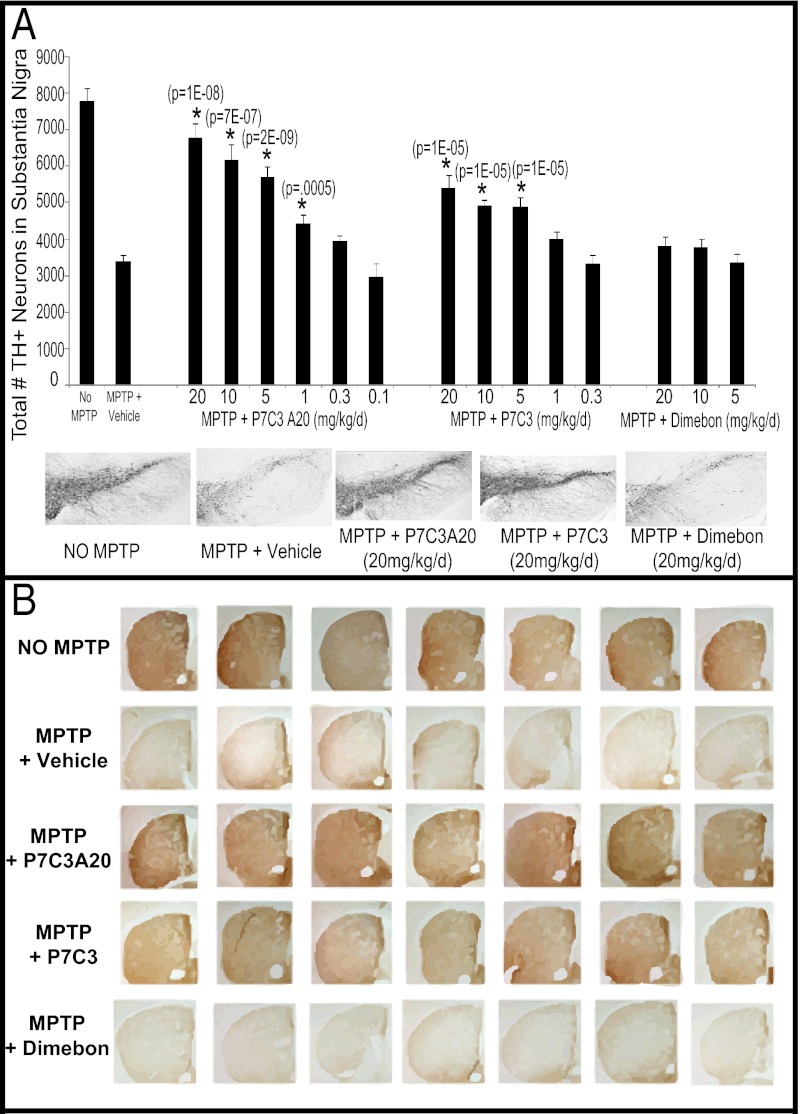
Fig. 2.
Neuroprotective efficacy of P7C3 and P7C3A20 from MPTP toxicity to SNc dopaminergic neurons. (A) Test compounds were evaluated by dose–response assay for their ability to block MPTP neurotoxicity in the SNc. P7C3A20 showed greater potency and CoE than P7C3, and Dimebon showed no protective efficacy. Fifteen animals were tested per group. The VEH group contained 30 animals: 15 animals that received the P7C3A20/P7C3 vehicle and 15 animals that received Dimebon vehicle (saline). These two control groups did not differ in number of surviving TH+ neurons and were thus combined. (Lower) Representative immunohistochemical pictures of TH staining in the SNc are shown. Dosing is expressed as total milligrams per day and was administered intraperitoneally in divided doses twice daily. Data are expressed as mean ± SEM. (B) Representative images of TH staining from the striata of individual animals demonstrate that 3 wk after a 5-d course of daily MPTP administration both P7C3 and P7C3A20 block the loss of dopaminergic axon terminals. P7C3A20 does so with greater effect, and Dimebon offers no protection. Striatal sections were obtained from the same mice used in Fig. 2, and compound treatment groups are from mice that received a dose of 20 mg⋅kg−1⋅d−1.
As has been observed by others (11, 12), MPTP administration reduced the number of TH+ neurons in the SNc by about 50% [vehicle (VEH)] (Fig. 2A). This neurotoxicity was blocked to varying degrees by both P7C3 and P7C3A20. P7C3 enhanced survival by about 40% over VEH at a dose of 5 mg⋅kg−1⋅d−1, and the highest dose of P7C3 (20 mg⋅kg−1⋅d−1) afforded almost 60% protection relative to vehicle. By contrast, the 20-mg⋅kg−1⋅d−1 dose of P7C3A20 preserved the number of dopaminergic neurons in the SNc to about 85% of that seen in normal mice not exposed to MPTP. At every dose tested, P7C3A20 provided superior protection to P7C3. Both CoE and potency of P7C3A20 were greater than those of P7C3, with the onset of P7C3A20 efficacy (30% over VEH) at a dose of 1 mg⋅kg−1⋅d−1. Dimebon failed to confer any measurable degree of protection from MPTP at any dose.
