Abstract
Histone deacetylases (HDACs) regulate inflammatory gene expression, as indicated by the potent antiinflammatory activity of pan-HDAC inhibitors. However, the specific contribution of each of the 11 HDAC proteins to the inflammatory gene expression program is unknown. Using an integrated genomic approach, we found that Hdac3-deficient macrophages were unable to activate almost half of the inflammatory gene expression program when stimulated with LPS. A large part of the activation defect was attributable to loss of basal and LPS-inducible expression of IFN-β, which maintains Stat1 protein levels in unstimulated cells and acts in an autocrine/paracrine manner after stimulation to promote a secondary wave of Stat1-dependent gene expression. Loss of Hdac3-mediated repression of nuclear receptors led to hyperacetylation of thousands of genomic sites and associated gene derepression. The up-regulation of the constitutively expressed prostaglandin endoperoxide synthase, Ptgs1 (Cox-1), a nuclear receptor target, had a causative role in the phenotype because its chemical inhibition reverted, albeit partially, the Ifn-β activation defect. These data indicate a central role for Hdac3 in inflammation and may have relevance for the use of selective Hdac inhibitors as antiinflammatory agents.
Keywords: chromatin, transcription
The inflammatory response involves the differential expression of hundreds of genes and is driven by well-defined stimulus-regulated transcription factors [e.g., NF-κB, activator protein-1 (AP-1), IFN regulatory factors (IRFs)] (1, 2). The interplay between these factors and the regulatory landscape specific to each cell type, which is generated by lineage-determining transcription factors, affects the final transcriptional output and the identity of the genes regulated by inflammatory stimuli (3).
The activation of the inflammatory gene expression program also involves a large number of coregulators acting at different steps of the transcription activation process (4), including chromatin modifiers (5–8), chromatin remodelers (9), and adaptors bridging chromatin-bound transcription factors or locally modified histones with the transcriptional machinery (10). Because of their role in inflammatory gene activation, these coregulators also represent potential drug targets, as exemplified by the attenuation of inflammatory responses by a small molecule that mimics acetylated histones and competes with them for binding to a bromodomain-containing adapter (11).
Chemical inhibitors of histone deacetylases (HDACis) have been reported to possess antiinflammatory properties in various in vitro and in vivo models (12, 13), in which they strongly reduce the production of inflammatory cytokines and mediators (14). Some of these compounds are already approved for use in humans in selected types of cancers (15), and therefore represent potential candidates for innovative antiinflammatory therapies.
Histone deacetylases (HDACs) are a family of lysine deacetylases targeting histones as well as a large number of nonhistone proteins (16). Lysine acetylation within the histone tail neutralizes the positive charge of the lysine residue, thus reducing electrostatic interactions with the DNA and increasing DNA accessibility. Acetylation also provides a docking platform for transcriptional regulators bearing complementary recognition domains, most notably the bromodomain (17). The classic HDAC family, whose components are all similarly sensitive to HDACis (15), includes class I (HDAC1, HDAC2, HDAC3, and HDAC8) and class II HDACs. Class I HDACs are nuclear enzymes constitutively expressed in virtually all cell types and associated with multimolecular complexes highly conserved from yeast to humans. Although Hdac1 and Hdac2 are part of at least three distinct complexes, Hdac3 seems to exist exclusively as a component of the nuclear receptor corepressor (NCoR)/silencing mediator for retinoid and thyroid hormone receptors (SMRT) corepressor complex (18). The most widely appreciated function of this complex is the constitutive repression (via histone deacetylation) of genes bound by unliganded nuclear hormone receptors (NRs), which directly interact with the NCoR and SMRT subunits (19, 20). On ligand binding, conformational changes in the NR promote the release of corepressor complexes and the recruitment of transcriptional activators (21).
Given this mechanism of action and their role in transcriptional repression, the notion that HDACs are required for inflammatory gene expression seems counterintuitive. However, yeast Hos2 deacetylase (the ortholog of mammalian Hdac3) was found to be required for expression of some genes, acting antagonistically toward Rpd3, the Hdac1/2 ortholog (22). Mechanistically, Hos2 (a component of the yeast Set3 complex, which may be related to the NCoR/SMRT complex) (23, 24) is recruited at the 5′ of transcribed regions, where it is required to keep acetylation levels low (25). It was speculated that increased histone acetylation at the 5′ of coding regions may interfere with early stages of transcriptional elongation, thus explaining the Hos2 requirement for transcription (25).
Two additional elements of complexity must be considered, which may dictate the net effect of HDACis on gene expression. First, HDACs control lysine acetylation at thousands of proteins, including transcriptional regulators (16), which may cause effects difficult to explain assuming that histones are the only relevant targets of these enzymes. Second, gene derepression caused by HDACis unavoidably causes secondary transcriptional effects that may eventually dominate over direct effects.
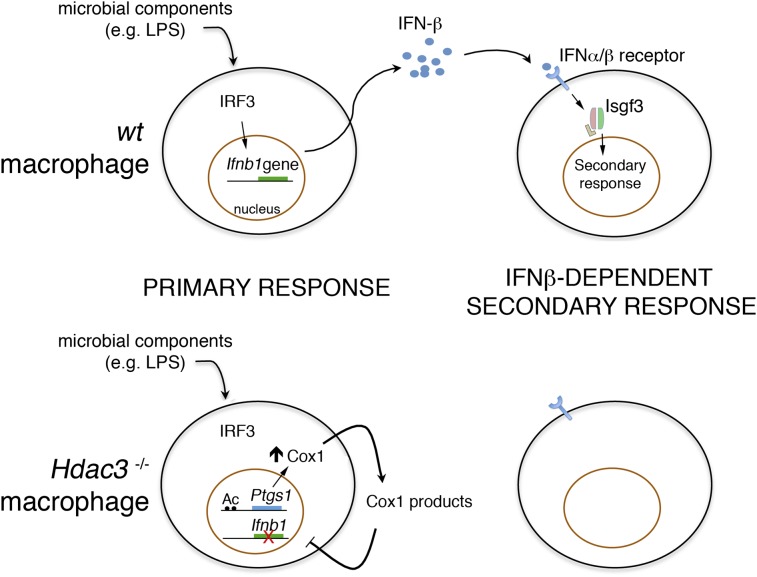
Starting from the prior notion that Hos2 is required for the expression of some inducible genes in yeast, we analyzed the role of its mammalian ortholog, Hdac3, in the induction of inflammatory genes in macrophages. We found that in the absence of Hdac3, expression of almost half of LPS-induced genes was severely impaired. Specifically, the IFN-β–dependent branch of the LPS response was almost completely abrogated because of reduced basal and LPS-inducible Ifn-β expression. In keeping with the described requirement for basal Ifn-β to maintain Stat1 expression (26), we found that Stat1 levels were strongly reduced in Hdac3−/− macrophages. However, Stat1 expression could be rescued by exogenous IFN-β treatment, indicating that the residual amount of Stat1 was functionally intact in Hdac3−/− cells. Finally, Hdac3−/− cells displayed greatly increased constitutive expression of Cox-1 [prostaglandin endoperoxide synthase (Ptgs1)], a key enzyme in the synthesis of prostaglandins and highly reactive electrophilic oxoderivatives (EFOXs) (27), probably attributable to hyperactive nuclear receptors binding to an enhancer upstream of the Ptgs1 gene. Two general Cox inhibitors, indomethacin and diclofenac, partially rescued Ifn-β and Stat1 levels, indicating a causative role for Cox-1 overexpression in the observed phenotype. The data described in this study may have practical therapeutic implications for the use of selective HDAC inhibitors in antiinflammatory therapies.
