Abstract
To develop more effective vaccines and strategies to regulate chronic inflammatory diseases, it is important to understand the mechanisms of immunological memory. Factors regulating memory CD4+ T helper (Th)-cell pool size and function remain unclear, however. We show that activation of type I invariant natural killer T (iNKT) cells with glycolipid ligands and activation of type II natural killer T (NKT) cells with the endogenous ligand sulfatide induced dramatic proliferation and expansion of memory, but not naïve, CD4 T cells. NKT cell-induced proliferation of memory Th1 and Th2 cells was dependent largely on the production of IL-2, with Th2-cell proliferation also affected by loss of IL-4. Type II NKT cells were also required for efficient maintenance of memory CD4 T cells in vivo. Activation of iNKT cells resulted in up-regulation of IFN-γ expression by memory Th2 cells. These IFN-γ–producing memory Th2 cells showed a decreased capability to induce Th2 cytokines and eosinophilic airway inflammation. Thus, activated NKT cells directly regulate memory CD4 T-cell pool size and function via the production of cytokines in vivo.
Keywords: α-galactosylceramide, CD1d KO mice, Jα18 KO mice, STAT5, allergy
Immunologic memory plays a central role in the immune system, in which memory CD4 T cells may provide protection against infections or cancer and provide the basis for successful vaccines (1, 2). On the other hand, memory CD4 T cells induce or prolong inflammatory diseases, such as allergic disorders (3, 4) and autoimmune diseases (5). After antigen recognition by the T-cell receptor (TCR), naïve CD4 T cells undergo clonal expansion and become functionally polarized effector T helper (Th) cells (e.g., Th1, Th2, and Th17 cells) within 1 or 2 wk (6, 7). After antigen clearance, most effector Th cells are thought to undergo apoptotic cell death during a period known as the contraction phase (8); however, some effector CD4 T cells escape cell death, differentiate into memory CD4 T cells, and survive for long periods in vivo. Once long-lived memory T cells are established, these cells persist for months and years, accompanied by slow basal turnover and homeostasis (9–12), while maintaining the ability to proliferate and produce polarized cytokines on antigen reencounter (13). In addition, heterogeneity in cytokine production potential is suggested in memory CD4 T cells (3, 13–15). However, once memory cells are established, factors that influence memory CD4+ Th-cell pool size and function remain poorly defined.
Natural killer T (NKT) cells belong to a unique lymphoid lineage distinct from T, B, and NK cells. Invariant NKT (iNKT) cells, or type I NKT cells, are characterized by the expression of a restricted TCR repertoire consisting of Vα14-Jα18 in mice and Vα24-Jα18 in humans, with a highly skewed set of Vβs, mainly Vβ8.2 in mice and Vβ11 in humans (16, 17). The most potent and well-analyzed ligand for the iNKT antigen receptor is a glycolipid, α-galactosylceramide (α-GalCer), which is presented exclusively by CD1d, a monomorphic class Ib molecule (18). Recently, several investigators have shown that iNKT cells are activated by microbial glycolipids from Shingomonas spp, Borrelia burgdorferi, Mycobacterium bovis, Helicobacter pylori, and Streptococcus pneumonie (19–22). Activated iNKT cells play critical roles in the regulation of various immune responses, including infection, allergic inflammation, antitumor immunity, and autoimmune responses, and thus represent a potential immunotherapeutic target with clinical potential (23, 24). In addition to iNKT cells, other CD1d-restricted, lipid antigen-reactive NKT cells, known as type II NKT cells, are present in humans and mice (25). Type II NKT cells express biased TCR repertoires and recognize a range of hydrophobic antigens, sulfatide, lysophosphatidylcholine, and even small aromatic molecules (26). Sulfatide is considered an endogenous ligand for type II NKT cells. Type II NKT cells have an activated or memory-like phenotype and the ability to modulate immune responses, including suppression of autoimmunity and inhibition of tumor rejection (27).
Given that the effects of NKT cells on T-cell memory remain to be fully defined, we examined the interplay between NKT cells and the memory CD4 Th-cell pool using an experimental system called “memory Th1/Th2 mouse,” in which antigen-specific memory CD4 T cells are efficiently generated and maintained in vivo (28).
Results
Activation of iNKT Cells with α-GalCer–Induced Proliferation of Memory CD4 T Cells, but Not Naïve CD4 T Cells, in Vivo.