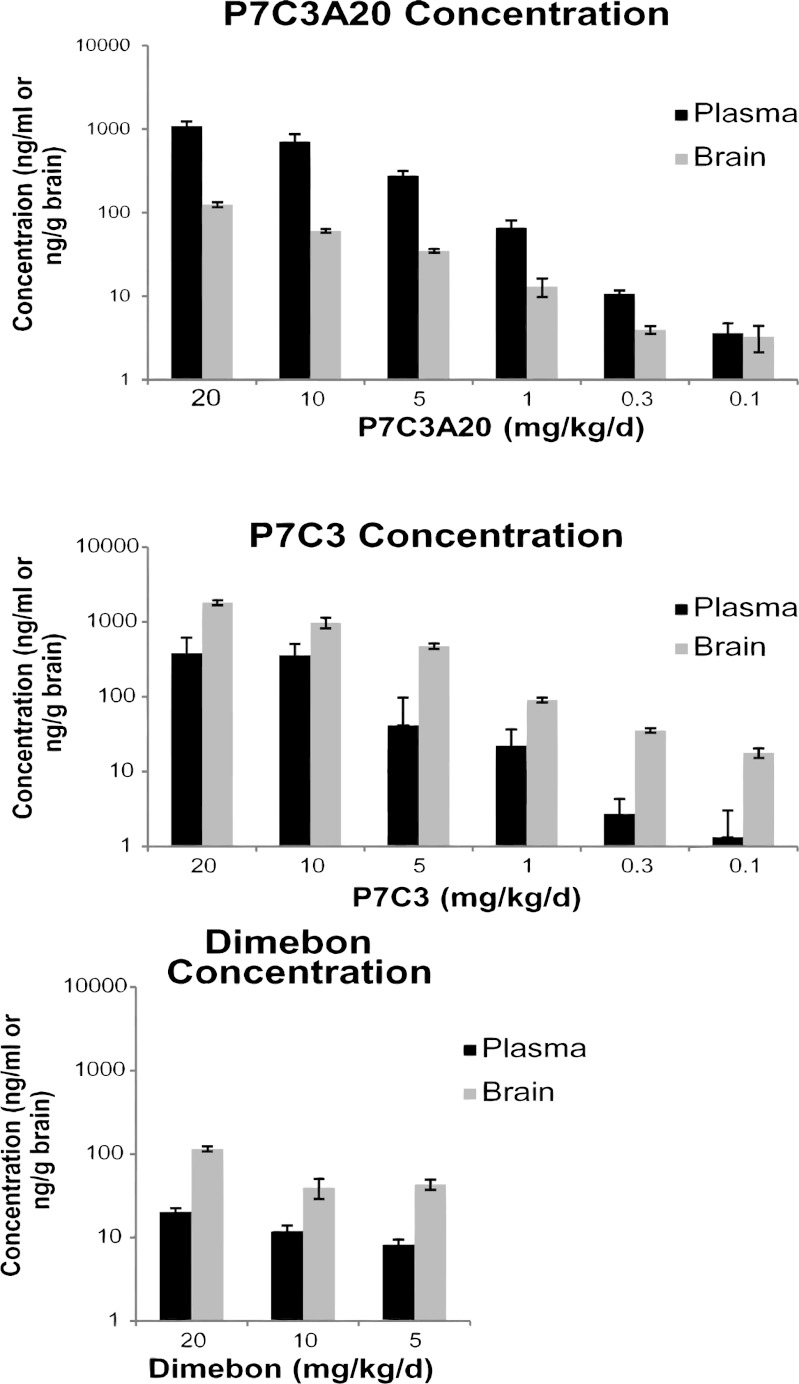
In addition to allowing quantification of dopaminergic cells in the SNc, TH staining is also routinely used to visualize the integrity of dopaminergic axonal protections from SNc cell bodies into the striatum (12). Fig. 2B shows that the highest dose of P7C3A20 (20 mg⋅kg−1⋅d−1) almost completely blocked depletion of dopaminergic axons in the striatum after MPTP exposure. The highest dose of P7C3 also revealed qualitatively notable protection. By contrast, Dimebon offered no protection of dopaminergic axons in the striatum, corresponding to its lack of neuroprotective efficacy in the SNc. As shown in Fig. 3, liquid chromatography-tandem mass spectrometry (LC/MS/MS) quantification of brain and blood levels of P7C3A20 and P7C3 confirmed that the neuroprotective efficacy for each compound correlated with brain and blood levels for each chemical. Notably, P7C3A20 displayed significantly greater protective efficacy than P7C3 despite the fact that P7C3A20 accumulated in brain tissue at less than one-tenth the concentration of P7C3. Dimebon, which displayed no neuroprotective efficacy in the MPTP model of dopaminergic neuron cell death, showed comparable levels of brain accumulation to those of P7C3A20.