Results
Hdac3 Requirement for Inflammatory Gene Expression.
To generate Hdac3-deficient macrophages we crossed C57/BL6 mice carrying a floxed Hdac3 allele (28) with an Mx-Cre strain, in which Cre expression is induced in response to poly-Inosinic:poly-Cytydylic (polyI:C) injections. After in vivo deletion, bone marrow cells were isolated and macrophages were differentiated in vitro. Deletion efficiency was reproducibly higher than 90% (SI Appendix, Fig. S1) and macrophage differentiation proceeded unperturbed in the absence of Hdac3, as evaluated from the expression of surface markers (Fig. 1A, Upper). However, analysis of activation markers, such as CD86, provided an initial indication of a defective response to LPS stimulation. To obtain a more global view of gene expression changes in the absence of Hdac3, we carried out a cDNA microarray analysis in Hdac3−/− macrophages, either untreated or stimulated with LPS for 4 h (Dataset S1). A gene set enrichment analysis showed a broad defect in LPS-induced gene expression in cells lacking Hdac3. We analyzed two gene sets: one that included the genes down-regulated and one that included the genes up-regulated (Fig. 1B, Lower Left) in LPS-treated Hdac3−/− macrophages. All genes were ranked on the x axis from left to right according to their relative level of expression in normal untreated macrophages vs. LPS-treated macrophages. The distribution of the gene set members relative to the ranked list indicated that genes requiring Hdac3 for expression were strongly overrepresented among those induced by LPS (Fig. 1B, Upper Left). Specifically, 313 (44.9%) of 697 LPS-induced genes showed significantly reduced expression in the absence of Hdac3 (Fig. 1B, Upper Right). Reduced activation of inflammatory genes did not reflect increased expression of LPS-inducible genes in the basal state (and therefore a reduced magnitude of the fold change in gene activation) because none of the genes down-regulated in LPS-treated Hdac3−/− macrophages displayed higher levels in the basal state. Because of the role of Hdac3 in the activity of NCoR/SMRT corepressor complexes, it is predicted that its loss will cause increased expression of a number of genes. However, when analyzing gene repression induced by LPS (Fig. 1B, Lower), the overall impact of Hdac3 loss was comparatively much smaller, with only 144 (15.4%) of 930 LPS-repressed genes partially or completely rescued by Hdac3 deletion. Therefore, the contribution of Hdac3 to LPS-induced repression is relatively marginal.
Fig. 1.
Hdac3 requirement for the inflammatory gene expression program in macrophages. (A) FACS analysis of Hdac3−/− bone marrow-derived macrophages. (Upper) Two classic differentiation markers, Cd11b and F4/80 (Emr1), are shown. (Lower) CD86 induction in response to LPS is shown. max, maximum. (B) Gene set enrichment analysis in Hdac3−/− macrophages stimulated with LPS for 4 h. Genes were sorted (x axis) from left to right based on their relative level of expression in untreated vs. LPS-treated macrophages. The two gene sets analyzed include the genes down-regulated (Upper) and those up-regulated (Lower) in Hdac3−/− cells. (Right) Venn diagrams indicate the overlap between LPS-inducible genes and Hdac3-dependent genes (Upper) or between LPS-repressed genes and genes whose expression is increased in LPS-stimulated Hdac3−/− cells (Lower). (C) Venn diagram shows the overlap between LPS-inducible HDAC3-dependent genes and genes whose activation in response to LPS requires IFN-β (the dataset used was from the study by Cheng et al. (36) and Pubmed identifier 20470404). (D) Heat map shows the effect of Hdac3 loss on the genes regulated (activated or repressed) by LPS stimulation. (E) Statistical overrepresentation of TFBS in the promoters of Hdac3-dependent and independent genes. (F) Quantitative RT-PCR shows the effects of Hdac3 loss at selected genes. (G) IL-6 and TNF protein levels were measured in the culture supernatants 24 h after LPS stimulation.
To determine if the genes affected by Hdac3 deletion belong to identifiable groups, we carried out an ingenuity pathway analysis (Dataset S2), which indicated the IFN signaling pathway as the most enriched one in the dataset (P < 3.54E-09) (SI Appendix, Fig. S2). In the LPS response, IFN-β is induced via a pathway dependent on the adapter TRIF, which controls the activation of the transcription factor IRF3 (29). IRF3 directly controls transcription of the Ifnb1 gene, whose product is rapidly released to activate an autocrine and paracrine loop that is ultimately responsible for a secondary wave of gene induction that includes classic IFN-β–regulated genes (30). Therefore, Irf3-dependent genes include direct IRF3 targets (e.g., Ccl5) (30) and IFN-β–regulated genes. The latter are activated by a trimeric complex composed of Stat1, Stat2, and Irf9, which was initially indicated as IFN-stimulated gene factor 3 (Isgf3). Because the binding specificity of Isgf3 is very similar to that of Irf family members, the expression of many genes that are initially activated by Irf3 is then sustained by IFN-β–activated Isgf3 (30).
A total of 222 (70.9%) of 313 LPS-inducible and Hdac3-dependent genes overlapped LPS-inducible and IFN-β–dependent genes (Fig. 1 C and D), indicating that the impact of Hdac3 loss on the LPS-induced gene expression program was largely dominated by an impaired IFN-β response. Consistent with these data, when the promoters of the 222 Hdac3-dependent and IFN-β–dependent genes were subjected to a statistical overrepresentation analysis to detect enriched transcription factor binding sites (TFBSs), the most overrepresented matrices corresponded to Irf sites (Fig. 1E and Dataset S3). Conversely, LPS-inducible Hdac3-independent genes were mainly enriched for the canonical E-box (CACGTG), a binding motif for basic helix–loop–helix transcription factors, such as the aryl-hydrocarbon receptor nuclear translocator and Myc proteins. Therefore, distinct groups of genes divided on the basis of their Hdac3 dependence clearly show different TFBS profiles.