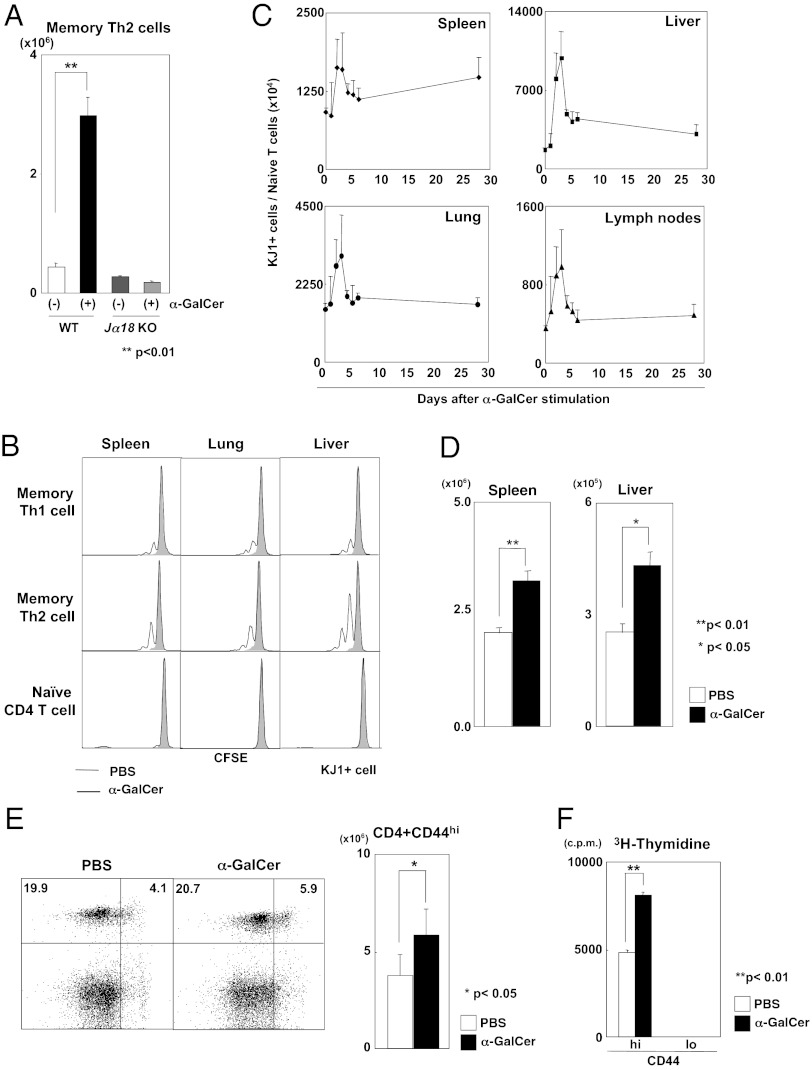
To examine whether iNKT cells control the generation and maintenance of memory Th2 cells, we used WT and Jα18-deficient (Jα18 KO) mice that lack iNKT cells and produced memory Th2 mice in which ovalbumin (OVA)-specific DO11.10 transgenic (Tg) memory Th2 cells are efficiently generated 1 mo after effector Th2-cell transfer (28). We administered α-GalCer i.p. to these memory Th2 mice at 30 d after cell transfer (Fig. S1A). The absolute numbers of memory Th2 cells (KJ1+ donor-derived cells) in the livers of these mice at 3 d after α-GalCer administration are shown in Fig. 1A. We found a dramatic increase in the number of memory Th2 cells in WT mice, but no increase in Jα18 KO mice, indicating iNKT cell-dependent increases in memory Th2 cells. We assessed cell division in memory Th1 and Th2 cells after α-GalCer administration using a carboxyfluorescein succinimidyl ester (CFSE)-labeling method (Fig. S1B). We found multiple rounds of cell division in memory Th1 and Th2 cells, but not in naïve CD4 T cells, in each organ tested (Fig. 1B); however, the magnitude of increase was smaller than that seen in memory Th2 cells. Cell division was observed in memory Th2 cells even at 3 d after α-GalCer administration (Fig. S1C). These results indicate that activation of iNKT cells with α-GalCer induced proliferation of memory Th1 and Th2 cells, but not of naïve CD4 T cells.
Fig. 1.
Activation of iNKT cells induced the proliferation of memory CD4 T cells in vivo. (A) Numbers of memory Th2 cells in the livers of WT and Jα18 KO mice at 3 d after α-GalCer administration. Values are mean ± SEM (n = 5). **P < 0.01. (B) CFSE analysis of memory Th2 cells. Memory Th2 cells (5 × 106) purified from Th2 memory mice and CD44loCD62Lhi naïve CD4 T cells purified from normal DO11.10 Tg mice were labeled with CFSE and then transferred into syngenic mice. One day later, α-GalCer was injected. On day 7, cell division of transferred cells in the liver was assessed by flow cytometry. (C) Monitoring of memory Th2 cells in various organs after α-GalCer administration into memory Th2 mice. Values are mean ± SEM (n = 3). (D) Numbers of memory Th2 cells in the spleens and livers at 4 wk after the last stimulation. (E) Percentages and absolute numbers of memory CD4 T cells (CD44hi) in the spleens of mice immunized with KLH and CFA. (F) 3H-thymidine uptake in CD44hi and CD44lo CD4 T cells purified from the spleens of mice immunized with KLH and CFA. The donor cells and recipient mice used in Fig.1 were BALB/c background. Similar data were obtained from at least three (A and B) or two (C–F) independent experiments. Values are mean ± SEM (n = 5). *P < 0.05; **P < 0.01.
We next monitored the number of memory Th2 cells in various organs of WT mice after α-GalCer administration, and found that memory Th2 cells increased and peaked in each organ at 3 d after α-GalCer administration (Fig. 1C). To determine whether the steady-state number of memory Th2 cells is maintained at high levels after iNKT-cell activation, we administered four injections of α-GalCer–pulsed bone marrow-derived dendritic cells (BMDCs) to memory Th2 mice, and 1 mo later assessed the number of memory Th2 cells (Fig. S1D). To minimize the induction of anergic changes in NKT cells by repeated stimulation (29), we used α-GalCer–pulsed BMDCs for NKT-cell stimulation in this experiment. We found significantly increased numbers of memory Th2 cells in the spleen and liver, indicating expansion of the memory Th2-cell pool (Fig. 1D).