Fig. 3.
Brain and blood levels of P7C3, P7C3A20, and Dimebon 3 wk after MPTP administration. Relative neuroprotective activity within a test compound correlated with brain levels of that compound, and brain levels correlated with blood levels of the test compounds. Only about 1/10th the amount of P7C3A20 accumulated in the brain compared with P7C3. Brain accumulation of Dimebon was equivalent to that of P7C3. Data are expressed as mean ± SEM. Three animals were tested per group.
Efficacy Assays of P7C3, P7C3A20, and Dimebon for Protection from MPP+ Toxicity to Dopaminergic Neurons in Caenorhabditis elegans.
Genes, metabolic signaling pathways, neurotransmitters, and receptor pharmacology are highly conserved between C. elegans and vertebrates (13), and exposure of C. elegans to MPP+ has been reported to selectively kill dopaminergic neurons and impair mobility (14). To investigate the neuroprotective efficacy of P7C3A20, P7C3, and Dimebon from MPP+ toxicity in C. elegans, we monitored dopaminergic cell death in a transgenic strain of worms in which dopaminergic neurons fluoresce green by virtue of GFP expression driven by the dopaminergic neuron-specific promoter dat-1 (15). As shown in Fig. 4, incubation of synchronized L1 larvae for 40 h with 5 mM MPP+ elicited virtually complete destruction of all four cephalic sensilla dopaminergic dendrites. For this assessment, GFP fluorescence was observed in 20 worms per group and was performed in triplicate. Cephalic sensilla dopaminergic dendrites were observed under 40× magnification (AMG; Evos fl microscope), and GFP signal was followed from the nerve ring to the tip of the nose, following established protocols (16). If any part of a dendrite was absent, as evidenced by loss of GFP signal, it was counted as degraded. All analyses were performed blind to treatment group.
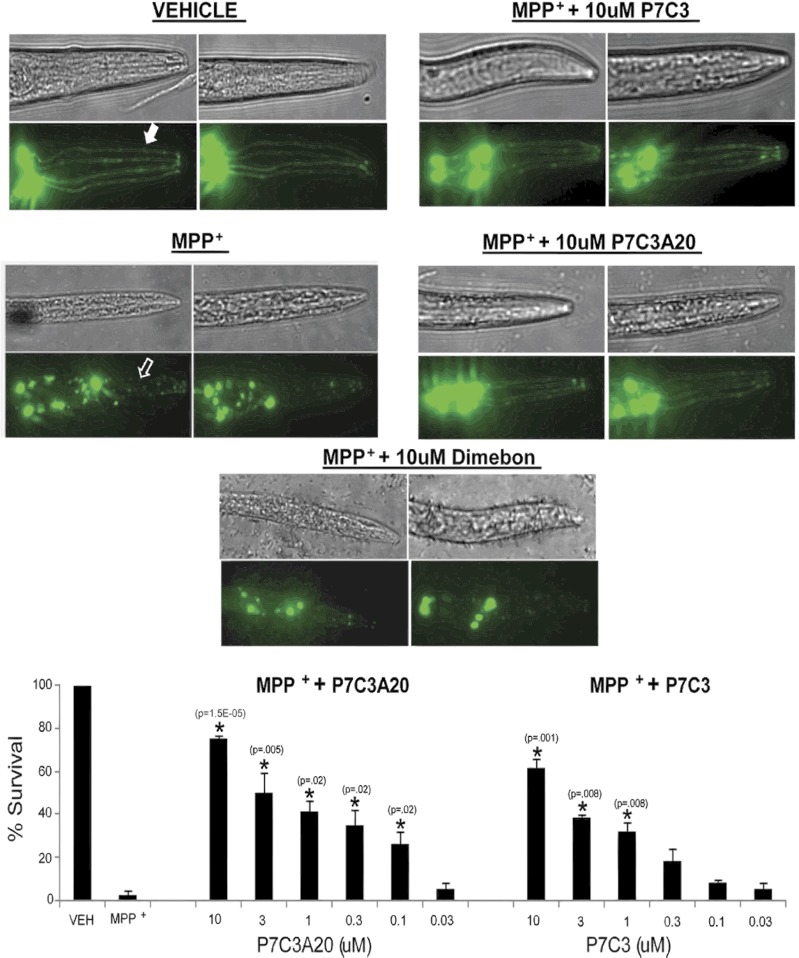
Fig. 4.