Quantitative PCR (Q-PCR) analysis on selected genes (Fig. 1F) confirmed the strong dependency of canonical IFN-β–dependent genes (e.g., Nos2, Ptgs2) on Hdac3. Among the IFN-β–independent genes down-regulated in Hdac3 mutant cells, IL-6 (Il6, which is a secondary response gene) was strongly affected, and the secretion of its protein product was almost completely abrogated (Fig. 1G). Ccl5, which is a direct Irf3 target, was unaffected by Hdac3 deletion, providing an initial indication that IRF3 activity is intact in Hdac3−/− cells.
Mapping Hdac3-Controlled Histone Acetylation in Macrophages.
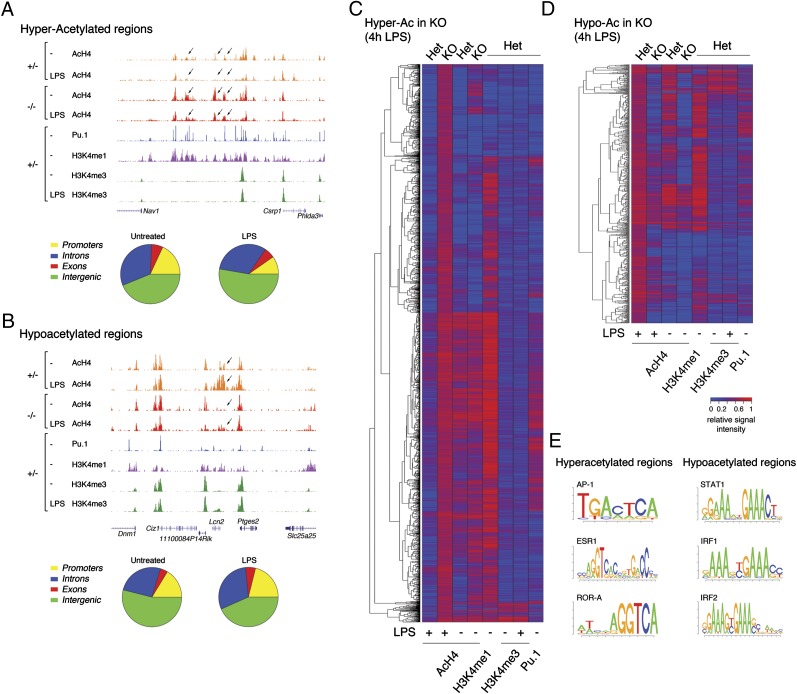
We next determined the impact of Hdac3 loss on histone H4 acetylation levels genome-wide using ChIP coupled to high-throughput sequencing (Seq). Using a stringent cutoff (P < 1e-10), 681 genomic regions were found to be hyperacetylated in untreated Hdac3−/− macrophages compared with their normal counterpart (Fig. 2A and Dataset S4). On LPS stimulation, the number of hyperacetylated regions (2,591 regions) was more than tripled (Fig. 2C and Dataset S4). At the same time, we detected a large number of regions (1,037 regions in untreated macrophages and 1,094 regions in LPS-treated macrophages) showing reduced H4 acetylation in mutant cells, likely as an indirect consequence of Hdac3 deletion (Fig. 2 B and D and Dataset S4). At many locations (e.g., genomic region shown in Fig. 2B), increased acetylation in response to LPS was greatly impaired in Hdac3−/− macrophages, indicating an underlying activation defect. Importantly, acetylation changes in both directions mainly occurred away from mapped promoters and transcription start sites (Fig. 2 and Dataset S4). Consistently, hyperacetylated regions commonly overlapped H3K4me1, a histone marker associated with enhancers (31), and Pu.1, the master regulator of macrophage differentiation that marks and activates a large fraction of the enhancer repertoire in these cells (32, 33).
Fig. 2.
Histone acetylation changes in Hdac3−/− macrophages. Snapshots of ChIP-Seq tracks show one hyperacetylated genomic region (A) and one hypoacetylated genomic region (B). In addition to AcH4, tracks from normal macrophages are shown (H3K4me1, H3K4me3, and Pu.1) for the localization of enhancer and promoter regions. The pie charts at the bottom of the two snapshots indicate the distribution of acetylation changes relative to RefSeq annotations. The heat maps display the genomic regions hyperacetylated (Hyper-Ac) (C) or hypoacetylated (Hypo-Ac) (D) in LPS-stimulated Hdac3−/− cells, respectively. The heat maps shown were generated using a cutoff of P < 1e-10 and considering only the genomic regions located distally (more than 2.5 kB) from annotated transcription start sites (TSS). Signal intensity was computed as follows. The raw reads counts for each region and dataset were retrieved and then normalized on the sequencing depth and on the length of the region in kilobases. Finally, for each different antibody, relative signals were rescaled from 0 to 1. (E) Top enriched TFBSs were overrepresented in genomic regions hyperacetylated or hypoacetylated in Hdac3−/− macrophages.
In Saccharomyces cerevisiae, deletion of the Hdac3 ortholog Hos2 results in gene activation defects (22) that have been linked to a possible inhibitory effect of hyperacetylation at the 5′ of the coding regions on RNA polymerase II elongation (25). Therefore, we assessed whether LPS-inducible Hdac3-dependent genes display hyperacetylation inside the coding region. Only 26 (8.3%) of 313 LPS-induced genes that required Hdac3 for activation displayed enhanced gene body acetylation in Hdac3−/− cells. Therefore, although these data are compatible with the possibility that Hdac3 is required at some genes for histone deacetylation at the 5′ of coding regions, they suggest that this mechanism does not account for the majority of the observed effects.
A statistical overrepresentation analysis of the TFBSs associated with regions hyperacetylated in LPS-stimulated Hdac3−/− macrophages identified AP-1 and nuclear receptor sites as the most enriched sequences (Fig. 2E and SI Appendix, Table S1). AP-1 is a family of homo- and heterodimeric transcription factors mainly belonging to the Jun and Fos families, whose activation is involved in inflammatory gene expression (34). In keeping with an overrepresentation of AP-1 sites in the hyperacetylated sequence set, it has been reported that AP-1 dimers in macrophages are under negative control by NCoR/SMRT complexes (35). Although we were unable to obtain robust Hdac3 ChIP results to determine its genomic distribution directly and confirm its overlap with hyperacetylated sites, these data strongly support the notion that loss of Hdac3 will lead to local hyperacetylation at regions basally bound by AP-1. Similarly, the observed enrichment of nuclear receptor sites indicates that in the absence of Hdac3, unliganded nuclear receptors associated with their cognate sites and bound to NCoR/SMRT corepressor complexes are unable to down-regulate local acetylation levels, which may lead to gene derepression.