We observed that the numbers of memory CD4 T cells generated from naïve cells by immunization in vivo are also increased by iNKT-cell activation (Fig. S1E). Furthermore, we found an increase in naturally occurring CD44hi phenotypic memory CD4 T cells (Fig. S1F). We assessed the percentages of CD44hi and CD44lo CD4 T cells in the spleen and liver and their cell division at 7 d after α-GalCer administration and found increased percentages of naturally occurring memory CD4 T (CD44hi) cells (Fig. S1G), and several cell divisions in these CD44hi cells (Fig. S1H). To study the effect of activated iNKT cells on endogenous polyclonal memory CD4 T cells, we immunized normal BALB/c mice with keyhole limpet hemocyanin (KLH) and complete Freund’s adjuvant (CFA) (Fig. S1I). The number of CD44hi CD4 T cells was increased slightly but significantly after α-GalCer administration (Fig. 1E). In addition, proliferation of memory phenotype CD44hi CD4 T cells in response to specific antigen was significantly enhanced by the activation of iNKT cells, whereas proliferation of CD44lo CD4 T cells was not detected (Fig. 1F). Taken together, these results indicate that the activation of iNKT cells with α-GalCer induces memory Th1/Th2-cell proliferation and expansion of the memory CD4 T-cell pool.
IL-2 Produced by Activated iNKT Cells Induced Proliferation of Memory Th1/Th2 Cells.
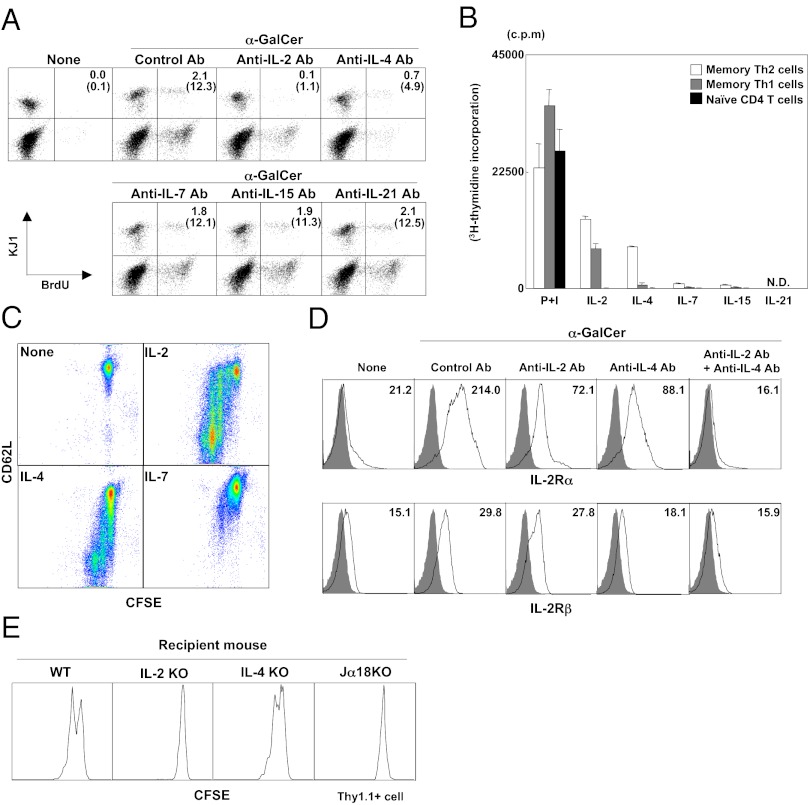
We next sought to identify functional molecules involved in the activated iNKT-cell–mediated proliferation of memory Th2 cells. Activated iNKT cells are known to regulate other immune cells by secreting various cytokines (16). We found that culture supernatant of splenocytes stimulated with α-GalCer was sufficient to induce proliferation of memory Th2 cells (Fig. S2A). We assessed the mRNA expression of NKT-cell– or memory T-cell–related cytokines in the liver at 3 d after α-GalCer administration (Fig. S2B) and found dramatically increased expression of IL-2 and IL-21 and significantly increased expression of IL-4, IL-7, IL-10, IL-12 (p35), IL-15, IFN-β, and IFN-γ. We conducted several in vitro experiments to examine whether α-GalCer or any of these cytokines can induce proliferation of memory Th2 cells. Memory Th2 cells purified from memory Th2 mice were cocultured with syngeneic whole splenocytes as a source of iNKT cells in the presence of α-GalCer. Neutralizing antibodies against these cytokines were added to the α-GalCer stimulation culture (Fig. 2A). Anti–IL-2 and –IL-4 mAbs inhibited the proliferation of memory Th2 cells, whereas anti–IL-7, –IL-15, and –IL-21 mAbs had no apparent effect. These results indicate that IL-2 and IL-4 predominantly induced the proliferation of memory Th2 cells in the α-GalCer stimulation culture.