Neuroprotective efficacy of P7C3 and P7C3A20 for MPP+ toxicity to dopaminergic neurons in C. elegans. Worms were treated with 5 mM MPP+ for 40 h, with preincubation for 30 min with different concentrations of test compounds or vehicle. VEH animals not exposed to MPP+ exhibited normal GFP expression in dopaminergic neurons (solid arrow). By contrast, GFP expression was lost after 40 h of MPP+ exposure (open arrow). Both P7C3A20 and P7C3 dose dependently protected dopaminergic neurons from MPP+ toxicity, with P7C3A20 exhibiting greater potency and ceiling of efficacy. Twenty worms were analyzed per group, and each group analysis was performed in triplicate. Data are expressed as mean ± SEM.
Cotreatment of MPP+-exposed worms with 10 μM P7C3A20 conferred 80% protection. By comparison, the neuroprotective efficacy of the same dose of P7C3 was only about 50%. Neuroprotective efficacy of both agents was diminished as the chemical dose was gradually reduced. By these measures, P7C3A20 showed greater potency than P7C3, with an onset of neuroprotective efficacy of 30% at the 0.1-μM dose. By comparison, P7C3 did not show efficacy (35%) until administered at 1.0 μM. Administration of Dimebon at the highest dose (10 μM) failed to protect dopaminergic neurons in worms from MPP+-induced toxicity.
Efficacy Assays of P7C3, P7C3A20, and Dimebon for Protection from MPP+-Induced Mobility Deficits in C. elegans.
As a behavioral measure of toxicity, worm mobility was assessed 32 h after MPP+ exposure. To quantify worm locomotion, video representations were recorded for 10 s at 4× magnification, using a Nikon Eclipse 80i microscope. Each video segment consisted of 160 frames, and the head of each worm (10–20 worms per group, repeated in triplicate) was manually tracked in each frame, using Imera software. The body length of each worm was also measured by Imera software. The ratio of distance traveled to body length was used to determine the movement index, defined as locomotion, as previously established (17). As shown in Fig. 5, locomotion was reduced in C. elegans by 50% after 32 h of exposure to 5 mM MPP+. Coincubation of MPP+-exposed worms with 10 μM P7C3A20 conferred 80% preservation of locomotion, whereas 10 μM P7C3 protected to about 60% of normal levels. Dimebon offered no protective efficacy in this behavioral assay. Movies of the worms shown in Fig. 5 are available online (Movies S1, S2, S3, S4, and S5).
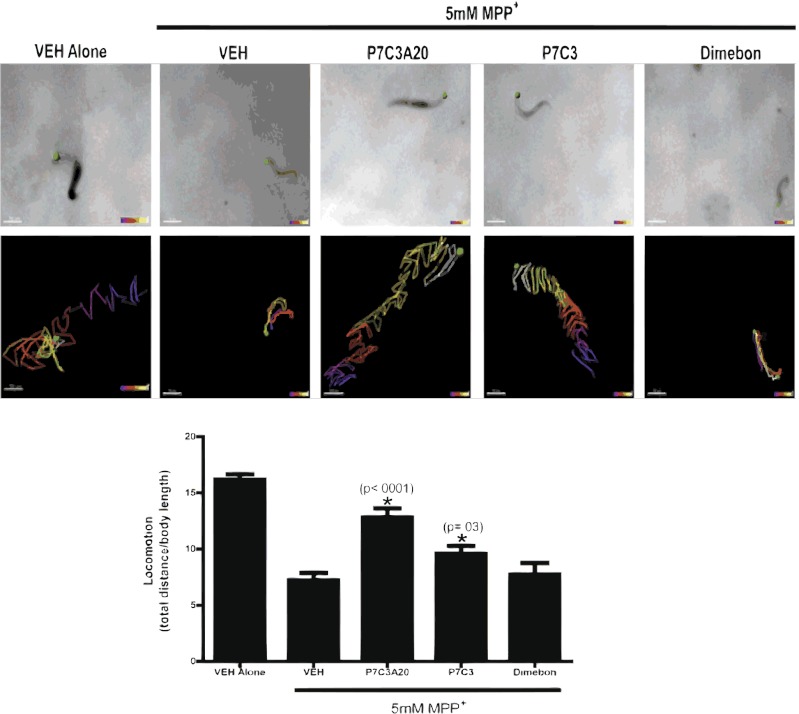
Fig. 5.
Protective efficacy of P7C3 and P7C3A20 for MPP+-induced mobility deficits in C. elegans. (Top) Worms with the head identified by a green dot. (Middle) Path taken by each worm in 10 s, determined by tracking the green dot. Tracking is visualized as starting with blue color and progressing to white by the completion of 10 s. The green dot was used to determine locomotion, defined as the distance traveled by the head of the worm in 10 s divided by body length. (Scale bars, 70 μm.) Quantitative analysis of locomotion showed that untreated VEH controls had a value of 16.2 ± 0.49 (n = 30). When worms were treated with MPP+, locomotion was reduced more than 50% (7.2 ± 0.68; n = 31, P < 0.0001). A total of 10 μM P7C3A20 protected mobility to almost 80% of normal (12.8 ± 0.81; n = 34, *P < 0.01), and 10 μM P7C3 protected mobility almost 60% (mobility index = 9.6 ± 0.72; n = 28, *P < 0.05). A total of 10 μM Dimebon did not offer any protection (7.7 ± 1.0; n = 30). Experiments were performed in triplicate and data are expressed as mean ± SEM.