When the same analysis was carried out on regions that were hypoacetylated in Hdac3−/− cells, the most enriched binding sites were recognition motifs for Irf family proteins and for Stat1. It should be noted that the Stat1 matrix identified as being overrepresented is, in fact, an Isgf3 binding site (whose binding preference largely reflects the contribution of the Irf9 component of the trimeric complex) and not a site for Stat1 homodimers [γ-activated sequence], which are selectively induced by IFN-γ.
Overall, Irf sites and the Irf-like Stat1 site were overrepresented both in the promoters of genes whose activation was impaired in Hdac3−/− macrophages and in putative enhancer regions displaying defective acetylation in LPS-treated KO cells. Moreover, Hdac3-dependent genes extensively overlapped IFN-β–dependent genes. These data prompted us to analyze the activity of the Irf3–Ifn-β–Stat1 (Isgf3) axis in cells lacking Hdac3.
Impaired Ifn-β–Stat1 Axis in Hdac3−/− Cells.
Activation of Irf3 in response to LPS stimulation, as measured using a phosphospecific antibody, was comparable in WT and Hdac3−/− cells (Fig. 3A). This result was in keeping with the normal activation of Ccl5 (Fig. 1F), a canonical Irf3 target gene (30), in macrophages lacking Hdac3. In contrast, Hdac3−/− cells showed dramatically reduced levels of both phosphorylated and total Stat1 (Fig. 1B). Stat1 protein down-regulation was associated with reduced mRNA levels, both in unstimulated cells (in which Stat1 mRNA was almost undetectable) and in LPS-stimulated macrophages (in which mRNA up-regulation in response to stimulation was severely attenuated) (Fig. 1C).
Fig. 3.
Impaired Ifn-β and Stat1 expression in Hdac3−/− macrophages. (A and B) Western blots were hybridized with the indicated antibodies. Vinculin was used as a loading control. Stat1 (C) and Ifnb (D) mRNA expression was measured by Q-PCR. TBP, TATA binding protein. (E) Tyk2 phosphorylation and mRNA levels (Right) in Hdac3−/− and control macrophages. (F) Reconstitution of WT Stat1 levels (Left) and LPS-induced Nos2 transcription (Right) on IFN-β treatment of Hdac3−/− macrophages. Histone H3 was used as a loading control. (G) Expression and phosphorylation of Stat3 and Stat5 in Hdac3−/− cells stimulated with LPS.
Maintenance of basal Stat1 mRNA and protein levels requires the constitutive production of small amounts of IFN-β, which is controlled by the AP-1 protein cJun in fibroblasts (26). Autocrine stimulation by constitutively secreted IFN-β activates the Isgf3 complex, which, in turn, induces Stat1 gene transcription (26). Consistent with this model, basal Stat1 levels are strongly reduced in the tissues of IFN-α/β receptor 1 (IFNAR1) mutant mice (26). In LPS-stimulated cells, the up-regulation of Stat1 expression requires the autocrine and/or paracrine activity of newly synthesized IFN-β (36). Therefore, we hypothesized that a defect in IFN-β production may underlie the observed reduction in Stat1 levels. Both basal and LPS-induced IFN-β mRNA levels were greatly reduced in Hdac3 mutant cells (Fig. 3D). As additional evidence of defective IFN-β production, we found that the phosphorylation of Tyk2, the Jak family kinase that mediates signaling from IFNAR1, was almost undetectable in Hdac3−/− macrophages, despite its normal expression (Fig. 3E). Impaired IFN-β expression was also detected using an alternative experimental approach, namely, retroviral shRNA-mediated depletion of HDAC3 in primary macrophages (SI Appendix, Fig. S3).
We next set out to obtain mechanistic insight into the defective IFN-β expression in Hdac3−/− macrophages. Basal histone acetylation at the Ifnb1 gene promoter was equally low in control and KO cells. Conversely, LPS-induced acetylation was almost completely abolished in Hdac3-deficient macrophages (SI Appendix, Fig. S4A). Therefore, the Hdac3 requirement for Ifnb1 activation was not linked to histone hyperacetylation and might be explained by an indirect effect. Despite impaired histone acetylation, recruitment of both p65 and IRF3, two transcription factors essential for Ifnb1 activation, was unaffected (SI Appendix, Fig. S4B). Together with data shown in Fig. 3A, these results confirmed that activation of IRF3, which is essential for Ifnb1 induction, and therefore for the secondary wave of Ifn-β–dependent gene expression, was normal in Hdac3−/− macrophages. Importantly, stimulation of Hdac3−/− macrophages with exogenous IFN-β for 24 h restored normal Stat1 levels (Fig. 3F, Left) and rescued LPS-induced Nos2 transcription (Fig. 3F, Right), suggesting that the residual amount of Stat1 in Hdac3−/− cells is fully functional and able to activate transcription if properly stimulated. Therefore, Hdac3 is not required for Stat1 activity.
We also tested the expression and activation of other Stat family members known to be induced in response to LPS stimulation. Stat3, which is phosphorylated and activated in response to IL-6, was expressed at normal levels, but its phosphorylation was strongly impaired (Fig. 3G), likely reflecting the defective IL-6 production (Fig. 1 F and G). Similarly, expression of Stat5 was comparable in WT and mutant cells, but its phosphorylation was almost completely abrogated in Hdac3−/− macrophages (Fig. 3G, Right), possibly attributable to loss of IL-15 induction, which is a Stat5-activating cytokine (Dataset S1).
Involvement of Ptgs1 (Cox-1) Up-Regulation in Ifnb1 Gene Repression.