Fig. 2.
IL-2 produced by activated iNKT cells induced the proliferation of memory Th1 and Th2 cells. (A) BrdU incorporation by memory Th2 cells in vitro. Memory Th2 cells (2 × 105) were purified from the spleens of memory Th2 mice and cocultured with BALB/c splenocytes (2 × 106) in the presence of α-GalCer (100 ng/mL) or absence of neutralizing antibodies (10 µg/mL) for 3 d. Values represent the number of cells as a percentage of total cells (in parentheses, as a percentage of KJ1+ cells). (B) [3H]-thymidine incorporation in memory Th cells and naïve CD4 T cells. Purified memory Th1 and Th2 cells (2 × 105) and naïve CD4 T cells (2 × 105) from DO11.10 Tg mice were stimulated with phorbol myristate acetate (50 ng/mL) plus ionomycin (500 nM) or each cytokine in the presence of IL-2 (25 U/mL), IL-4 (100 U/mL), IL-7 (100 U/mL), IL-15 (100 ng/mL), or IL-21 (100 ng/mL). (C) Purified CD62Lhi memory Th2 cells were labeled with CFSE and stimulated with each cytokine for 48 h with the same concentration of cytokines used in B. (D) The expression of IL-2Rα and IL-2Rβ on memory Th2 cells stimulated with splenocytes (2 × 106) and α-GalCer (100 ng/mL) in presence of anti–IL-2 and/or anti–IL-4 antibody (10 μg/mL). (E) Memory Th2 cells (Thy1.1+; C57BL/c background) cells (5 × 106) were labeled with CFSE and transferred into WT, IL-2 KO, IL-4 KO, or Jα18 KO mice (Thy1.2+; C57BL/c background). On day 1, the mice were given α-GalCer. The divisions of memory Th2 cells in the liver were determined by flow cytometry on day 7. The donor cells and recipient mice used in these experiments were BALB/c background except for those shown in Fig. 1E. Similar data were obtained from at least three (A–D) or two (E) independent experiments.
We next purified memory Th2 cells by cell sorting and directly stimulated these cells with IL-2, IL-4, IL-7, IL-15, and IL-21, and then evaluated their proliferation by [3H]-thymidine incorporation (Fig. 2B). IL-2 and IL-4 induced substantial memory Th2-cell proliferation, IL-7 and IL-15 induced slight proliferation, and IFN-β, IL-10, TNF-α, and IFN-γ induced no proliferation (Fig. S2C). IL-2 induced memory Th1-cell proliferation. Naïve CD4 T cells did not proliferate in response to these cytokines. IL-2–induced [3H]-thymidine incorporation by CD62Lhi memory Th1/Th2 cells was greater than that observed for CD62Llo memory Th2 cells (Fig. S2D). We labeled the sorted CD62Lhi memory Th2 cells with CFSE and assessed cell division in the presence of IL-2, IL-4, and IL-7. CD62Lhi memory Th2 cells underwent several rounds of cell division after culture with IL-2 and IL-4, accompanied by reduced CD62L expression; IL-7 also had a slight effect in this culture (Fig. 2C). The α-GalCer–induced up-regulation of IL-2Rα expression was inhibited by anti–IL-2 and anti–IL-4 mAbs, and was almost completely inhibited in the presence of both mAbs (Fig. 2D). The α-GalCer–induced up-regulation of IL-2Rβ appeared to be more dependent on IL-4. These results indicate that IL-2Rα and IL-2Rβ expression by memory Th2 cells is up-regulated by IL-2 and/or IL-4. IL-2 stimulation did not induce cytokine production (IL-4, IFN-γ, or IL-2) from memory Th2 cells (Fig. S2 E and F), indicating that autocrine stimulation via IL-2 produced by activated memory Th2 cells is unlikely.
To confirm the importance of IL-2 and IL-4 produced from activated iNKT cells in vivo, we examined cell division of memory Th2 cells after α-GalCer administration in IL-2–deficient mice and IL-4–deficient mice (Fig. 2E). We found no cell division of memory Th2 cells in IL-2 KO and Jα18 KO mice, but moderate cell division in IL-4 KO mice. These results indicate that IL-2 is the major cytokine responsible for the induction of α-GalCer–mediated proliferation of memory Th2 cells. The proliferation of memory Th1 cells after α-GalCer administration also appeared to be dependent largely on IL-2 produced by activated iNKT cells (Fig. S3). Memory CD8 T cells, such as memory Tc1 and Tc2 cells generated in our effector T-cell transfer system (28), also showed a dramatic α-GalCer–induced proliferation that was also largely dependent on IL-2 (Fig. S4).
We also found that another iNKT-cell ligand, α-glucoronsylceramide (GSL-1), a glycosphingolipid derived from Sphingomonas (22), induced similar effects on the memory T-cell population. After splenocyte coculture with GSL-1′–pulsed BMDCs, IL-2 and IFN-γ production was lower than that induced by α-GalCer, but IL-4 production was comparable (Fig. S5A). GSL-1′ also induced proliferation of memory Th1 and Th2 cells, which was inhibited by anti–IL-2 (Fig. S5B). GSL-1′–pulsed CD1d KO BMDCs did not induce the proliferation of memory Th2 cells (Fig. S5C), whereas both α-GalCer– and GSL-1′–pulsed BMDCs induced cell division of memory Th1 and Th2 cells in the liver (Fig. S5D).