Correlation of Efficacy of Analogs of P7C3 in the in Vivo Hippocampal Neurogenesis Assay with Neuroprotective Efficacy in MPTP-Mediated Dopaminergic Cell Death.
Over the past 2 y, we have conducted a comprehensive SAR study to improve the potency, efficacy, and physical properties of the P7C3 series of molecules, as well as to eliminate real or perceived chemical liabilities. To date, we have synthesized over 250 analogs of P7C3, all of which have been evaluated by primary screening in the in vivo hippocampal neurogenesis assay. Our efforts include, but are not limited to, eliminating the bromines and aniline ring, increasing biologic activity, decreasing lipophilicity, eliminating toxicities such as hERG channel binding, increasing solubility, and reducing molecular weight. Here, we show the results of evaluation of 8 of these analogs (Fig. 6A) in both the hippocampal neurogenesis assay (4 mice for each compound) and the MPTP protection assay (10 mice for each compound) (Fig. 6B). All analyses were conducted blind to treatment group.
Fig. 6.
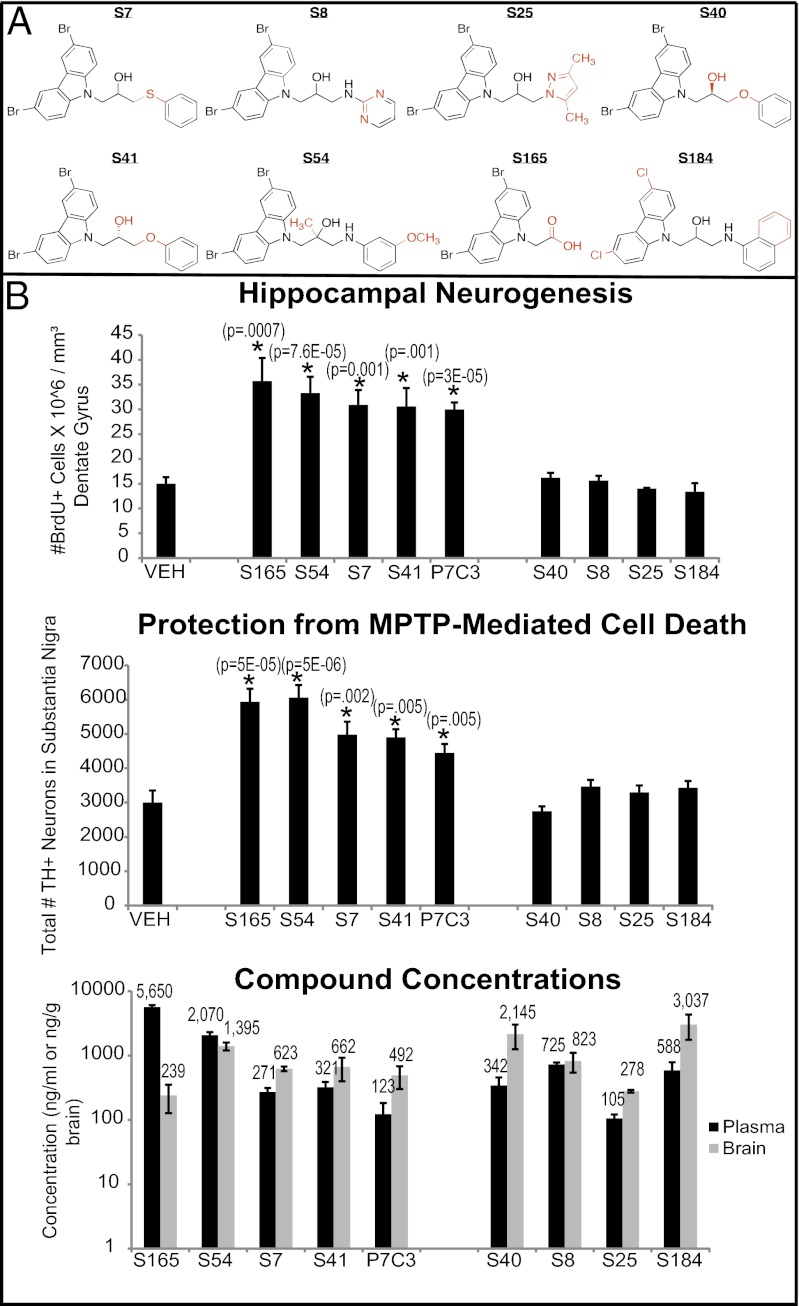
Efficacy of unique P7C3 analogs in the in vivo hippocampal neurogenesis assay correlates with activity in the in vivo MPTP-neuroprotection assay. (A) P7C3-S7 differs from P7C3 by replacing the aniline NH with sulfide linker. P7C3-S8 differs from P7C3 by replacing the aniline phenyl ring with a pyrimidine. P7C3-S25 differs from P7C3 by replacing the aniline moiety with a dimethyl pyrazole. P7C3-S40 and S41 differ from P7C3 by replacing the aniline NH with an oxygen linker, and they are R and S single enantiomers, respectively. P7C3-S54 differs from P7C3 mainly by the addition of a methyl group to the central carbon of the propyl linker and an OMe group on the aniline. P7C3-S165 differs from P7C3 by replacing the aniline and carbinol fragments with a carboxylic acid. P7C3-S184 differs from P7C3 by replacing the bromines on the carbazole with chlorines and replacing the aniline with a naphthyl amine. (B) Unique analogs of P7C3 were subjected to in vivo testing in the hippocampal neurogenesis (4 mice each) and MPTP-protection (10 mice each) assays. Results show that activity in these two assays correlates, such that the in vivo neurogenesis screen is useful for predicting neuroprotective efficacy of P7C3 analogs for dopaminergic neurons in the substantia nigra. LC/MS/MS assay of blood and brain levels of all compounds administered a single time to C57BL/6 mice at 10 mg/kg (i.p.) indicates that they cross the blood–brain barrier following i.p. administration.