The data described above point to a central role of reduced Ifn-β in the observed transcriptional phenotype. The simplest mechanism that may account for reduced Ifnb1 transcription is that some gene(s) de-repressed because of the absence of Hdac3 might negatively control its transcription. Therefore, we analyzed the list of genes overexpressed in Hdac3-deficient macrophages to identify possible candidates. This list includes a large number of targets of Nrf2, the pivotal transcription factor in the antioxidant response (37) (Dataset S1), which are constitutively overexpressed already in unstimulated Hdac3-deficient macrophages. Consistently in the ingenuity pathway analysis (SI Appendix, Fig. S2), the Nrf2-mediated oxidative stress response is one of the five most significant pathways deregulated in the KO, a result confirmed by the analysis of individual genes by Q-PCR (SI Appendix, Fig. S5). Nrf2 target up-regulation suggests ongoing oxidative stress in Hdac3-deficient cells. This result is also consistent with the notion that Vorinostat, a pan-HDACi, induces Nrf2 activation and antioxidant gene expression in promyelocytic leukemia cells (38).
Among the top up-regulated genes (both in unstimulated and stimulated Hdac3-null macrophages) (Dataset S1), two were of potential relevance to the observed phenotype: Ptgs1, encoding the constitutive isoform of prostaglandin-endoperoxide synthase (commonly known as Cox-1) (27), and Ptger2, encoding EP2, one of the four prostaglandin E2 (PGE2) receptors (39). Whereas Cox-1 is a constitutively expressed protein, Cox-2 is inducible in response to inflammatory stimuli; however, the catalytic properties of Cox-1 and Cox-2 are virtually identical, and their main physiological role is to catalyze the conversion of arachidonic acid (AA) to the PGH2 endoperoxide, which is then transformed by specific synthases into an array of products, which include PGE2, PGD2, and PGF2 and thromboxane A2 (TXA2) (27). Moreover, Cox-1 and Cox-2 also catalyze the synthesis of a broad family of EFOXs (40) that are extremely reactive and able to exert antiinflammatory effects via at least two different groups of mechanisms: by acting as agonists of the peroxisome proliferator activated receptor-γ (PPARγ) nuclear receptor (41) and by interfering with various components of the NF-κB activation cascade (42, 43). Because Ifnb1 transcription is exquisitely sensitive to the inhibitory activity of PGE2 (44) and EFOXs inhibit the activation of Ifn-β–dependent genes (e.g., Nos2) in macrophages (40), we considered the possibility that up-regulation of Cox-1 might be causally involved in down-regulation of Ifnb1 transcription.
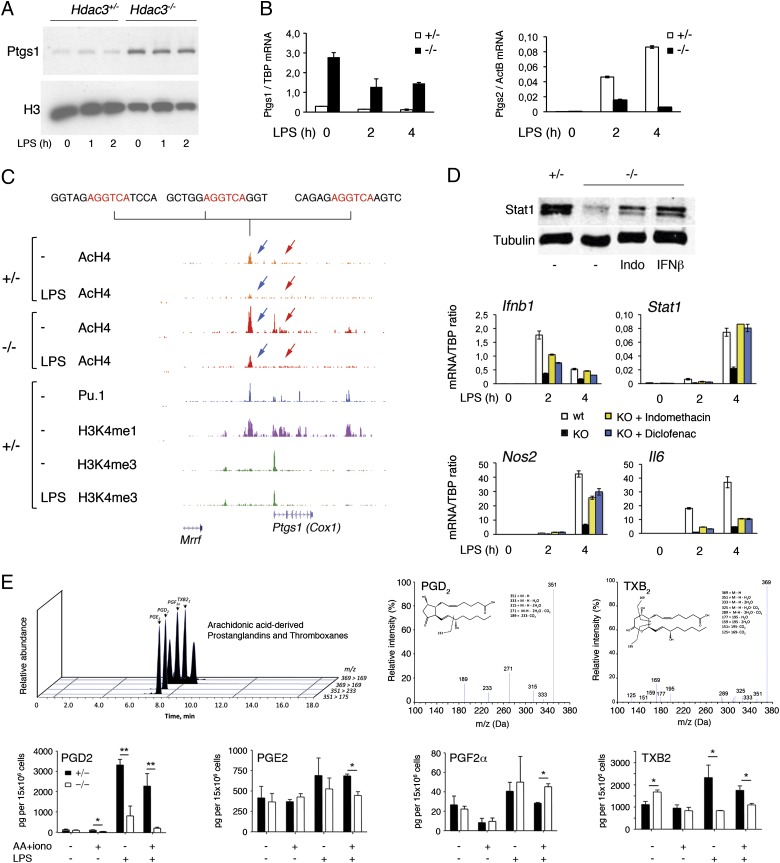
First, we confirmed the strong overexpression of Cox-1 in Hdac3-null macrophages, both at the protein (Fig. 4A) and mRNA (Fig. 4B, Left) levels. Conversely LPS induction of Cox-2 (Ptgs2), which is undetectable in basal conditions, was strongly attenuated in Hdac3−/− macrophages (Fig. 4B, Right). Because Cox-1 is a constitutively active enzyme, these data suggest that Hdac3−/− macrophages might be chronically exposed to its product(s). Second, we explored the genomic region surrounding the Ptgs1 gene and found that both the promoter and an upstream enhancer region (marked by H3K4me1 and Pu.1) were basally hyperacetylated in Hdac3-null macrophages (Fig. 4C). The hyperacetylated enhancer contained several sites matching the core nuclear receptor motif 5′-AGGTCA-3′ (Fig. 2E), indicating that loss of nuclear receptor-associated Hdac3 may lead to local histone hyperacetylation and derepression of the downstream gene. These data are also in keeping with the previously reported induction of Cox-1 by glucocorticoids (45), which act through nuclear receptors.
Fig. 4.
Ptgs1 (Cox1) up-regulation and IFN-β repression in Hdac3−/− macrophages. (A) Overexpression of Ptgs1 (Cox-1) protein in macrophages lacking Hdac3. Antibodies against histone H3 were used as a loading control (total lysate). (B) Ptgs1 (Left) and Ptgs2 (Right) mRNA expression in Hdac3-null macrophages. TBP, TATA-binding protein. (C) Genomic snapshot of the Ptgs1 locus. Blue arrows indicate a hyperacetylated region overlapping an upstream enhancer; red arrows indicate hyperacetylation at the Ptgs1 promoter and gene body. (D) Effects of the Cox-1/2 inhibitors indomethacin and diclofenac on Hdac3−/− macrophages. (Upper) Total Stat1 levels in control cells and Hdac3-null cells treated for 48 h with indomethacin. Tubulin was used as a loading control. For comparison, the effect on Stat1 levels of overnight treatment with IFN-β is shown. (Lower) Effects of indomethacin and diclofenac on Ifnb, Stat1, Nos2, and Il6 transcription. (E) Measurement of AA-derived prostaglandins and thromboxanes in WT and Hdac3−/− macrophages using LC-MS/MS. Extracts were made from cells in standard medium or supplemented with AA and ionomycin (iono) before extract preparation. (Upper) High performance liquid chromatography (HPLC) chromatogram (Left) and LC-MS/MS spectra for PGD2 and TXB2 (Right). (Lower) Amounts of the indicated species are shown in picograms per 12 × 106 cells (y axis). Data were obtained from three independent biological replicates (cells from three different mice). Error bars indicate ±SD.