IL-21 Enhanced IL-2–Mediated Memory CD4 T-Cell Proliferation and STAT5 Signaling.
Although IL-21 alone did not induce the proliferation of memory Th1 or Th2 cells, IL-21 significantly augmented IL-2–induced proliferation of purified memory Th1 and Th2 cells (Fig. S6A). In fact, IL-21R expression was up-regulated on memory Th1 and Th2 cells by IL-2 (Fig. S6B). Stimulation of memory Th1 and Th2 cells with IL-2, IL-21, and IL-2 plus IL-21 for 30 min revealed that STAT5 phosphorylation was up-regulated by IL-2 or IL-21 and was greatly enhanced by IL-2 and IL-21 together (Fig. S6C). STAT3 phosphorylation was induced by IL-21 in memory Th2 cells, but was not enhanced by costimulation with IL-2 (Fig. S6D). Thus, IL-2 appears to up-regulate the IL-21R on memory Th1 and Th2 cells, and IL-21 enhances IL-2–induced proliferation accompanied by enhanced activation of the STAT5 signaling pathway.
Type II NKT Cells Contributed to the Maintenance of Memory Th1 and Th2 Cells.
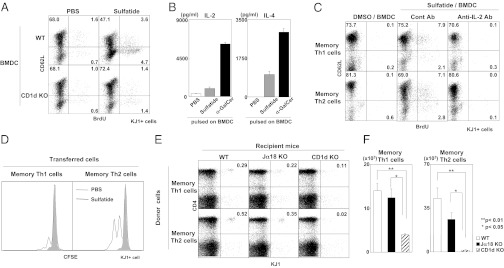
We next examined whether type II NKT cells also contribute to the proliferation of memory Th1 and Th2 cells when activated with an endogenous ligand, sulfatide (30). Memory Th2 cells were cultured with sulfatide-pulsed BMDCs and splenocytes as a source of type II NKT cells. Increased numbers of BrdU-incorporated memory Th2 cells were observed in the culture with sulfatide-pulsed WT BMDCs, but not in the culture with sulfatide-pulsed CD1d KO BMDCs (Fig. 3A). Sulfatide stimulation induced the production of IL-2 and IL-4, but at lower levels than those induced by α-GalCer (Fig. 3B). The sulfatide-induced proliferation of memory Th1 and Th2 cells was also dependent on IL-2, as demonstrated by the finding that the addition of anti–IL-2 mAbs to the culture inhibited proliferation (Fig. 3C). Moreover, sulfatide-pulsed BMDCs induced cell division of both memory Th1 and Th2 cells in vivo (Fig. 3D).
Fig. 3.
Type II NKT cells contributed to the maintenance of memory Th1 and Th2 cells. (A) BrdU incorporation by memory Th2 cells stimulated with sulfatide was examined by flow cytometry. Memory Th2 cells (2 × 105) prepared from memory Th2 mice were cultured with BALB/c splenocytes (2 × 106) and sulfatide-pulsed WT or CD1d KO BMDCs (4 × 105) for 72 h. (B) Splenocytes from BALB/c mice were cultured with sulfatide or α-GalCer–pulsed BMDCs for 72 h. Cytokine concentrations in the culture supernatants were determined by ELISA. (C) Memory Th1 and Th2 cells were prepared and stimulated with sulfatide-pulsed BMDCs in the presence of BALB/c splenocytes and anti–IL-2 mAb (10 µg/mL). (D) Memory Th1 or Th2 cells (5 × 106) were labeled with CFSE and adoptively transferred into naïve syngenic mice. Sulfatide-pulsed BMDCs (5 × 105) were transferred the next day. On day 7, cell division of memory Th1 or Th2 cells in the liver was assessed. (E and F) Memory Th1 or Th2 cells (5 × 106) prepared from the spleens of memory Th1 and Th2 mice were transferred into syngeneic WT, Jα18 KO, and CD1d KO mice. Sixty days later, percentages and absolute number of memory Th1 and Th2 cells in the liver were assessed. No specific stimulation, such as with sulfatide/BMDCs, was used for this experiment. The donor cells and recipient mice used in this experiments were BALB/c background. Similar data were obtained from at least three independent experiments. Values are mean ± SEM (n = 5). *P < 0.05; **P < 0.01.