With respect to the original P7C3 scaffold, P7C3-S7 differs by replacing the aniline NH with a sulfide linker, P7C3-S8 differs by replacing the aniline phenyl ring with a pyrimidine, and P7C3-S25 differs by replacing the aniline moiety with a dimethyl pyrazole (Fig. 6A). As shown in Fig. 6B, all eight test molecules crossed the blood–brain barrier. P7C3-S7 and P7C3-S25 were active in both the hippocampal neurogenesis assay and the MPTP protection assay. By contrast, P7C3-S8 was devoid of activity in both assays. We also compared the efficacy of members of an enantiomeric pair in these assays. P7C3-S40 and P7C3-S41 differ from P7C3 by replacing the aniline NH with an oxygen linker (Fig. 6A). P7C3S40 and P7C3S41 are the R and S single enantiomers, respectively, and Fig. 6B shows that neuroprotective activity in both assays resides exclusively in the S enantiomer. P7C3-S54 differs from P7C3 mainly by the addition of a methyl group to the central carbon of the propyl linker and additionally has an OMe group on the aniline ring (Fig. 6A). This analog was observed to retain neuroprotective activity in both assays. P7C3-S165 differs from P7C3 by replacing the aniline and carbinol fragments with a carboxylic acid, a change that dramatically increases polarity (Fig. 6A). Encouragingly, neuroprotection was observed in both assays (Fig. 6B).
Finally, P7C3-S184 differs from P7C3 by replacing the bromines on the carbazole with chlorines and by replacing the aniline with a naphthyl amine (Fig. 6A). This molecule was inactive in both in vivo assays. P7C3-S184 has been reported as a β-secretase (BACE1) inhibitor (18). BACE1 is an aspartate proteolytic enzyme that catalyzes the formation of Aβ peptide from amyloid precursor protein, and inhibition of this enzyme has been proposed as a therapeutic approach for Alzheimer’s disease (19). We have analyzed BACE1 inhibition with P7C3A20 and an inactive analog of P7C3 (P7C3A35) (2) and have found no correlation between neuroprotective efficacy in our in vivo models and BACE1 inhibition (Fig. S1).
Discussion
The results of a target-agnostic, unbiased screen of 1,000 chemically diverse, drug-like compounds led to the identification of an aminopropyl carbazole endowed with the capacity to enhance adult neurogenesis (2). This compound, designated P7C3, was found to act by blocking the death of newborn neurons in the dentate gyrus of adult mice. Here we have sought to answer a simple question. If P7C3 is capable of preventing the death of newborn neurons during hippocampal neurogenesis in adult mice, could this compound also prevent death of existing neurons in animal models of neurodegenerative disease? More specifically, we have administered MPTP to mice as a means of killing dopamine neurons. Fully 24 h after removal of the toxin, mice were treated with varying doses of one of three compounds for a period of 3 wk. Thereafter mice were killed and assayed for evidence of neuroprotective efficacy. As a second, related animal model of neuron death, C. elegans worms were cotreated with MPP+ and varying doses of P7C3, P7C3A20, and Dimebon as a means of assessing neuroprotective activity.