Finally, we tested the effect of two nonselective Cox-1/2 inhibitors, indomethacin and diclofenac, on Ifnb1 and Stat1 expression. A 48-h pretreatment with indomethacin increased basal Stat1 protein levels in Hdac3−/− macrophages (Fig. 4D, Upper). Moreover, pretreatment with either indomethacin or diclofenac partially rescued basal and inducible Ifnb1 mRNA expression and almost completely restored Stat1 induction by LPS (Fig. 4D). Consistently, transcription of the LPS-inducible and IFN-β–dependent gene Nos2 returned to levels comparable to those observed in control cells. Conversely, the effects of these two drugs on activation of Il6, which is dependent on Hdac3 but not on IFN-β, were minimal. We also attempted to down-regulate Cox-1 levels by RNAi-mediated depletion. However, although WT macrophages tolerated Cox-1 depletion, Hdac3−/− macrophages rapidly died (SI Appendix, Fig. S6), indicating that these cells are addicted to increased Cox-1 levels or to increased production of Cox-1–generated metabolites.
To determine the identity of the metabolites generated by overexpressed Cox-1 in Hdac3−/− cells, we carried out a liquid chromatography-tandem MS (LC-MS/MS) analysis (46) for the identification of AA-derived prostaglandins and thromboxanes (Fig. 4E). In unstimulated cells, the level of none of the prostaglandins measured (PGD2, PGE2, and PGF2a) was significantly affected (either in the absence or presence of exogenously added ionomycin and AA). At 2 h poststimulation, the amount of PGE2 and, even more, PGD2 produced by Hdac3−/− macrophages was significantly reduced compared with WT cells, a result readily explained by the impaired LPS-induced expression of Cox-2 (Fig. 4B). Differently from prostaglandins, thromboxane synthesis (measured by levels of TXB2, the stable metabolite of the active species TXA2) was reproducibly increased in Hdac3−/− cells (Fig. 4E). These data suggest that increased production of PGH2 by Cox-1 in unstimulated Hdac3−/− macrophages could not be funneled toward prostaglandin production, probably because of limiting amounts of downstream prostaglandin synthases. Nevertheless, increased TXB2 production indicates that overexpressed Cox-1 in Hdac3-deficient macrophages is functional, a result in keeping with the known constitutive activity of this enzyme. Taken together, these data point to a role of Cox-1–generated nonprostaglandin metabolites in the negative regulation of Ifnb1 expression.
Discussion
In this study, we report a major role for Hdac3 in the activation of the inflammatory gene expression program in macrophages, which may be of practical relevance for the possible use of Hdac3-selective inhibitors in the therapy of inflammatory diseases.
From a mechanistic point of view, the central (yet not the only) component of the observed transcriptional phenotype is the lack of basal and LPS-inducible Ifn-β expression. The autocrine and paracrine activity of newly synthesized Ifn-β controls a secondary wave of gene induction (30) that includes many genes essential for several aspects of the inflammatory response, including microbial killing (e.g., Nos2, multiple GTPases) (47), antigen presentation (MHC class II genes), and T-cell costimulation (e.g., CD86). The strong (albeit partial) rescue effect of two Cox inhibitors indicates that Ifn-β down-regulation was dependent on the catalytic activity of Cox-1, whose expression was increased in macrophages lacking Hdac3 (possibly as a direct consequent of the loss of Hdac3-mediated control of nuclear receptor activity). From a biochemical point of view, Cox-1 up-regulation in unstimulated Hdac3−/− macrophages did not result in increased prostaglandin biosynthesis but was associated with increased production of TXA2, likely reflecting a limited availability of prostaglandin synthases acting downstream of Cox-1–generated PGH2. Because there is no evidence that TXA2 can exert any antiinflammatory activity on macrophages, the role of other Cox-1 products must be advocated. Indeed, Cox enzymes are also responsible for the enzymatic synthesis of EFOXs (40), highly reactive electrophilic molecules that are endowed with potent antiinflammatory activity and negatively regulate the production of IFN-β–regulated genes, such as Nos2 (40, 42). Consistent with this interpretation, the gene class most up-regulated in Hdac3-deficient macrophages was represented by Nrf2-dependent antioxidant genes. Activation of this antioxidant response may be required to enable Hdac3-deficient cells to cope with the oxidative stress caused by Cox-1–dependent production of EFOX.
It is clear that because HDACs target thousands of substrates, including many transcription factors and general transcriptional regulators (e.g., chromatin remodelers) (16), attempts to reduce the consequences of Hdac3 loss to a single mechanism would be unrealistic and unreasonable. The fact that about one-third of the observed effects (including the impaired IL-6 transcription) cannot be ascribed to defective Ifn-β production (and cannot be reversed by Cox-1/2 inhibitors) indicates the contribution of additional mechanisms to the transcriptional phenotype of Hdac3-deficient macrophages. For instance, Hdac3 was reported to be required for Cxcl2 induction by cJun/AP-1 (48). Because AP-1 sites are strongly overrepresented at genomic regions hyperacetylated in Hdac3−/− macrophages, an obvious possibility is that dysregulated AP-1 activity contributes to the phenotype.
It has recently been reported (and confirmed by our own data; SI Appendix, Fig. S7) that Hdac3 loss in macrophages results in hyperresponsiveness to IL-4 (49), which drives macrophages toward a specific polarized state known as alternative activation. Compared with classically activated macrophages, which are induced by IFN-γ through Stat1 (50), alternatively activated macrophages produce lower amounts of inflammatory cytokines and metabolites (e.g., nitric oxide) and are relevant for the response to parasites and for wound healing (51). Importantly, IL-4–activated Stat6 and IFN-γ–activated Stat1 act in an antagonistic fashion to control macrophage polarization (50). Our data showing that Hdac3−/− macrophages lack expression of Stat1 might contribute to explain their increased responsiveness to IL-4. Although pan-HDACis are already available for therapy (15), the search for more specific compounds targeting subsets of HDACs or even single enzymes of the family is actively ongoing (52). Specific Hdac3 inhibitors with an aminobenzamide moiety have recently been identified in a chemoproteomic screening (53), suggesting the possibility of selectively targeting Hdac3-containing corepressor complexes in contexts in which global Hdac inhibition may not provide additional benefits and, instead, may increase side effects.