To examine the physiological role of NKT cells in the maintenance of memory Th1 and Th2 cells, we transferred memory CD4 T cells into NKT-cell–deficient mice. Given the unavailability of an appropriate specific marker for type II NKT cells, the most reliable way to study the in vivo function of type II NKT cells is by comparing the results obtained using CD1d KO mice (which lack both iNKT cells and type II NKT cells), Jα18 KO mice (which lack only iNKT cells), and WT mice. In this experiment, we generated memory Th1 or Th2 cells in WT mice; transferred these cells into WT, Jα18 KO, or CD1d KO mice; and then evaluated the numbers of memory Th1 or Th2 cells maintained in the liver after 2 mo. The percentages of memory Th1 and Th2 cells were lower in CD1d KO mice compared with WT mice, whereas the percentages of memory Th1 and Th2 cells in Jα18 KO mice were similar to that of WTmice (Fig. 3E). The absolute numbers of memory Th1 and Th2 cells were significantly reduced in CD1d KO mice compared with WT and Jα18 KO mice, but the reduced numbers of these cells in Jα18 KO mice was not significant compared with WT mice (Fig. 3F). The decreases in memory Th1 and Th2 cells in the CD1d KO mice might be related to rejection, given that memory Th1 and Th2 cells express CD1d molecules (Fig. S7A), and CD1d KO mice might not be tolerant of CD1d. However, the maintenance of CD1d KO memory Th2 cells was also significantly impaired in CD1d KO mice, which excludes the possibility of rejection of CD1d-expressing memory Th2 cells in CD1d KO mice (Fig. S7B). Moreover, CD1d KO memory Th2 cells were normally generated in WT mice. Therefore, NKT cells play an important role in the maintenance of memory Th2 cells. Taken together, these data indicate that type II NKT cells may be activated by endogenous ligands presented on CD1d and contribute to the maintenance of memory Th1 and Th2 cells.
α-GalCer Administration Altered the Function of Memory Th2 Cells and Attenuated Memory Th2-Cell–Dependent Allergic Airway Inflammation.
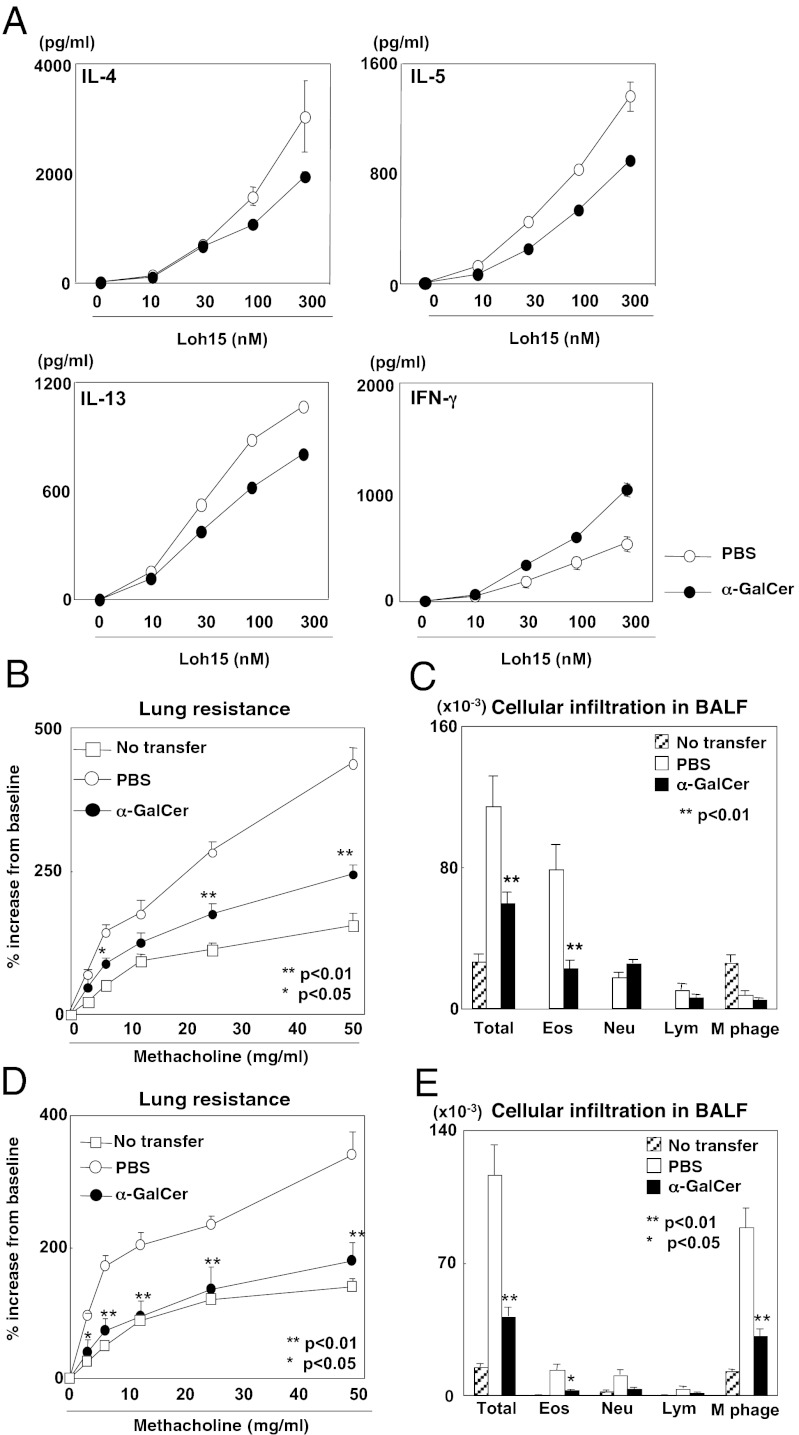
To assess the function of memory Th2 cells after iNKT-cell activation, we sorted memory Th2 cells from the memory Th2 mice at 30 d after α-GalCer treatment and stimulated these cells with OVA peptide and irradiated allophycocyanin. After iNKT-cell activation in vivo, IL-4, IL-5, and IL-13 production was reduced, but IFN-γ production was increased (Fig. 4A), indicating acquisition of the Th1 phenotype by memory Th2 cells. The production of IL-2 and IFN-γ by memory Th1 cells was not obviously altered by the α-GalCer treatment (Fig. S8A).