The three compounds chosen for extensive testing, P7C3, its structurally related analog P7C3A20, and Dimebon, were selected by virtue of the knowledge that they demonstrate distinct proneurogenic activities. Among dozens of chemical analogs of P7C3 evaluated in the study first reporting this category of proneurogenic compounds, P7C3A20 displayed the highest potency and ceiling of proneurogenic efficacy (2). In addition to the P7C3 and P7C3A20 chemicals, we included Dimebon in the present study for two reasons. First, even though P7C3 and Dimebon both contain three-ring heterocycles, Dimebon was found to display significantly diminished levels of potency and efficacy relative to P7C3 in our original study (2). Its proneurogenic activity in the hippocampus of adult mice was observed only at relatively high doses, and its ceiling of efficacy was clearly diminished relative to that of both P7C3 and P7C3A20. Likewise, when tested for its ability to protect mitochondrial membrane integrity following exposure of cultured cells to a calcium ionophore, Dimebon exhibited a protective potency between 100- and 1,000-fold lower than that of P7C3 (2). Second, Dimebon has been the subject of extensive clinical studies in both Alzheimer’s disease and Huntington disease. Despite early indications of efficacy in a phase 2 trial for Alzheimer’s disease (20), Dimebon failed in two independent phase 3 trials. By testing the properties of these three compounds in the present study of neuroprotective efficacy, one having a very favorable efficacy profile as a proneurogenic chemical (P7C3A20), another having an intermediate efficacy profile (P7C3), and a third having a far more modest efficacy profile (Dimebon), we sought to determine whether this hierarchy of activities might be preserved.
Encouragingly, we observe that the A20 chemical variant of P7C3 displays significant neuroprotective efficacy in the MPTP model of dopaminergic neuron cell death in both rodents and worms. P7C3, the original compound discovered in the unbiased screen of 1,000 drug-like chemicals for proneurogenic activity (2), displayed a lower level of neuroprotective activity in the mouse and worm models of dopaminergic neuron death than P7C3A20. By contrast, Dimebon showed no protective activity in either assay. Although others have recently demonstrated that treatment of an Alzheimer’s disease mouse model (TgCRND8 mice) with Dimebon improves memory and lowers accumulation of insoluble Aβ42 in the brain (21), Dimebon appears too weakly active to afford neuroprotection in the models of Parkinson disease that we have used.
We tentatively conclude that P7C3 and P7C3A20 protect dopaminergic neurons from MPTP-induced cell death with a hierarchy of activity that is analogous to their abilities to protect newborn hippocampal neurons from cell death. If correct, this interpretation offers the possibility that the relatively straightforward assay we have used to monitor the activities of hundreds of chemical variants of P7C3, wherein adult neurogenesis is monitored over a 7-d period following direct administration of test compounds into the adult mouse brain, may represent a trusted surrogate for the refinement of drug-like chemicals having broad neuroprotective activity. Indeed, we hereby observe that an unbiased, blinded analysis of nine analogs of P7C3 confirms this correlation. Five of these molecules showed significant efficacy in our standard in vivo hippocampal neurogenesis assay, and these same five molecules also showed significant neuroprotective efficacy from MPTP-mediated neurotoxicity to dopaminergic neurons. The four analogs that were inactive in the in vivo neurogenesis likewise showed no efficacy in the in vivo MPTP neurotoxicity assay. Taken together, these results show that the relatively rapid evaluation of molecules in the in vivo neurogenesis assay is predictive of their neuroprotective efficacy in the MPTP assay.
Ongoing SAR efforts with our P7C3 series of molecules using the in vivo neurogenesis assay appear to qualify as a rapid and accurate way to guide the refinement of this series of molecules into a neuroprotective drug for Parkinson disease. In this context, several of the analogs shown in Fig. 6 represent potential improvements to P7C3. We have been primarily concerned with the presence of an aniline ring, as this functionality can be associated with toxicity. Encouragingly, three of the analogs (P7C3S7, -S41, and -S165) lack aniline rings and display potency equal to or greater than that of P7C3 in both in vivo assays. We further note that P7C3 was originally identified as a racemic mixture and that racemic mixtures may require additional characterization for clinical development. P7C3S41 and P7C3S165 address this limitation because the former is a single enantiomer whereas the latter lacks stereochemistry altogether. Finally, we have sought to reduce lipophilicity and molecular mass of these neuroprotective compounds. P7C3S165 is significantly lighter than P7C3 (molecular mass = 383 Da vs. 474 Da for P7C3) and, by virtue of the carboxylic acid, substantially more polar. These results suggest that it should be possible to further improve the physical properties of these analogs in efforts to optimize derivatives suitable for clinical testing.