In this context, an interesting therapeutic opportunity is provided by our observation that the Ifn-β defect is partially rescued by Cox-1/2 inhibitors. The combination of an Hdac3-selective inhibitor with a Cox-1/2 inhibitor may result in the down-regulation of only a subset of Hdac3-dependent genes, including some of great relevance in the pathogenesis of inflammatory disease and inflammation-associated cancer, such as IL-6 (54). Moreover, Cox-1/2 inhibitors may also be used as partial antidotes to alleviate the negative consequences of Hdac3 inhibition that may occur in some individuals.
In conclusion, our data demonstrate an essential role of Hdac3 in the deployment of the inflammatory gene expression program, which is, in part, linked to a defect in Ifn-β production and may be of therapeutic relevance for treatment of inflammatory diseases.
Materials and Methods
Antibodies.
The following antibodies were used: acetyl histone H4 (06-866; Millipore), phospho-IRF-3 (Ser396, CST 4947S; Cell Signaling Technology), IRF-3 (4302; Cell Signaling Technology), Stat1 (9172L; Cell Signaling Technology), phospho-Stat1 (9171L; Cell Signaling Technology), phospho-Stat3 (9145; Cell Signaling Technology), Stat3 (9132; Cell Signaling Technology), Stat5 (C-17; Santa Cruz), phospho-Stat5 (Tyr694, 14H2, 9356S Cell Signaling Technology), Cox-1 (4842; Cell Signaling Technology), phospho-Tyk2 (Tyr1054/1055, 9321; Cell Signaling Technology), allophycocyanin (APC) rat anti-mouse CD11b (553312; BD Biosciences), phycoerythrin (PE) rat anti-mouse CD86 (553692; BD Biosciences), PE rat anti-mouse CD40 (553791; BD Biosciences), and anti-mouse F4/80 antigen FITC (48-4801-82; E-Biosciences).
Hdac3-Deficient Mice.
Hdac3fl/fl mice (28), in which Hdac3 exon 7 (encoding the deacetylase domain) is flanked by loxP sites, were crossed to an Mx-Cre strain. To induce Cre expression, 12-wk-old mice received two rounds of i.p. injections of 250 μg of poly(I:C) (27-4732-01; GE Healthcare) in PBS every 2 d. Mice were killed 2 d after the last injection for bone marrow cell isolation. Mice carrying a floxed exon 7, WT exon 7, and deleted exon 7 were identified by PCR screening (SI Appendix, Fig. S1). The following primers were used: Hdac3 1263T 5′-CCACTGGCTTCTCCTAAGTTC-3′ and Hdac3 2158B 5′-CCCAGGTTAGCTTTGAACTCT-3′.
Cell Culture and Retroviral Infections.
Bone marrow cells from mice were plated in 10-cm plates in 6 mL of bone marrow medium (high glucose DMEM supplemented with 20% (vol/vol) low-endotoxin FBS, 30% (vol/vol) L929-conditioned medium, 1% glutamine, 1% penicillin/streptomycin, 0.5% sodium pyruvate, and 0.1% β-mercaptoethanol). Cultures were fed with 2.5 mL of fresh medium every 2 d. Stimulation and harvesting of the cells were carried out at days 6 and 7. LPS from Escherichia coli serotype 055:B5 (Sigma) was used at 100 ng/mL. IFN-β was from PBL International (12400-1). Ten IU/mL indomethacin (25 mM) or diclofenac (1 mM) (Sigma) was given for 48 h before stimulation.
FACS Analysis.
Cells were stained with the following antibodies: F4/80 (used at a 1:50 dilution, ab6640; Abcam) and biotin-labeled CD11b (used at a 1:50 dilution, 557395; BD Biosciences) detected with streptavidin-PE-Cy5 (554062; BD Biosciences). The cells were analyzed by means of a FACSCalibur 4- or 10-channel flow cytometer (BD Biosciences).
Quantitative RT-PCR.
RNA was extracted from macrophages using TRIzol (Invitrogen) and reverse-transcribed with random hexamers. The sequences of the primers used are provided in Dataset S4.
Cytokine Measurements.
Culture supernatant was collected from LPS-treated (14 h) control and Hdac3-null macrophages. IL-6, and TNF-α concentrations were determined by a commercially available ELISA (R&D Systems). Optical densities were measured on a Bio-Rad Dynatech Laboratories ELISA reader at a wavelength of 450 nm.
ChIP.
For ChIP experiments, bone marrow macrophages were left untreated or stimulated for 4 h with LPS. Fixation with formaldehyde and sonication was carried out as described previously (32). ChIP lysates were generated from 2 × 107 cells. Lysates were immunoprecipitated with 10 mg of the anti-AcH4 antibody (06-866; Millipore). Antibodies were prebound overnight to 100 μL of G protein-coupled paramagnetic beads (Dynabeads, Invitrogen) in PBS/BSA 0.5%. Beads were then added to lysates (the preclearing step was omitted), and incubation was allowed to proceed overnight. Beads were washed six times in a modified radioimmunoprecipitation assay (RIPA) buffer [50 mM Hepes (pH 7.6), 500 mM LiCl, 1 mM EDTA, 1% Nonidet P-40, and 0.7% Na-deoxycholate] and once in TE containing 50 mM NaCl. DNA was eluted in TE containing 2% SDS, and cross-links were reversed by incubation overnight at 65 °C. DNA was then purified by means of Qiaquick columns (Qiagen) and quantified using PicoGreen (Invitrogen).
Preparation of ChIP DNA Libraries, Sequencing, and Computational Analyses.
ChIP DNA was prepared for Solexa 2G sequencing using a standard protocol consisting of blunting, addition of dA overhangs, ligation of Illumina adapters, selection on gel, and PCR. A mixture of T4 DNA polymerase, DNA polymerase I, and T4 kinase was used according to the manufacturer’s instructions (Illumina). The PCR was recovered using a Qiaquick PCR purification kit (Qiagen) according to manufacturer’s recommendations. Adaptors for the genome analyzer were added by ligation; the ligated fragments were subjected to PCR amplification and then gel-purified using Qiagen columns. The purified DNA was quantified both with an Agilent Bioanalyzer and Picogreen and diluted to a working concentration of 10 nM. Cluster generation was performed and loaded into individual lanes of a flow cell (4 pM per sample).
Basic data processing was carried out using the Fish the ChIPs pipeline (55). A detailed description of the computational analyses is provided in the SI Appendix. Raw datasets are available for download at the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE33164, which comprises expression data (accession no. GSE33162) and ChIP-Seq data (accession no. GSE33163).
cDNA Microarrays.