Fig. 4.
α-GalCer administration altered the function of memory Th2 cells and attenuated memory Th2-dependent allergic airway inflammation. (A) Memory Th2 cells were purified from memory Th2 mice treated with or without α-GalCer (100 µg/kg) at 30 d before analysis. Memory Th2 cells (4 × 104) were purified from the spleens of these mice and then stimulated with irradiated splenocytes (2 × 105) and antigenic peptide (Loh15) at the indicated concentrations. Three days later, culture supernatants were collected, and cytokine levels were determined by ELISA. (B and C) Thirty days after α-GalCer administration, memory Th2 mice were exposed to OVA aerosol to induce airway inflammation. One day after the last OVA challenge, AHR was assessed by measuring lung resistance. The absolute numbers of leukocytes in the bronchoalveolar lavage fluid are shown. (D and E) Memory Th2 cells were transferred as shown in Fig. S8F, and AHR and leukocyte infiltration were assessed as B and C. (B–E) Values are mean ± SEM (n = 5). The donor cells and recipient mice used in these experiments were BALB/c background. Similar data were obtained from at least three independent experiments. *P < 0.05; **P < 0.01.
To assess the effect of activation of iNKT cells on memory Th2-cell function in vivo, we examined memory Th2 cell-dependent allergic airway inflammation (Fig. S8B). Airway hyperreactivity (AHR) and eosinophillic infiltration were reduced in the α-GalCer–injected OVA-challenged memory Th2 mice compared with PBS-injected OVA-challenged control mice (Fig. 4 B and C). The leukocytes accumulated in the peribronchiolar regions of the lungs were greatly decreased in the memory Th2 mice treated with α-GalCer (Fig. S8C). In addition, reduced periodic acid-Schiff staining was evident in the bronchial epithelium of the asthmatic lungs (Fig. S8D). Furthermore, reduced IL-4, IL-5, and IL-13 production and increased IFN-γ production in bronchoalveolar lavage fluid were detected in samples from α-GalCer–treated memory Th2 mice (Fig. S8E). Finally, we transferred memory Th2 cells into normal mice and then exposed these mice to OVA to induce allergic responses (Fig. S8F). AHR and eosinophil infiltration into the airways were reduced in the mice that received memory Th2 cells prepared from mice treated with α-GalCer compared with the mice that received cells from mice treated with PBS (Fig. 4 D and E). These results indicate that activation of iNKT cells alters memory Th2-cell function and attenuates memory Th2 cell-dependent allergic airway inflammation.
Discussion
Here we report that iNKT cells activated with glycolipid ligands selectively induced proliferation of memory Th1 and Th2 cells and increased the steady-state numbers of memory Th1 and Th2 cells in vivo. IL-2 produced by activated iNKT cells plays a major role in inducing the proliferation of both memory Th1 and Th2 cells, with IL-4 also playing a role in the proliferation of Th2 cells. Type II NKT cells appear to be required to maintain the steady-state population of memory Th1 and Th2 cells, possibly in response to recognition of an endogenous ligand. Furthermore, activated iNKT cells alter the function of memory Th2 cells, resulting in the attenuation of memory Th2-dependent allergic airway inflammation. Thus, NKT cells appear to control memory CD4 T-cell pool size and also function in vivo.
We found that IL-2 produced by activated NKT cells induced the proliferation of memory Th1 and Th2 cells, but not of naïve CD4 T cells (Fig. 1). Because this proliferation is independent of antigen recognition by TCRs, exposure to cytokines such as IL-2 appears to be sufficient to induce memory CD4 T-cell proliferation. IL-2 up-regulated IL-2R components, particularly IL-2Rα, on memory Th2 cells (Fig. 2) and facilitated the IL-2/STAT5–mediated proliferation of these cells (Fig. S6C). We found that memory Th1 and Th2 cells proliferated in response to IL-2 produced by NKT cells activated with GSL-1′, a component of the cell wall of the Gram-negative bacteria Sphingomonas (22) (Fig. S5). Therefore, iNKT cells likely are activated during bacterial infection, leading to the bystander proliferation of memory Th1 and Th2 cells. In addition to IL-2, both IL-4 and IL-21, which are produced by activated iNKT cells, contributed to the proliferation of memory CD4 T cells (Fig. S6). IL-4 alone induced the proliferation of memory Th2 cells (Fig. 2B), but the level was lower than that induced by IL-2. α-GalCer–induced up-regulation of IL-2Rα and IL-2Rβ expression was inhibited by anti-IL-4 (Fig. 2D), indicating that IL-4 may facilitate the IL-2–mediated proliferation of memory Th2 cells. IL-4 had only a marginal role in the induction of memory Th1-cell proliferation, probably because of the low expression of IL-4R on these cells. IL-21 augmented the IL-2–mediated proliferation of memory Th1 and Th2 cells, accompanied by increased phosphorylation of STAT5 (Fig. S6). Thus, although IL-2/STAT5–mediated proliferation appears to be of central importance, several cytokines produced by activated iNKT cells may cooperate to induce the proliferation of memory Th1 and Th2 cells.