Consistent with the interpretation that the activity of P7C3 analogs in the in vivo neurogenesis assay correlates with neuroprotective efficacy in mature neurons are the results of assays of P7C3, P7C3A20, and Dimebon in a mouse model of amyotrophic lateral sclerosis (discussed in companion article, ref. 22). In this model, using mice expressing a high level of a human transgene encoding a mutated variant of the gene encoding human Cu,Zn superoxide dismutase (23–27), we have observed the same hierarchy of activities wherein P7C3A20 is active, P7C3 is intermediately active, and Dimebon is inactive (22). If the more active variants of this class of compounds indeed possess neuroprotective properties, and if we can rely on the relatively rapid in vivo assay of enhanced neurogenesis to rank order compounds for SAR scoring, it should be possible to optimize variants with the goal of selecting an appropriately qualified chemical to advance for human testing. To date, no safely tolerated, neuroprotective chemical is available for the treatment of any of a wide range of neurodegenerative diseases, including Parkinson disease, Alzheimer’s disease, and amyotrophic lateral sclerosis. On the basis of the observations reported herein, we propose that a properly optimized variant of the P7C3 class of proneurogenic, neuroprotective chemicals may offer promise for the future treatment of neurodegenerative disease.
Materials and Methods
The details of the 30-d survival assay of newborn hippocampal neurons, assessment of MPTP-mediated neurotoxicity to murine SNc neurons, synthesis of P7C3 compounds, pharmacokinetic analysis, maintenance of C. elegans, assessment of MPP+ dopaminergic neuron toxicity in C. elegans, locomotion analysis of C. elegans, and BACE1 activity assay techniques are available in SI Materials and Methods. Approval for the animal experiments described herein was obtained by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. For statistics, all P values were obtained with Student’s t test.
Supplementary Material
Acknowledgments
We thank Iryna Zubovych for valuable help in establishing the C. elegans system; Abhijit Bugde and Arnaldo Carreira-Rosario of the University of Texas Southwestern Medical Center Live Cell Imaging Center for assistance in quantifying worm mobility; Latisha McDaniel, Ruth Starwalt, and Jieqi Wang for technical assistance; and Drs. Jay Baraban, Martin Raff, and Dale Boger for critical review of the manuscript. This work was supported by grants from the Edward N. and Della C. Thome Memorial Foundation and the Welch Foundation (I-1612) (to J.M.R.); the Ted Nash Long Life Foundation, The Hartwell Foundation, the Staglin Family Fund, and the Morton H. Meyerson Family Tzedakah Fund (A.A.P.); a National Institutes of Health transformative R01 grant (National Institute of Mental Health Grant R01MH087986) (to A.A.P and S.L.M.); National Cancer Institute Program Project Grant P01CA095471(to S.L.M.); and funds provided by an anonymous donor (to S.L.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213956109/-/DCSupplemental.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Pieper AA, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieper AA, et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacMillan KS, et al. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc. 2011;133:1428–1437. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKnight SL, Pieper AA, Ready JM, De Brabander J. 2010. Proneurogenic compounds. US Patent 2010/020681.
- 6.Bachurin S, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 7.Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344, discussion 345–349. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda T. Neurotoxicity of MPTP. Neuropathology. 2001;21:323–332. doi: 10.1046/j.1440-1789.2001.00402.x. [DOI] [PubMed] [Google Scholar]
- 9.Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amato RJ, Lipman ZP, Snyder SH. Selectivity of the parkinsonian neurotoxin MPTP: Toxic metabolite MPP+ binds to neuromelanin. Science. 1986;231:987–989. doi: 10.1126/science.3080808. [DOI] [PubMed] [Google Scholar]
- 11.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 12.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 13.Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 14.Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener Dis. 2004;1:175–183. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- 15.Pu P, Le W. Dopamine neuron degeneration induced by MPP+ is independent of CED-4 pathway in Caenorhabditis elegans. Cell Res. 2008;18:978–981. doi: 10.1038/cr.2008.279. [DOI] [PubMed] [Google Scholar]
- 16.Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:3264–3269. doi: 10.1073/pnas.042497999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, et al. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000350. doi: 10.1371/journal.pgen.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asso V, et al. Alpha-Naphthylaminopropan-2-ol derivatives as BACE1 inhibitors. ChemMedChem. 2008;3:1530–1534. doi: 10.1002/cmdc.200800162. [DOI] [PubMed] [Google Scholar]
- 19.John V, Beck JP, Bienkowski MJ, Sinha S, Heinrikson RL. Human beta-secretase (BACE) and BACE inhibitors. J Med Chem. 2003;46:4625–4630. doi: 10.1021/jm030247h. [DOI] [PubMed] [Google Scholar]
- 20.Doody RS, et al. dimebon investigators Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 21.Steele JW, et al. 2012. Latrepirdine improves cognition and arrests progression of neuropathology in an Alzheimer’s mouse model. Mol Psych, 10.1038/mp.2012.106.
- 22.Tesla R, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2012;109:17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 24.Letiner M, Menzies S, Lutz C. Working with ALS Mice. Guidelines for Preclinical Testing & Colony Management. Bar Harbor, ME: The Jackson Laboratory; 2010. [Google Scholar]
- 25.Sulston JE, Hodgkin JA. Methods, the Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1988. [DOI] [PubMed] [Google Scholar]
- 26.Emmons SW, Klass MR, Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 1979;76:1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nass R, Hamza I. 2007. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Curr Protoc Toxicol Chap 1:Unit1.9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.