A biological triplicate was used for cDNA microarray analyses. RNA was purified using an RNeasy-Maxi kit (Qiagen). Quality analysis of total RNA, cRNA synthesis, hybridization, and data extraction were performed at the Cogentech Microarray Core Facility (European Institute of Oncology-Italian Foundation for Cancer Research Institute of Molecular Oncology Foundation). The mouse gene ST1.0 Affymetrix array (Affymetrix) was used for gene expression screening.
shRNA-Mediated Depletion of Hdac3.
Two constructs were generated using the pLMP miR-30–based backbone (56). The following 22-mer Hdac3-specific sequences were used: 5′-CTGGCATTGACTCATAGCCTAG-3′ and 5′-GCGTGGCTCTCTGAAACCTTAA-3′.
Retroviral infection of mouse bone marrow cells was carried out as described (57).
Lipidomics.
Murine bone marrow-derived macrophages (15 × 106) were incubated with AA (10 μM) and ionomycin (AA23187, 5 μM) for 30 min at 37 °C. In some cases, cells were stimulated with LPS for 2 h at 37 °C, after which AA (10 μM) and ionomycin (5 μM) were added (30 min at 37 °C). Following incubations, cells were immediately scraped on ice, snap-frozen, and stored at − 80 °C until extraction. LC-MS/MS was carried out as described (46). Briefly, all samples for LC-MS/MS analysis were extracted with solid phase extraction columns. Before sample extraction, 500 pg of deuterium-labeled d4-PGE2 was added to facilitate the quantification of sample recovery. Extracted samples were analyzed by a UV LC-MS/MS system, QTrap 5500 (ABSiex), equipped with an Agilent HP1100 binary pump and diode-array detector. An Agilent Eclipse Plus C18 column (50 mm × 4.6 mm × 1.8 m) was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (vol/vol/vol) to 100:0:0.01 at a flow rate of 0.5 mL/min. Identification was conducted using previously published criteria, including matching retention time to synthetic standard and a minimum of six diagnostic ions. Quantification was carried out based on the peak area of the multiple reaction monitoring transition and the linear calibration curve for each compound.
Supplementary Material
Acknowledgments
We thank Charles Serhan (Brigham and Women’s Hospital and Harvard Medical School) for advice and support on the LC-MS/MS analyses. Work in the laboratory of G.N. was supported by the European Community [Sixth Framework Programme (FP6) Grant TRANS-TAR]. The work of J.D. was supported by National Institutes of Health GM Grant 5P01GM095967 (to Charles Serhan).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.T.S. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE33162–GSE33164).
See Author Summary on page 16768 (volume 109, number 42).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121131109/-/DCSupplemental.
References
- 1.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 2.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Natoli G, Ghisletti S, Barozzi I. The genomic landscapes of inflammation. Genes Dev. 2011;25:101–106. doi: 10.1101/gad.2018811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amit I, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 6.Covic M, et al. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-kappaB-dependent gene expression. EMBO J. 2005;24:85–96. doi: 10.1038/sj.emboj.7600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Santa F, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Essen D, Zhu Y, Saccani S. A feed-forward circuit controlling inducible NF-κB target gene activation by promoter histone demethylation. Mol Cell. 2010;39:750–760. doi: 10.1016/j.molcel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez-Carrozzi VR, et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicodeme E, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leoni F, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabiec AM, et al. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J Immunol. 2010;184:2718–2728. doi: 10.4049/jimmunol.0901467. [DOI] [PubMed] [Google Scholar]
- 14.Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32:335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 17.Dhalluin C, et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 18.Wen YD, et al. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc Natl Acad Sci USA. 2000;97:7202–7207. doi: 10.1073/pnas.97.13.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hörlein AJ, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 20.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 21.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: Evolving models of co-repressor action. Nat Rev Genet. 2010;11:109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Kurdistani SK, Grunstein M. Requirement of Hos2 histone deacetylase for gene activity in yeast. Science. 2002;298:1412–1414. doi: 10.1126/science.1077790. [DOI] [PubMed] [Google Scholar]
- 23.Pijnappel WW, et al. The S. cerevisiae SET3 complex includes two histone deacetylases, Hos2 and Hst1, and is a meiotic-specific repressor of the sporulation gene program. Genes Dev. 2001;15:2991–3004. doi: 10.1101/gad.207401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T, Buratowski S. Dimethylation of H3K4 by Set1 recruits the Set3 histone deacetylase complex to 5′ transcribed regions. Cell. 2009;137:259–272. doi: 10.1016/j.cell.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gough DJ, et al. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol. 2010;8:e1000361. doi: 10.1371/journal.pbio.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 28.Bhaskara S, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008;30:61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 30.Doyle S, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 31.Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 32.Ghisletti S, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis. 2011;70(Suppl 1):i109–i112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa S, et al. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci USA. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng CS, et al. The specificity of innate immune responses is enforced by repression of interferon response elements by NF-κB p50. Sci Signal. 2011;4:ra11. doi: 10.1126/scisignal.2001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, et al. Overcoming resistance to histone deacetylase inhibitors in human leukemia with the redox modulating compound β-phenylethyl isothiocyanate. Blood. 2010;116:2732–2741. doi: 10.1182/blood-2009-11-256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 40.Groeger AL, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 42.Straus DS, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 44.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL, Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun H, Sheveleva E, Chen QM. Corticosteroids induce cyclooxygenase 1 expression in cardiomyocytes: role of glucocorticoid receptor and Sp3 transcription factor. Mol Endocrinol. 2008;22:2076–2084. doi: 10.1210/me.2007-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011 doi: 10.1002/0471142735.im1426s95. Chapter 14:Unit 14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim BH, et al. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 48.Wolter S, et al. c-Jun controls histone modifications, NF-kappaB recruitment, and RNA polymerase II function to activate the ccl2 gene. Mol Cell Biol. 2008;28:4407–4423. doi: 10.1128/MCB.00535-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullican SE, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 51.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 52.Bieliauskas AV, Pflum MK. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev. 2008;37:1402–1413. doi: 10.1039/b703830p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bantscheff M, et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol. 2011;29:255–265. doi: 10.1038/nbt.1759. [DOI] [PubMed] [Google Scholar]
- 54.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barozzi I, Termanini A, Minucci S, Natoli G. Fish the ChIPs: A pipeline for automated genomic annotation of ChIP-Seq data. Biol Direct. 2011;6:51. doi: 10.1186/1745-6150-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickins RA, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37:1289–1295. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 57.De Santa F, et al. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]