The maintenance of memory Th1 and Th2 cells was significantly impaired in CD1d KO mice lacking both type I and type II NKT cells, whereas no obvious effect was observed in Jα18 KO mice lacking only iNKT cells (Fig. 3). This finding indicates that type II NKT cells play an important role in maintaining the steady-state pool size of memory CD4 T cells independent of activation by infectious agents. It is possible that type II NKT cells are activated by endogenous ligands in vivo, thereby controlling the steady-state numbers of memory Th1 and Th2 cells. In fact, sulfatide, an endogenous ligand for type II NKT cells, induced the proliferation of memory Th1 and Th2 cells, again via IL-2 (Fig. 3). These data suggest a possible role for sulfatide in the maintenance of memory CD4 T cells in vivo. Although further specific studies are needed to identify the most important endogenous ligands recognized by NKT cells to maintain the memory CD4 T-cell pool, our data indicate an implicit role for NKT cells in the maintenance of T-cell memory.
TCR stimulation in concert with type I and type II IFNs and IL-12 during lymphocytic choriomeningitis virus infection has been found to reprogram effector Th2 cells to Th2+1 phenotype cells by the induction of T-bet (31), indicating that polarized Th cells display a greater degree of functional plasticity than previously believed (6). Our findings also may indicate that the Th-cell function can be modulated in the microenvironment in which memory T cells reside, and that a mixed cytokine secretion phenotype in memory Th cells can be induced particularly in the peripheral tissues, where various microorganisms interact with the regional immune system under physiological conditions. It will be interesting to study the factors induced in the microenvironment that can modulate the function of memory Th cells in various in vivo infectious and disease models.
In summary, we have demonstrated that activation of NKT cells induces the proliferation of memory CD4+ Th1 and Th2 cells through the production of IL-2. This may occur during infection and also even in steady state to control maintenance of the memory CD4 T-cell pool in the body. In addition, activated NKT cells alter the function of memory Th2 cells. Therefore, NKT cells control the quantity and quality of memory CD4 T cells in vivo, and thus the current study may provide insight into the immunoregulatory role of NKT cells in vaccine development for infectious diseases, as well as the pathogenesis of chronic allergic disorders and autoimmune diseases.
Materials and Methods
Generation of Memory Th1 and Th2 Cells in Vivo.
Memory Th1 and Th2 cells were generated as described previously (28). In brief, CD4 T cells from DO11.10 OVA-specific TCR Tg mice or OT-II Tg mice were stimulated with specific OVA peptides (Loh15) plus allophycocyanin for 6 d in vitro under either Th1 or Th2 conditions. These effector Th1 or Th2 cells (3 × 107) were transferred i.v. into syngeneic recipient mice (BLAB/c, C57BL/6, BALB/c nu/nu, or TCR-βδ KO mice).
Cell Division Assay.
For analysis of cell division by CFSE staining, donor memory Th1 or Th2 cells were generated in BALB/c nu/nu or TCR-βδ KO mice, isolated with a CD4 T-cell isolation kit and AutoMACS separator (Miltenyi Biotec), and then labeled with CFSE (Invitrogen). The day after adoptive transfer into syngenic mice, recipient mice were injected i.p. with α-GalCer (100 μg/kg) or PBS. Six days later, donor T cells were analyzed by flow cytometry.
Supplementary Material
Acknowledgments
We thank Chizuka Obara, Kaoru Sugaya, Hikari Asou, Miki Kato, and Toshihiro Ito for their excellent technical assistance. This work was supported by the Global Center for Education and Research in Immune System Regulation and Treatment Program and by the City Area Program (Kazusa/Chiba Area) of the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by grants from Ministry of Education, Culture, Sports, Science, and Technology of JapanGrants-in-Aid for Scientific Research (B) 21390147 and for Young Scientists (B) 22790452; the Ministry of Health, Labor and Welfare of Japan; the Uehara Memorial Foundation; the Mochida Foundation; and the Naito Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203494109/-/DCSupplemental.
References
- 1.Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186:1325–1331. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo Y, et al. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 2011;35:733–745. doi: 10.1016/j.immuni.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007;119:280–294. doi: 10.1016/j.jaci.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Hiepe F, et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 6.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 9.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 11.Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: Functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama T, Yamashita M. Initiation and maintenance of Th2 cell identity. Curr Opin Immunol. 2008;20:265–271. doi: 10.1016/j.coi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 14.MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do? Semin Immunol. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010;130:1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 17.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 19.Chang YJ, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J Exp Med. 2011;208:1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinjo Y, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12:966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 23.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 24.Motohashi S, Okamoto Y, Yoshino I, Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. 2011;140:167–176. doi: 10.1016/j.clim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist M, et al. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol. 2009;39:1726–1735. doi: 10.1002/eji.200839001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T, Yamashita M. Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin Immunol. 2009;21:78–83. doi: 10.1016/j.smim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahng A, et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegazy AN, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.